Abstract
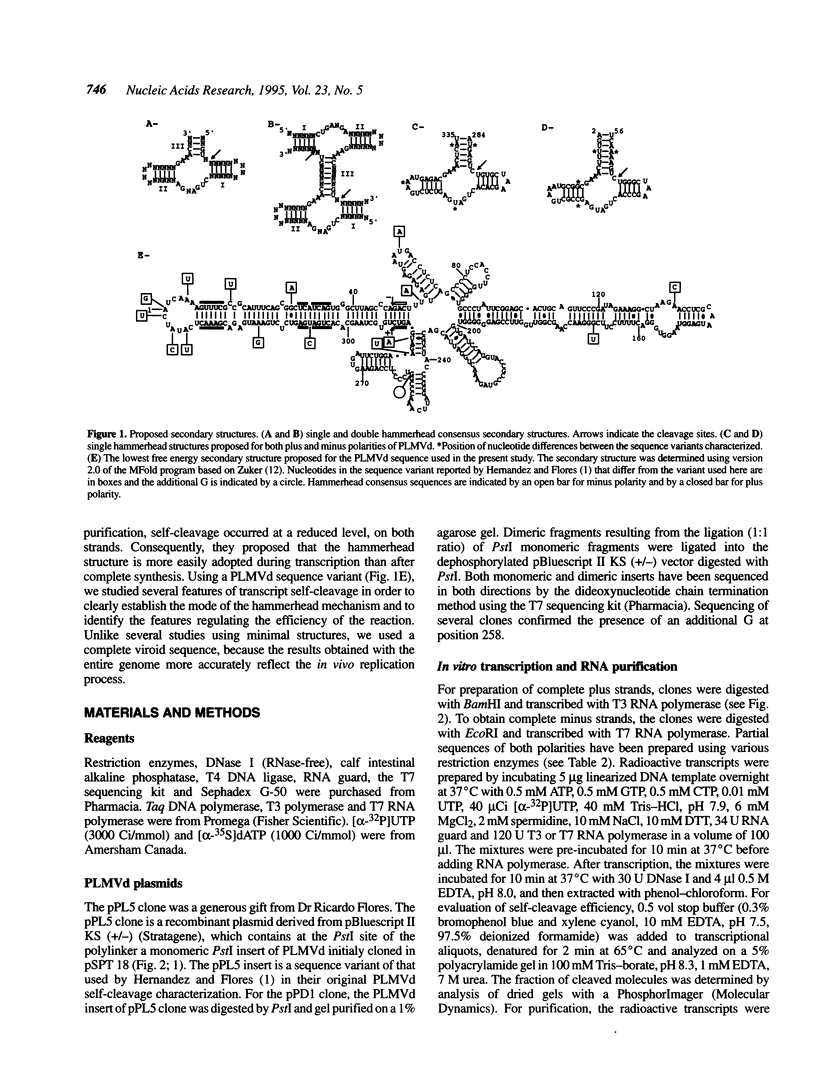
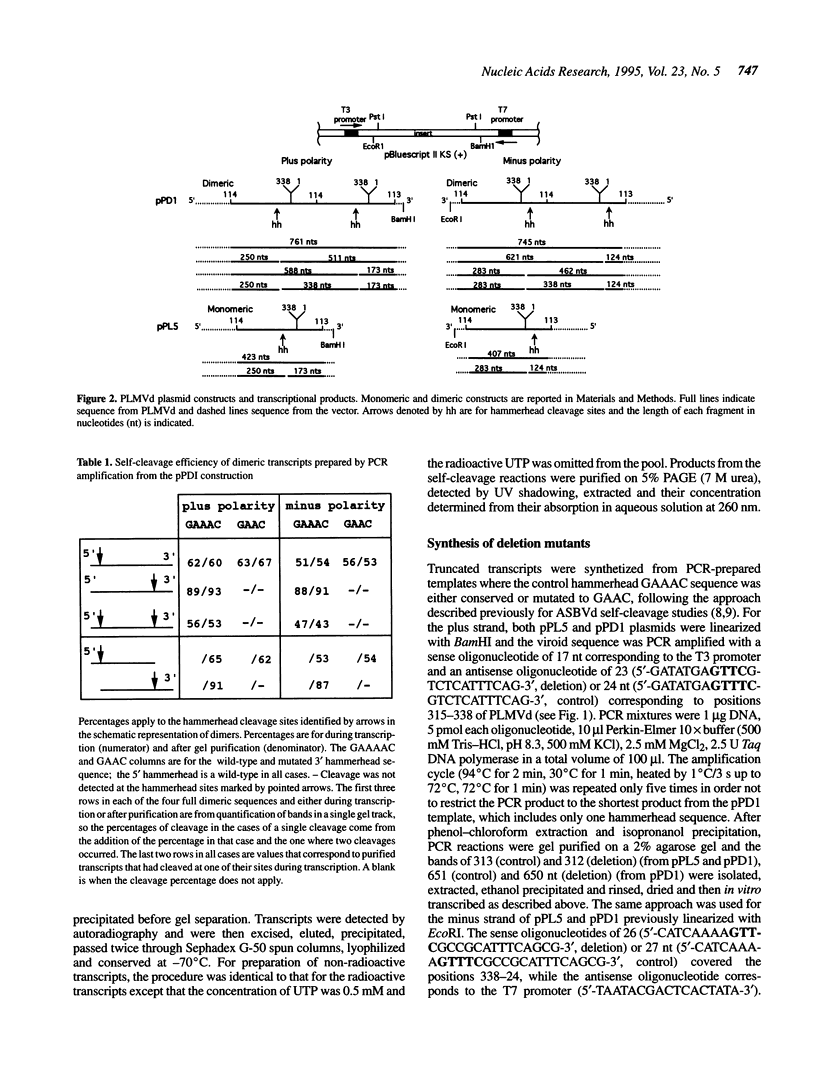
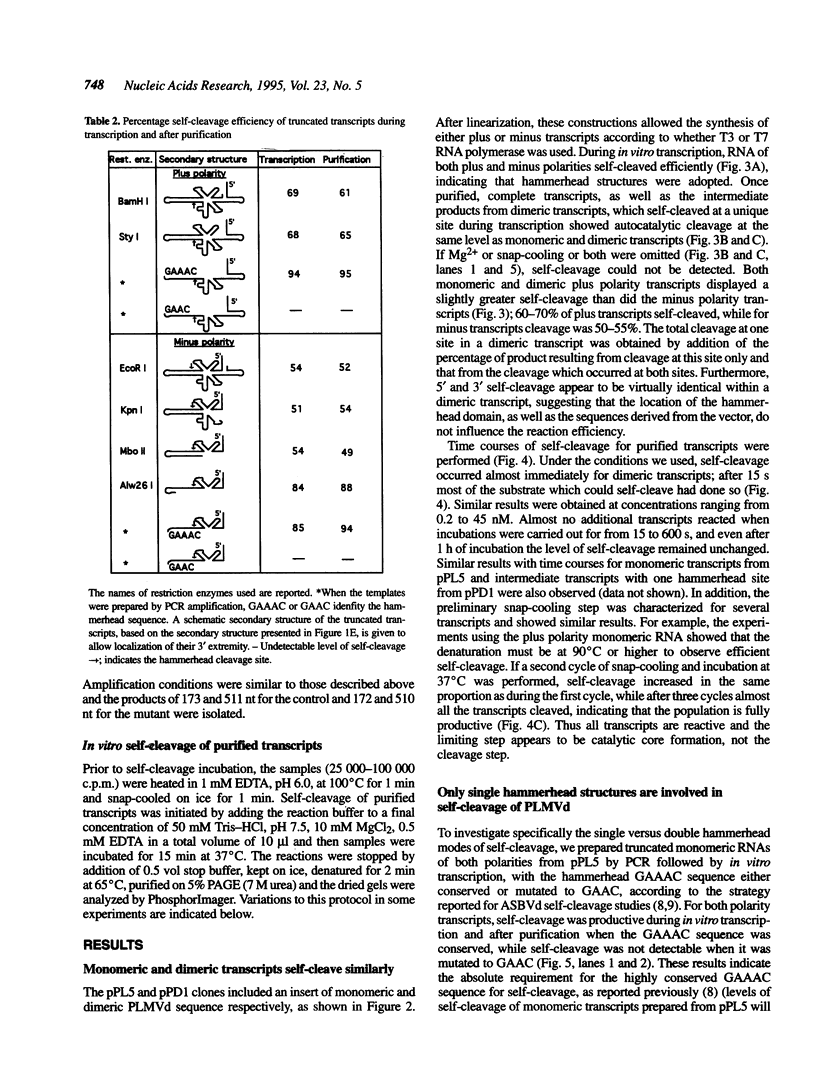
Hammerhead self-cleavage of dimeric, monomeric, truncated and mutated transcripts derived from both polarities of the peach latent mosaic viroid (PLMVd) were characterized. In contrast to some results previously published for a very close sequence variant (see ref. 1), these RNAs exhibit a virtually identical self-cleavage during transcription and after purification. By self-cleavage of dimeric transcripts with normal and mutated hammerhead domains and by complementation experiments, we show that the cleavage reactions involve only single hammerhead structures. This observation contrasts with the case of avocado sunblotch viroid (ASBVd), the other self-cleaving viroid, whose mechanism involves mostly double hammerhead structures, whereas single hammerhead cleavage is associated with viroid-like plant satellite RNAs. The difference in stability between the native secondary structures adopted by viroids and the autocatalytic structures, including the hammerhead motif, governs the efficiency of the self-cleavage reaction. The transition between these conformers is the limiting step in catalysis and is related exclusively to the left arm region of PLMVd secondary structure, which includes the hammerhead sequences. Most of the mutations between the variant we used and the sequence variant previously published are located in this left arm region, which may explain to a great extent the differences in their cleavage efficiency. No interactions with long-range sequences contributing to the autocatalytic tertiary structure were revealed in these experiments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Bratty J., Chartrand P., Ferbeyre G., Cedergren R. The hammerhead RNA domain, a model ribozyme. Biochim Biophys Acta. 1993 Dec 14;1216(3):345–359. doi: 10.1016/0167-4781(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Davies C., Sheldon C. C., Symons R. H. Alternative hammerhead structures in the self-cleavage of avocado sunblotch viroid RNAs. Nucleic Acids Res. 1991 Apr 25;19(8):1893–1898. doi: 10.1093/nar/19.8.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Circular RNAs: relics of precellular evolution? Proc Natl Acad Sci U S A. 1989 Dec;86(23):9370–9374. doi: 10.1073/pnas.86.23.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena S. F., Dopazo J., Flores R., Diener T. O., Moya A. Phylogeny of viroids, viroidlike satellite RNAs, and the viroidlike domain of hepatitis delta virus RNA. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5631–5634. doi: 10.1073/pnas.88.13.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Pabón-Peña L. M. Alternative modes of self-cleavage by newt satellite 2 transcripts. Nucleic Acids Res. 1991 Apr 11;19(7):1699–1705. doi: 10.1093/nar/19.7.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores R., Hernández C., Desvignes J. C., Llácer G. Some properties of the viroid inducing peach latent mosaic disease. Res Virol. 1990 Jan-Feb;141(1):109–118. doi: 10.1016/0923-2516(90)90060-v. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Davies C., Sheldon C. C., Jeffries A. C., Symons R. H. Self-cleaving viroid and newt RNAs may only be active as dimers. Nature. 1988 Jul 21;334(6179):265–267. doi: 10.1038/334265a0. [DOI] [PubMed] [Google Scholar]
- Hernández C., Flores R. Plus and minus RNAs of peach latent mosaic viroid self-cleave in vitro via hammerhead structures. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3711–3715. doi: 10.1073/pnas.89.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D. M., Uhlenbeck O. C. Kinetic characterization of intramolecular and intermolecular hammerhead RNAs with stem II deletions. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6977–6981. doi: 10.1073/pnas.91.15.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. RNA stem stability in the formation of a self-cleaving hammerhead structure. Nucleic Acids Res. 1989 Jul 25;17(14):5665–5677. doi: 10.1093/nar/17.14.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Sänger H. L. Ribonuclease T1 generates circular RNA molecules from viroid-specific RNA transcripts by cleavage and intramolecular ligation. Nucleic Acids Res. 1991 Apr 11;19(7):1605–1612. doi: 10.1093/nar/19.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]