Abstract
Heterozygous reeler mice (HRM) haploinsufficient for reelin express ≈50% of the brain reelin content of wild-type mice, but are phenotypically different from both wild-type mice and homozygous reeler mice. They exhibit, (i) a down-regulation of glutamic acid decarboxylase 67 (GAD67)-positive neurons in some but not every cortical layer of frontoparietal cortex (FPC), (ii) an increase of neuronal packing density and a decrease of cortical thickness because of neuropil hypoplasia, (iii) a decrease of dendritic spine expression density on basal and apical dendritic branches of motor FPC layer III pyramidal neurons, and (iv) a similar decrease in dendritic spines expressed on the basal dendrite branches of CA1 pyramidal neurons of the hippocampus. To establish whether the defect of GAD67 down-regulation observed in HRM is responsible for neuropil hypoplasia and decreased dendritic spine density, we studied heterozygous GAD67 knockout mice (HG67M). These mice exhibited a down-regulation of GAD67 mRNA expression in FPC (about 50%), but they expressed normal amounts of reelin and had no neuropil hypoplasia or down-regulation of dendritic spine expression. These findings, coupled with electron-microscopic observations that reelin colocalizes with integrin receptors on dendritic spines, suggest that reelin may be a factor in the dynamic expression of cortical dendritic spines perhaps by promoting integrin receptor clustering. These findings are interesting because the brain neurochemical and neuroanatomical phenotypic traits exhibited by the HRM are in several ways similar to those found in postmortem brains of psychotic patients.
Brain postmortem studies from patients with schizophrenia reveal a characteristic pattern of neuroanatomical and neurochemical abnormalities including: (i) enlarged cerebral ventricles (1, 2), (ii) altered cortical distribution of NADPH-diaphorase positive cells (3), (iii) decreased cortical thickness (4), (iv) increased cell-packing density associated with a neuropil hypoplasia in absence of gliosis (5), (v) decreased expression of dendritic spine in frontal, temporal, and subicular cortex (5–7), and (v) decreased expression of glutamic acid decarboxylase 67 (GAD67) mRNA in prefrontal cortex neurons, particularly evident in layers II and III (8–11).
Patients with schizophrenia or bipolar disorder with psychosis express about 50% of the normal brain reelin mRNA levels in every cortical structure so far investigated, as well as in hippocampus, cerebellum, and caudate nucleus (9, 10). Although the number of GABAergic neurons that express reelin in prefrontal and temporal cortices (9, 10) and in the hippocampus (12) of these patients is reduced, the number of these interneurons is unchanged (8). Thus, it has been suggested that the decrease of reelin in neurons is probably because of the down-regulation in the expression of GAD67 mRNA and protein in neurons rather than a reduction of the number of neurons per se (9, 10, 12). To evaluate whether the down-regulation of GAD67 mRNA expression is associated with a down-regulation of reelin expression, we studied the expression of the mRNAs encoding for reelin and GAD67 in the brain of reelin haploinsufficient heterozygote reeler mice (HRM) and in heterozygote GAD67 knockout mice (HG67M) and compared them to their respective wild-type background mice (WTM). In the present study, we have also quantified the number of neurons immunopositive for reelin in the motor area of the frontoparietal cortex (FPC) of HRM, as well as the total number of neurons immunopositive for NeuN and the number of glial cells stained by Nissl (13) expressed in each of the six layers of this cortical area. In WTM, HRM, and HG67M, we have also quantified the laminar expression of GAD67-immunopositive neurons and the dendritic spine density expressed by pyramidal neurons of layer III FPC and of CA1 hippocampus.
Materials and Methods
Colonies of HRM and HG67M.
We have established an HRM breeding colony (obtained from The Jackson Laboratory) and more recently an HG67M colony (obtained from the Institute of Molecular Medicine and Genetics, Augusta, GA). HRM (B6C3Fe strain) express a normal and a defective reelin allele with a deletion of approximately 150 kb at the 3′ end (Edinburg mutation) (14), and HG67M are heterozygous for a targeted allele of GAD67 (15). HG67M were originally on a mixed 129/C57BL6J background and have been backcrossed for four to five generations with HRM background.
The offspring of both heterozygous mice were genotyped by PCR as previously described for reelin (16) and GAD67 (17). The primer sequences (from 5′-3′) for reelin were, forward: taatctgtcctcactctgcc; reverse: acagttgacataccttaatc; reverse mutated: tgcattaatgtgcagtgttgtc. The primer sequences for GAD67 were, forward: tagaagctctcccggcacagctctc; reverse: gcgcaggttggtagtattaggatccg; reverse mutated: cgtgttcgaattcgccaatgacaagac. The WTM, HRM, or HG67M used in the experiments were 60- to 80-day-old males and were randomly sampled from several contemporaneous litters.
Quantitative Reverse Transcription–PCR Analysis of Reelin, GAD67, and GAD65 mRNAs.
Primers and internal standards to quantify reelin mRNA were previously described (17); the amplification primers used were forward base pairs 9211–9231 and reverse base pairs 9549–9569 (Gen Bank accession no. HSU79716). Primers for GAD67 were: forward 1855–1878; reverse 2246–2269 base pairs (Gen Bank accession no. M81883); the internal standard contained a BglII restriction endonuclease, which on digestion generated fragments of 199 and 216 base pairs. Primers for GAD65 were: forward 82–103; reverse 507–532 (Gen Bank accession no. M72422); the internal standard contains an XbaI restriction endonuclease, which on digestion generated fragments of 215 and 235 base pairs. The assay was conducted as described by Grayson and Ikonomovic (18).
Immunohistochemistry.
The brains used in these studies were obtained from mice anesthetized for about 1 min in a CO2 chamber and perfused intracardially with 10 ml of PBS followed by 10 ml of ice-cold fixative (for light microscopy: 4% paraformaldehyde in PBS; for Golgi impregnation: 4% paraformaldehyde + 0.25% glutaraldehyde in PBS). Brains were removed and left in fresh fixative for 24 h at 4°C before storage in 30% sucrose at 4°C.
Forty-micrometer sections were cut with a cryostat, and diaminobenzidine immunostaining was performed as previously described (17). The following antibodies were used: (i) mouse monoclonal antibody G-10 (1:1,000), which was raised against the N-terminal region of Reelin (amino acid residue 40–189); a generous gift from A. Goffinet (Department of Human Physiology, Facultés Universitaires Notre-Dame de la Paix School of Medicine, Namur, Belgium): (ii) rabbit GAD67 (1:2,000, Chemicon); (iii) mouse anti-NeuN, a neuronal nuclei-specific marker (1:500, Chemicon).
Stereological Cell-Counting Method.
In different cortical layers of motor FPC, cell density was quantified by a microscopist blind with respect to the mouse genotype, by using variations of the three-dimensional cell-counting method described by Selemon and Goldman-Rakic (4). Briefly, cells were counted with a ×40 Leica TCSNT laser confocal microscope by using the “optical dissector” method in a cortical probe consisting of an uninterrupted series of three-dimensional counting boxes (100 × 100 × 40) that span from the pial surface to the underlying white matter. For each box, the generated confocal image stacks (approximately 16) allow counting of stained cells that come into view (or, alternatively, disappear) through a known depth (Z) of the tissue section by using the Stereo Investigator Confocal Software (Microbright Field, Colchester, VT) and the Optical Fractionator Tool from the Stereo Investigation Program described by Williams and Rakic (19). Cells located completely inside the counting box and those crossing the top, right, or back sides were counted, whereas those crossing other planes were excluded. In each section, the number of reelin-immunopositive neurons was counted in five to six cortical probes in a total of three sections (one of every five sections). Similarly, in serial sections NeuN-immunopositive cells and GAD67-immunopositive cells were quantified, as were Nissl-stained neurons and glial cells; glial cells were differentiated from neurons by using the criteria described by Benes et al. (13). The number of reelin-positive cells, NeuN-positive cells, Nissl-stained neurons, and glial cells are expressed as the mean ± SEM of the number of cells per cubic millimeter, whereas the number of GAD67-positive cells are expressed per area, as defined in Fig. 2.
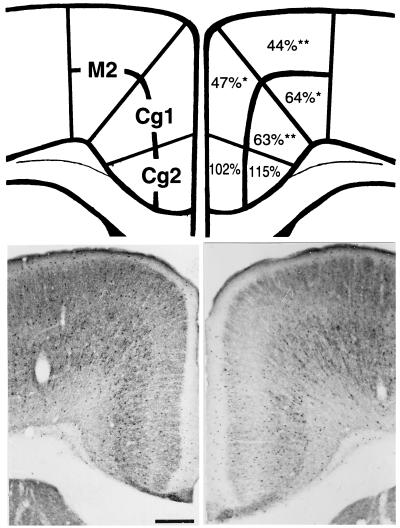
Figure 2.
GAD67-immunoreactive neurons in FPC of HRM and WTM. (Upper Left) A schematic representation of a coronal section of FPC (0.74 mm anterior to Bregma) illustrating the subdivisions of superficial layers (I–IV) and deep cortical layers (V–VI) of the M2, Cg1, and Cg2 regions of the FPC, and Right is the percentage of GAD67-immunoreactive cells in the HRM, as compared with the WTM. Student's t test comparing HRM with WTM. *, P < 0.01;**, P < 0.001. (Lower) Photomicrographs of 40-μm sections through the FPC of the WTM (Left) and HRM (Right) immunolabeled for GAD67. Note that in the HRM there is a decreased number of GAD67-immunopositive cells in the Cg1 and M2 regions, as well as a decrease in the surrounding neuropil expression of GAD67. Each value represents the mean of eight to nine animals. Standard error range from 2–10% of the mean. (Bar = 300 μm.)
Two Independent Methods to Measure Dendritic Spine Density.
Golgi- impregnation technique.
Tissue blocks of desired cortex area about 1–2 mm thick were cut with a razor blade. The blocks were rapid-Golgi stained, as previously described (20). Briefly, the blocks were immersed in a solution of 2% osmium tetroxide in buffer for 24 h, followed by 2-day immersion in a 4% solution of potassium dichromate. Then the blocks were washed briefly in dH2O and fresh solution of 0.75% silver nitrate and stored for 24 h in fresh solution of 0.75% silver nitrate. Finally, the blocks were transferred to a graded series of increasing glycerol concentration and stored in pure glycerol at 4°C. These blocks were embedded in 7% agar and cut at 80 μm by using a vibratome. Sections were kept for 30 min under an illuminated device and studied under the Zeiss Axioskope microscope at ×40 objective with a video camera.
For the quantification of dendritic spine expression density.
Three-dimensional reconstruction of dendrites and their spines at high magnification was obtained by using a Zeiss Axioskope connected to a live-image monitor. Only pyramidal neurons in layer III of the motor cortex that satisfied the following criteria were included: (i) complete impregnation (including all dendrites), not obscured by other neurons or artifacts; (ii) clear image; and (iii) visibility of at least three basal dendrites. For each neuron, the apical and at least three basilar dendrites (and all of their branches) were traced to their natural or artificial ends. Each branch was numbered with reference to its proximity to the cell body. For instance, the basal branch originating from the cell body is B1, which will eventually branch off to form B2, and so on. For each mouse, five neurons from the FPC were selected. For each neuron, the number and length of dendrites were quantified.
Laser confocal microscope fluorescence image technique.
Layer III pyramidal motor neurons from ≈400 μm coronal sections of FPC, serially sectioned 0.5 to 3 mm anterior to the Bregma (21), were stained by pressure injection of microdrops of 1,1′dioctadecyl-3,3,3′,3′-tetramethylindocarbocyamine perchlorate dissolved in menhaden fish oil (22). A total of four to five slices were used from each animal, and four to six motor neurons were stained on each slide. Cells were visualized by a Leica confocal laser-scanning microscope by using a ×40 water-immersion objective. Scans were made of each individual cell somata followed by four to eight serial zoomed optical sections of three to four regions of secondary dendrites around each cell. Individual dendrites were traced three dimensionally from the computer analyses of the optical serial sections, and the entire pyramidal neurons and their dendritic shafts and spines were reconstructed. Quantification of spine density was determined as described for the Golgi impregnation technique.
Results
Reelin, GAD67, and GAD65 mRNA Expression in the HRM and HG67M Models.
Table 1 shows that reelin mRNA expression is decreased by about 50% in the FPC of HRM. This decrease is accompanied by a down-regulation in GAD67 mRNA, with no change in the expression of GAD65 mRNA. Table 1 also shows that in FPC of HG67M, the expression of GAD67 mRNA is down-regulated by about 50%, whereas the expression of GAD65 and reelin mRNAs is unchanged. However, despite the low content of GAD67 mRNA, the down-regulation of GAD67 by 50% is associated with a 20% decrease of FPC GABA content (unpublished data).
Table 1.
Reelin, GAD67 and GAD65 mRNA levels (attomol/μg total RNA) in FPC of WTM, HRM and HG67M
| Type of mice | Reelin | GAD67 | GAD65 |
|---|---|---|---|
| WTM | 190 ± 9.0 | 7.0 ± 0.80 | 48 ± 14 |
| HRM | 99 ± 16* | 4.2 ± 0.59* | 40 ± 12 |
| HG67M | 145 ± 24 | 3.2 ± 0.38* | 42 ± 11 |
Mean ± SEM for WTM (n = 6), HRM (n = 6), and HG67M (n = 4). Student's t test, two-tailed. *, P < 0.01.
Expression Density of Reelin-Positive Neurons in the FPC of the HRM Model.
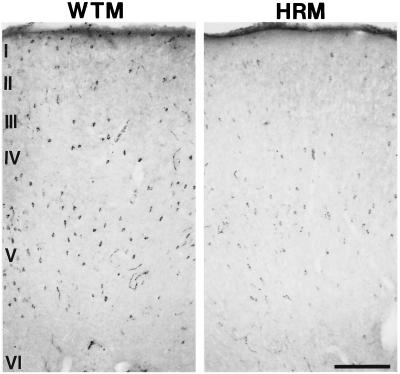
In Table 2, we report that in motor FPC of HRM, stereological counts of reelin-immunopositive neurons were significantly decreased in five of the six cortical layers; the nonsignificant decrease in the expression of reelin-positive neurons in layer VI reflects the extremely low density of reelin-immunopositive neurons expressed by this cortical layer. Fig. 1 shows that in the WTM (Left), as previously described in rats (17, 23), the neurons containing reelin are scattered throughout the cortical mantle. In the HRM (Right), the reelin-expressing neurons are scattered throughout the cortex, but their density is significantly lower than that of WTM (Fig. 1 and Table 2). In addition, the diffuse reelin immunostaining expressed in the neuropil of HRM appears to be lower than that in WTM (Fig. 1).
Table 2.
Measurement of cell density and cortical thickness in motor FPC* of WTM and HRM
| Reelin positive neurons (no.
neuron/μm3 × 10−3)
|
Neurons
(no. neuron/μm3 ×
10−3)
|
Glial cells (no.
neuron/μm3 × 10−3)
|
Cortical
layer thickness, μm
|
|||||
|---|---|---|---|---|---|---|---|---|
| WTM | HRM | WTM | HRM | WTM | HRM | WTM | HRM | |
| Layer I | 5.8 ± 0.6 | 2.0 ± 0.5† | 24.5 ± 1.3 | 32.4 ± 1.8† | 50.7 ± 5.6 | 50.0 ± 4.8 | 106 ± 8.5 | 92.0 ± 7.8 |
| Layer II | 7.2 ± 2.2 | 0.3 ± 0.2† | 55.1 ± 5.1 | 63.5 ± 4.6 | 47.7 ± 6.2 | 45.9 ± 4.4 | 75.0 ± 0.9 | 72.7 ± 1.4 |
| Layer III | 3.6 ± 1.2 | 0 ± 0† | 45.4 ± 1.7 | 81.5 ± 4.4† | 33.9 ± 3.2 | 31.3 ± 4.4 | 98.3 ± 0.8 | 93.3 ± 1.4† |
| Layer IV | 2.2 ± 0.6 | 0.3 ± 0.2† | 40.2 ± 1.4 | 78.5 ± 3.7† | 25.6 ± 1.3 | 37.3 ± 3.0† | 130 ± 1.1 | 113.7 ± 2.4† |
| Layer V | 1.6 ± 0.6 | 0 ± 0† | 41.9 ± 2.5 | 87.8 ± 3.7† | 21.9 ± 1.6 | 34.1 ± 3.0† | 346.7 ± 2.3 | 316 ± 3.7† |
| Layer VI | 0.5 ± 0.3 | 0 ± 0 | 45.1 ± 3.2 | 86.4 ± 5.2† | 21.6 ± 1.6 | 32.9 ± 1.9† | 433.8 ± 3.1 | 406 ± 4.0† |
Each value is the mean ± SEM of five animals.
Coronal sections between 0.5 and 1 mm anterior to Bregma.
Indicated there is a significant different between WTM and HRM; Student's t test, P < 0.05.
Figure 1.
Photomicrographs of reelin-immunostaining through the motor FPC of WTM (Left) and HRM (Right) of 20-μm coronal sections taken 0.74 mm anterior to Bregma. Note that there is a decrease in the number of reelin-immunopositive neurons as well as an apparent decrease in the expression of reelin in the neuropil. (Bar = 200 μm.)
Expression Density of GAD67-Immunoreactive Neurons in the FPC Cortex of the HRM Model.
The number of GAD67 immunoreactive neurons was quantified in various FPC regions (Fig. 2), which were labeled as M2, Cg1, and Cg2, following the nomenclature of Franklin and Paxinos (21). Fig. 2 Upper shows all counts of GAD67-immunopositive neurons in the HRM model as percentage of those of the WTM. The percentage of GAD67-immunopositive neurons in the HRM model is significantly decreased in the M2 and Cg1 regions of the FPC, and this decrease is more pronounced in the superficial layers (I–IV). GAD67 immunoreactivity in the neuropil of M2 and Cg1 regions in FPC sections of HRM is also lower than those of WTM.
Neuronal Packing Density in the FPC of HRM Model.
The stereological counts of neurons and glial cells and the estimation of cortical layer thickness in the motor FPC area of WTM and HRM are reported in Table 2. The HRM model shows a significant increase in neuronal packing density, evidenced by an increase in neuronal density and a concomitant decrease in cortical thickness, which is particularly striking in cortical layers III–VI. In addition, deeper cortical layers IV–VI also show a modest increase in glial cell density, present only in layers that have a significantly reduced thickness.
Dendritic Spine Expression Density in the HRM Model.
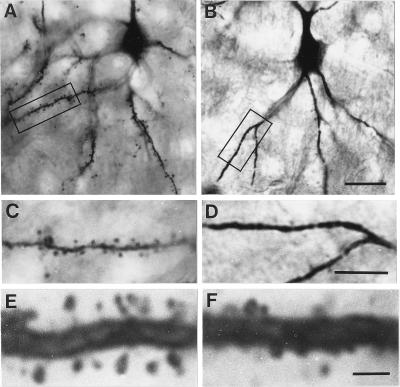
As seen in Fig. 3, spine density on layer III pyramidal cells of the FPC of the HRM model (B and D) is significantly lower than in WTM (A and C). Bar graphs in Fig. 4 show that the expression density of dendritic spines in various apical and basal branches of layer III pyramidal cells in FPC of HRM is significantly lower than in WTM. In addition, the morphology of the dendritic spines expressed in layer III pyramidal neurons of HRM differs from the spine morphology of homologous branches of WTM: the HRM spines appear somewhat smaller and have shorter necks than those of WTM. It is possible also to detect filopodia-like spines in WTM but not in HRM (Fig. 3 E and F). However, neither the length nor the thickness of dendritic branches differs in these two groups of mice (data not shown). Although less dramatic, there is also a significant decrease in the expression density of dendritic spines on most basal branches, but not on the apical branches of CA1 hippocampal pyramidal neurons of the HRM model (Fig. 4). Furthermore, there is a modest but significant decrease in dendritic spine expression on the distal branches of Purkinje neurons in the cerebellum (WTM = 15 ± 0. 3 and HRM = 12 ± 0.3 spines per 10 μm dendritic length; n = 5; P < 0.001). Note that in Purkinje neurons, only the spine expression on distal branches could be quantified because the dendritic arborization of the proximal branches was very intricate and too voluminous to allow a confident quantification of spine expression. Spine density was also determined on basilar or apical dendrites of layer III pyramidal neurons of the FPC of HG67M model. In this model, there were no significant differences in expression density of spines in basal or apical dendrite branches when compared with the respective WTM. The average number of spines was 1.8 ± 0.3 (basal) and 3.0 ± 0.4 (apical) per 10 μm of dendrite in 3 WTM, and 1.5 ± 0.2 (basal) and 2.2 ± 0.5 (apical) in the homologous dendritic branches of 3 HG67M.
Figure 3.
Photomicrographs showing a Golgi-impregnated FPC layer III pyramidal cell from a WTM (A) and a HRM (B). C and D are higher magnifications of the area boxed in A and B, respectively. Note the decreased number of spines on the dendrites of the HRM. High magnification of B2 dendritic branches shows that the spines of the HRM (F) appear to be smaller and have a shorter neck than the spines of the WTM (E). (Bars in A and B = 20 μm; in C and D = 10 μm; in E and F = 2.5 μm.)
Figure 4.
Number of dendritic spines expressed on basal or apical branches of motor FPC layer III pyramidal neurons (Left) and on basal or apical branches of hippocampal (HIP) CA1 pyramidal neurons (Right). Coronal FPC sections 0.5–1 mm anterior to the Bregma, □, WTM; ■, HRM. Each value is the mean ± SEM of five animals. Student's t test comparing HRM with WTM *, P < 0.02.
Similar results were obtained by laser confocal microscope imaging of cortical pyramidal neurons stained with Δ9 1,1′dioctadecyl-3,3,3′,3′-tetramethylindocarbocyamine perchlorate to visualize dendritic spines. Fig. 5 shows that the apical and basal dendrites branches of cortical pyramidal neurons of HRM have fewer spines than the WTM. The average number of spines in apical dendrite branches is: 6.1 ± 0.6 per 10 μm of dendrite in the WTM and 2.9 ± 0.2 in the HRM (n = 5, P < 0.001). This result provides an independent confirmation of the results obtained by Golgi impregnation.
Figure 5.
Laser confocal microscope fluorescent image of FPC pyramidal neurons stained with Δ9 1,1′dioctadecyl-3,3,3′,3′-tetramethylindocarbocyamine perchlorate. (A and C) Pyramidal neuron of motor FPC area (1.0 mm anterior to Bregma) of WTM. (B and D) Pyramidal neuron from the homologous FPC area of HRM. (Lower C and D) are the higher magnification of the area boxed in A and B. (Bars: A and B = 10 μm; C and D = 5 μm.)
Discussion
The present experiments have been conducted to establish whether the haploinsufficiency of reelin of the HRM, which is similar in extent to that measured in the cortex of psychotic (schizophrenia and bipolar depressed) patients (9, 10), is associated with a neuropil hypoplasia and a down-regulation of GAD67 similar to that detected in the brains of these patients.
Decreased Dendritic Spine Density in the HRM Model.
In this study, we showed that the HRM model exhibits a decreased dendritic spine density. This characteristic is similar to what is seen in postmortem brains of schizophrenic and bipolar disorder patients (5–7). Because dendritic spines are critical for mammalian brain synaptic plasticity, a reduction of dendritic spine density has important implications for brain function.
A decreased dendritic spine density may also have important functional consequences, because about 80% of the excitatory synaptic afferents to cortical pyramidal neurons impinge on dendritic spines (24). These cortical pyramidal cells also receive local inhibitory GABAergic innervation from several subtypes of GABAergic interneurons (25), including horizontal, bitufted and Martinotti cells, which are reelin-immunopositive (23). Conversely, basket and chandelier cells, which inhibit pyramidal cells at the level of the aspiny cell body and axon initial segment, respectively, do not express reelin (23). Because reelin is synthesized and secreted from the subpopulation of GABAergic interneurons that impinge directly on dendritic spines and shafts, and because electron microscopy reveals that reelin is found to adhere to and presumably act on dendritic spines (26), it seems possible that a decrease in reelin expression may be related to the decrease in dendritic spine expression. Our finding that dendritic spines are not decreased in HG67M suggests that a down-regulation of GAD67 is not driving the decreased spine expression.
In the HRM hippocampus, dendritic spine density is decreased on basal dendrites of CA1 pyramidal cells, although not on apical dendrites. This fact is in keeping with our previous findings that in the rat hippocampal formation, the majority of GABAergic interneurons expressing reelin are located in the stratum oriens (17), around the basal dendrites of pyramidal neurons. If reelin plays a role in neuropil plasticity regulation via a trophic action on dendritic spines, it is not surprising that a down-regulation of reelin would have a greater impact on dendritic spine expression on nearby basal dendrites than on the apical dendrites of the reelin-scarce stratum radiatum.
In the cerebellum, reelin is synthesized by glutamatergic granule cells and secreted by their axon parallel fibers onto Purkinje cell dendritic trees (17). Dendritic spines are also decreased, albeit modestly, on the distal dendrites of cerebellar Purkinje cells in the HRM. Overall, dendritic spine density was decreased in several brain areas of the HRM in a manner that may be related to the level of reelin that is normally released into the extracellular matrix surrounding their dendrites (26). In contrast, dendritic spine expression does not appear to be reduced in HG67M, suggesting that the decrease of GAD67 present in HRM may not likely contribute significantly to the decrease in dendritic spine expression observed in the HRM.
Reelin Expression.
We have reported that reelin haploinsufficient HRM express about 50% of the normal amount of reelin in various brain structures, in peripheral organs, and in plasma (16, 27). Here we show that the HRM also express about half of the normal levels of reelin mRNA in FPC (Table 1) and have a significant decrease in the number of cells expressing immunodetectable levels of reelin. Because there is no loss of cells in the FPC of these animals, it appears that the decrease in the number of reelin-positive cells is not because of a decrease of GABAergic interneurons (see Fig. 1). Furthermore, by using electron microscopy, we have shown that the amount of reelin expressed around dendritic spines and their shafts is also decreased in the HRM FPC (G. Pappas, personal communication). Interestingly, postmortem brains of schizophrenia or bipolar disorder patients with psychosis exhibit a similar loss of reelin immunolabeled cells and reelin expression in the extracellular matrix, with no loss of neurons (9, 10). Because schizophrenics and bipolar disorder patients with psychosis consistently manifest a decrease of GAD67, without changes of GAD65 expression (8–11), we investigated whether a primary defect of GAD67 gene expression in GABAergic neurons is directly or indirectly responsible for the down-regulation of reelin expression we have consistently detected in psychotic patients' postmortem brains. Here we report that reelin mRNA levels are normal in the HG67M (Table 1), suggesting that the down-regulation of GAD67 is not likely driving the down-regulation of reelin in brains of psychotic patients.
GAD67 Down-Regulation in the HRM Model.
Similar to the postmortem brains of schizophrenia and bipolar disorder patients with psychosis (10), the brains of HRM also exhibit a down-regulation in the expression of GAD67 mRNA and in the number of FPC neurons in which we detected GAD67 immunoreactivity. However, because in the HRM, as in the brain of schizophrenic patients (9–11), there is no apparent loss of neurons (see Table 2), it is likely that GABAergic neurons are still present but do not express immunodetectable levels of GAD67. In addition, like in the brains of psychotic patients, there is a loss of GAD67 immunoreactivity in the surrounding neuropil. This likely reflects decreased GAD67 expression in the axon terminals of these interneurons, presumably in response to the decreased number of synaptic targets associated with the dendritic spine. Interestingly, M2 and Cg1 areas of the PFC have also been implicated in the pathophysiology of schizophrenia (28).
From these data, we cannot determine whether the GABAergic interneurons that are not expressing normal levels of GAD67 are reelin-containing cells. However, because reelin is expressed primarily in horizontal, bitufted, and Martinotti cells (23), and because the location of GABAergic interneurons that are down-regulated in M2 and Cg1 is mostly in the superficial layers where these GABAergic neurons are located (17, 23), it is possible that reelin-containing cells are among those that exhibit a down-regulation of GAD67 expression.
It is unlikely that the decrease of GAD67 mRNA expression in HRM is caused by a GAD67 genomic defect associated with the reelin gene mutation, because in the HRM cerebellum, the expression of GAD67 is virtually identical to that in the cerebellum of WTM (data not shown).
It does not appear that in the HRM model GAD65 is compensating for the down-regulation of GAD67, because GAD65 levels are virtually unchanged. Although GAD65 expression is also not up-regulated in the postmortem brains of schizophrenia and bipolar disorder patients with psychosis (10), one should be prudent in making analogies between human and mouse brain models, because GAD65 is the most predominantly expressed GABA synthesizing enzyme in the rat (29) and mouse brain (see Table 1), whereas GAD67 is the most predominantly expressed GABA synthesizing enzyme in the human brain (10).
Reduction of Cortical Thickness in the HRM Model.
As in the brains of schizophrenia patients (25), HRM have a decrease in cortical thickness that appears to be the result of a neuropil hypoplasia rather than a consequence of a pathological neuronal necrosis. This finding is in line with the apparent absence of reactive gliosis, which would be expected if neuronal necrosis or apoptosis has taken place. Although we found a modest increase in glial cells in the deeper cortical layers, this appears to be the result of an increased glial cell-packing density, because it is observed only in the layers where thickness is significantly reduced. Furthermore, neuronal counts are significantly increased in almost every cortical layer, which, given the reduction in cortical thickness, likely reflects an increased cell-packing density rather than an increase in the expression of neurons per se. Because the neuropil consists mainly of axons, dendritic trees, and spines, the decreased spine density reported here to occur in the FPC of HRM could potentially account for the diminished neuropil. In fact spines express receptors, which are targets for GABAergic, glutamatergic, and monaminergic axon terminals; reduction of spine-associated receptors triggers compensatory pruning of these axon terminals.
Possible Role of Reelin on Dendritic Spines Expression.
Although it appears that a reelin deficiency is associated with a decrease in dendritic spine density in both the HRM and in patients with schizophrenia (5–7), one may speculate that in the adult brain, reelin may play a role in dendritic spine dynamics. In fact, the HRM model shows not abnormalities in dendritic length or thickness but rather changes in the shape, size, and number of spines and filopodia (Fig. 5). Our electron microscopy studies in monkeys show that reelin released into the extracellular matrix decorates dendritic spines and shafts of their presumed target cells (26). Reelin's colocalization with α3 integrin receptor subunits on these dendritic spines and shafts suggests that integrins, which function selectively to interconnect extracellular matrix proteins with intracellular cytoskeletal proteins, are the putative receptors for reelin (30). On the basis of converging evidence from several laboratories, our current working hypothesis postulates that in adult brain, reelin adheres to dendrites and spines presumably as a homopolymeric aggregate (31). The reelin–integrin receptor signal transduction may trigger an intracellular transduction cascade involving the adaptor protein mouse disabled 1 or mDab1 (32), which activates the phosphorylation of Dab1 by a focal adhesion tyrosine kinase (for details of this proposed model, see refs. 10 and 33). Phosphorylated Dab1 may then transfer specific proteins to the polyribosomes and mRNAs resident at the confluence of spines and dendrites and directly or indirectly activate the translation of these mRNAs, which often encode cytoskeletal proteins. The ability of spines to synthesize proteins suggests not only that the spine protein composition, shape, and volume can change as a function of this synthesis, but also that spines, via the synthesis of new proteins, can send specific messages to the nucleus and thereby modify nuclear DNA transcription.
Concluding Remarks.
In the HRM, as in the brains of patients with schizophrenia, a reelin haploinsufficiency is associated with neuropil hypoplasia, which we suggest is an important factor in psychosis vulnerability. This hypoplasia may also underlie behavioral abnormalities, including the disruption of prepulse inhibition (16), increased anxiety in the elevated plus-maze test (16), cognitive impairments in an eight-arm radial maze task, and abnormal responses to an intruder mouse (G.C., unpublished work). The seemingly analogous neuroanatomical and behavioral abnormalities of the HRM and the schizophrenia patient, which at present do not appear to have other transmitter mechanism commonalties except for the decreased reelin and GAD67, suggest that reelin haploinsufficiency may provide a model to study a new pharmacology of the psychosis vulnerability and perhaps of psychotic symptoms.
Acknowledgments
We thank Dr. Floyd E. Bloom, Department of Neuropharmacology, the Scripps Research Institute, La Jolla, CA, for constructive criticisms and suggestions in the preparation of the manuscript. This work was in part supported by National Institute of Mental Health Grants MH 49486 and MH 62188 (to A.G.)
Abbreviations
- GAD67
glutamic acid decarboxylase 67
- HRM
heterozygous reeler mouse
- WTM
wild-type mice
- FPC
frontoparietal cortex
- HG67M
heterozygous GAD67 knockout mice
References
- 1.Weinberger D R, Berman K F, Zec R F. Arch Gen Psychol. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger D R, Lipska B K. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- 3.Akbarian S, Kim J J, Potkin S G, Hetrick W P, Bunney W E, Jr, Jones E G. Arch Gen Psychol. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- 4.Selemon L D, Goldman-Rakic P S. Biol Psychol. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 5.Garey L J, Ong W Y, Patel T S, Kanani M, Davis A, Mortimer A M, Barnes T R, Hirsch S R. J Neurol Neurosurg Psychol. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glantz L A, Lewis D A. Arch Gen Psychol. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Rosoklija G, Toomayan G, Ellis S P, Keilp J, Mann J J, Latov N, Hays A P, Dwork A J. Arch Gen Psychol. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 8.Akbarian S, Kim J J, Potkin S G, Hagman J O, Tafazzoli A, Bunney W E, Jr, Jones E G. Arch Gen Psychol. 1995;552:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 9.Impagnatiello F, Guidotti A R, Pesold C, Dwivedi Y, Caruncho H, Pisu M G, Uzunov D P, Smalheiser N R, Davis J M, Pandey G N, et al. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti A, Auta J, Davis J M, DiGiorgi-Gerenini V, Dwivedi J, Grayson D R, Impagnatiello F, Pandey G N, Pesold C, Sharma R F, et al. Arch Gen Psychol. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 11.Volk D W, Austin M C, Pierri J N, Sampson A R, Lewis D A. Arch Gen Psychol. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 12.Fatemi S H, Earle J A, McMenomy T. Mol Psychol. 2000;5:654–663. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 13.Benes F M, McSparren J, Bird E D, Sangiovanni J P, Vincent S L. Arch Gen Psychol. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 14.D'Arcangelo G, Miao G G, Curran T. Mol Brain Res. 1996;39:223–236. doi: 10.1016/0169-328x(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 15.Condie B G, Bain G, Gottlieb D I, Capecchi M R. Proc Natl Acad Sci USA. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tueting P, Costa E, Dwivedi J, Guidotti A, Impagnatiello F, Manev R, Pesold C. NeuroReport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- 17.Pesold C, Impagnatiello F, Pisu M G, Uzunov D P, Costa E, Guidotti A, Caruncho H J. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grayson D R, Ikonomovic S. In: In-Vitro Neurochemical Techniques. Boulton A A, Baker G B, Bateson A N, editors. Vol. 34. Totowa, NJ: Humana; 1998. pp. 127–151. [Google Scholar]
- 19.Williams R W, Rakic P. J Comp Neurol. 1998;278:233–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- 20.Kisvarday Z F, Gulyas A, Beroukas D, North J B, Chubb I W, Somogyi P. Brain. 1990;113:793–812. doi: 10.1093/brain/113.3.793. [DOI] [PubMed] [Google Scholar]
- 21.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic; 1997. [Google Scholar]
- 22.Hosokawa T, Bliss T V, Fine A. NeuroReport. 1992;3:477–480. doi: 10.1097/00001756-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Pesold C, Liu W S, Guidotti A, Costa E, Caruncho H J. Proc Natl Acad Sci USA. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberwine J H. In: Dendrites. Stuart G, Spruston N, Häusser M, editors. New York: Oxford Univ. Press; 1999. pp. 35–67. [Google Scholar]
- 25.Lewis D A, Lieberman J A. Neuron. 2000;289:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez M A, Pesold C, Liu W S, Kriho V, Guidotti A, Pappas G D, Costa E. Proc Natl Acad Sci USA. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. . (First Published March 21, 2000; 10.1073/pnas.050589797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smalheiser N R, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, Kriho V, Pappas G. Proc Natl Acad Sci USA. 2000;97:1281–1286. doi: 10.1073/pnas.97.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benes F M, Vincent S L, Alsterberg G, Bird E D, San Giovanni J P. J Neurosci. 1992;12:924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E. Proc Natl Acad Sci USA. 2001;98:3483–3488. doi: 10.1073/pnas.051628698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dulabon L, Olson E C, Taglienti M G, Eisenhuth S, McGrath B, Walsh C A, Kreidberg J A, Anton E S. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 31.Utsunomiya-Tate N, Kubo K, Tate S, Kainosho M, Katayama E, Nakajima K, Mikoshiba K. Proc Natl Acad Sci USA. 2000;97:9729–9734. doi: 10.1073/pnas.160272497. . (First Published August 1, 2000; 10.1073/pnas.160272497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice D S, Curran T. Gene Dev. 1999;13:2758–2773. doi: 10.1101/gad.13.21.2758. [DOI] [PubMed] [Google Scholar]
- 33.Guidotti A, Pesold C, Costa E. Neurochem Res. 2000;25:1207–1218. doi: 10.1023/a:1007635927069. [DOI] [PubMed] [Google Scholar]