Abstract
Dendritic cells are sentinels in innate and adaptive immunity. Upon virus infection, a complex program is in operation, which activates IκB kinase (IKK), a key regulator of inflammatory cytokines and costimulatory molecules. Here we show that the γ134.5 protein, a virulence factor of herpes simplex viruses, blocks Toll-like receptor-mediated dendritic cell maturation. While the wild-type virus inhibits the induction of major histocompatibility complex (MHC) class II, CD86, interleukin-6 (IL-6), and IL-12, the γ134.5-null mutant does not. Notably, γ134.5 works in the absence of any other viral proteins. When expressed in mammalian cells, including dendritic cells, γ134.5 associates with IKKα/β and inhibits NF-κB activation. This is mirrored by the inhibition of IKKα/β phosphorylation, p65/RelA phosphorylation, and nuclear translocation in response to lipopolysaccharide or poly(I:C) stimulation. Importantly, γ134.5 recruits both IKKα/β and protein phosphatase 1, forming a complex that dephosphorylates two serine residues within the catalytic domains of IκB kinase. The amino-terminal domain of γ134.5 interacts with IKKα/β, whereas the carboxyl-terminal domain binds to protein phosphatase 1. Deletions or mutations in either domain abolish the activity of γ134.5. These results suggest that the control of IκB kinase dephosphorylation by γ134.5 represents a critical viral mechanism to disrupt dendritic cell functions.
Herpes simplex virus 1 (HSV-1), a member of the Herpesviridae, establishes both latent and lytic infection (45). Upon infection of the mucosal tissues, HSV encounters a variety of cells, including dendritic cells (DCs), that bridge innate and adaptive immunity (7). Immature DCs are able to capture and process viral antigens. When activated, DCs express a high level of costimulatory molecules. Moreover, DCs release inflammatory cytokines to promote DC maturation. A prominent feature of DCs is to activate naive T cells, where myeloid mucosal and lymph node-resident DCs are responsible for HSV-specific T cell activation (1, 40, 47).
A complex program is in operation upon DC maturation, which is coupled to Toll-like receptor (TLR)-related pathways (34). For example, when exposed to lipopolysaccharide (LPS) or viral proteins (3, 24, 41), TLR4 activates the two arms of downstream signaling. In this process, TLR4 recruits TRIF via an adaptor, TRAM, and migrates to the endosomal membrane, where it activates TANK binding kinase 1 (TBK1) and interferon (IFN) regulatory factor 3 (IRF3). In parallel, TLR4 engages with MyD88 through the adaptor TIRAP (also called Mal) at the plasma membrane, which facilitates the formation of a complex consisting of TRAF6, TAK1, TAB2, and IRAK. This relays signals to IκB kinase (IKK), containing the IKKα, ΙΚΚβ, and IKKγ subunits. Activated IκB kinase phosphorylates IκB proteins to trigger their ubiquitination and proteosome-mediated degradation, leading to the nuclear translocation of NF-κB, which usually exists as a p65 (RelA)/p50 heterodimer (12). Accordingly, TLR activation upregulates the expression of inflammatory cytokines and costimulatory molecules.
While DCs can recognize HSV through TLR-related mechanisms (15, 22, 27, 33), viral replication compromises DC functions. Several lines of evidence demonstrate that HSV interactions with immature DCs result in the downregulation of costimulatory molecules and inflammatory cytokines (19, 29, 32, 36). Recently, we reported that the γ134.5 protein of HSV-1 is required to suppress DC maturation, resulting in impaired T cell activation (18). This phenotype fits with efficient viral replication (18). HSV γ134.5 is a virulence factor that promotes viral pathogenesis (4, 44). In infected cells, it precludes translational arrest by the double-stranded RNA-dependent protein kinase PKR (5). In doing so, HSV γ134.5 redirects protein phosphatase 1 (PP1) to dephosphorylate the α-subunit of translation initiation factor 2 (13, 14). Additionally, γ134.5 inhibits TBK1-mediated type I interferon induction early in infection (43). HSV γ134.5 also interacts with Beclin 1 and blocks autophagy (30). Nevertheless, the mechanism through which γ134.5 impairs DC maturation remains largely unknown. Here, we demonstrate that HSV γ134.5 abrogates the induction of inflammatory cytokines and costimulatory molecules by TLR in infection. Remarkably, HSV γ134.5 recruits both PP1 and IκB kinase, forming a complex that dephosphorylates the α/β-subunit of IκB kinase and inactivates NF-κB. These results highlight a novel HSV-mediated mechanism.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Harlan Sprague Dawley Inc. and housed under specific-pathogen-free conditions in biosafety level 2 containment. Groups of 5-week-old mice were selected for this study. Experiments were performed in accordance with the guidelines of the University of Illinois, Chicago.
Cells and viruses.
HeLa and 293T cells were obtained from the American Type Culture Collection and propagated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Myeloid DCs were generated as previously described (17). Briefly, bone marrow cells were removed from the tibias and femurs of BALB/c mice. Following red blood cell lysis and washing, progenitor cells were plated in RPMI 1640 medium (Invitrogen, Auckland, New Zealand) supplemented with 10% fetal bovine serum (FBS), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Biosource, Camarillo, CA) in 6-well plates at 4 × 106 cells/well. Cells were supplemented with fresh medium every other day. On day 8, DCs were positively selected for surface CD11c expression by using magnetic beads (Miltenyi Biotech, Auburn, CA) to give a >97% pure population of CD11c+ major histocompatibility (MHC) class II (MHCII)-positive cells. DCs displayed low levels of CD40, CD80, CD86, and MHC class II molecules, which is characteristic of immature DCs. Purified CD11c+ DCs were cultured in fresh medium with FBS and GM-CSF and used in subsequent experiments. HSV-1(F) is a prototype HSV-1 strain used in this study (9). In recombinant virus R3616, a 1-kb fragment from the coding region of the γ134.5 gene was deleted (4). In the recombinant virus HSV-1(F)R, the deletion in R3616 was restored with a wild-type (WT) γ134.5 gene (4).
Viral infection.
HeLa cells were infected with viruses at an indicated multiplicity of infection. Purified CD11c+ DCs were plated into 12-well plates (5 × 105 cells/well) or in 96-well round-bottom plates (5 × 104 cells/well) and infected with viruses. After 2 h of incubation, cells were washed with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium supplemented with 10% FBS and 20 ng/ml GM-CSF. At different time points after infection, cells were harvested for analysis.
Plasmids.
Plasmids pcDNA3, pTK-luc, dN200, FLAG-γ134.5, and N159 were described elsewhere previously (10, 43). Plasmids HA-MyD88, HA-TRAF6, and FLAG-PP1 were constructed by the cloning of PCR fragments into pcDNA3. To construct FLAG-γ1MT, a DNA fragment encoding mutant γ134.5 with V193E and F195L substitutions in the PP1 binding site was cloned into pcDNA3. To construct ΔN146, a DNA fragment encoding amino acids 146 to 263 of γ134.5 was cloned into pcDNA3. For retrovirus transduction, DNA fragments of wild-type γ134.5, γ1MT, ΔN146, and N159 were cloned into pSIN-Ova_GFP, a dual-promoter human immunodeficiency virus type 1 vector from Mary Collins (University College London, London, United Kingdom) (35). Plasmids FLAG-IKKβ, pNFκB-Luc, IKKα, p65/RelA, and FLAG-TRIF were gifts from Warner Greene (University of California, San Francisco), Zuoming Sun (Beckman Research Institute of City of Hope, Duarte, CA), David Knipe (Harvard Medical School), and Jurg Tschopp (University of Lausanne, Lausanne, Switzerland).
Western blot and immunoprecipitation analyses.
To analyze protein expression, cell lysates were subjected to electrophoresis, transferred onto nitrocellulose membranes, and reacted with primary antibodies (10). The membranes were rinsed in phosphate-buffered saline and reacted with either goat anti-rabbit or goat anti-mouse antibody conjugated to horseradish peroxidase (Amersham Pharmacia Biotech Inc.).
To examine protein interactions, cells were transfected with the indicated plasmids or transduced with retroviral constructs. Cells were then harvested and lysed in 50 mM Tris-HCl (pH 7.4) buffer containing 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 1 μg/ml aprotinin/leupeptin/pepstatin, 1 mM Na3VO4, and 1 mM NaF. Lysates were incubated overnight at 4°C with anti-FLAG M2 affinity gel (Sigma) or anti-hemagglutinin (HA) antibody (Applied Biological Materials Inc.) plus protein A/G-agarose beads (Santa Cruz Biotechnology). Immunocomplexes captured on the affinity gel or protein A/G-agarose beads were subjected to electrophoresis and immunoblotting analysis.
Reporter assays.
Luciferase reporter assays were performed as described previously (10). Briefly, 293T cells grown on 12-well plates were transfected with a control plasmid or a plasmid vector expressing TRIF, TRAF6, MyD88, IKKβ, IKKα, and γ134.5 variants, along with an NF-κB reporter plasmid expressing firefly luciferase using Lipofectamine 2000 (Invitrogen). Total levels of transfected DNA were kept constant with an empty vector plasmid. As a control for transfection efficiency, a plasmid containing the Renilla luciferase gene driven by the HSV-1 TK promoter was included. At 36 h after transfection, cells were harvested, and luciferase activities were measured by using the dual-luciferase assay system from Promega.
Flow cytometry.
Flow cytometry of the cell surface markers CD11c, MHCII, CD80, and CD86 on DCs was performed according to a standard protocol, with some modifications (18). Cells were blocked with 1 μl of Fcγ monoclonal antibody (MAb) (0.5 μg/ml) for 30 min at 4°C. After washing with PBS, cells were stained with isotype-matched antibodies, anti-CD11c-phycoerythrin (PE), anti-MHCII-fluorescein isothiocyanate (FITC), anti-CD80-FITC, and anti-CD86-FITC antibodies (eBioscience) for 30 min on ice with gentle shaking. Samples were processed and screened by using a FACSCalibur instrument, and data were analyzed with Cell Questpro software (BD). To determine viral infectivity, DCs mock infected or infected with viruses were fixed in 4% paraformaldehyde (Sigma) and permeabilized in permeabilizing buffer (eBioscience, San Diego, CA). Cells were blocked with 5% normal mouse serum (Sigma), incubated with a MAb against HSV-1 ICP27 (Virusys, Sykesville, MD), and allowed to react with a goat anti-mouse FITC-conjugated antibody (Santa Cruz Biotech, CA). ICP27 expression was evaluated by flow cytometry.
Transduction and ELISA.
Plasmids were cotransfected along with HIVtrans and the vesicular stomatitis virus G protein (VSV-G) into 293T cells using Lipofectamine 2000 (Invitrogen) as described previously (10). At 48 h after transfection, the supernatant was collected, and the titers were determined by green fluorescent protein (GFP) expression. Immature DCs were transduced with retroviral constructs, and cells were grown with fresh RPMI 1640 medium containing GM-CSF (20 ng/ml) every 2 days. On day 5, GFP-positive DCs were sorted by fluorescence-activated cell sorter (FACS) analysis. Levels of interleukin-6 (IL-6) and IL-12 in supernatants of cell cultures were quantified by enzyme-linked immunosorbent assay (ELISA) using kits from R&D Systems according to the manufacturer's instruction.
Cell fractionation assays.
Cells were lysed in phosphate-buffered saline containing 0.4% Nonidet P-40 and protease inhibitor mixture (Sigma) and kept on ice with gentle inversion. After brief centrifugation, the nuclei were pelleted, and supernatants were collected. After washing, the nuclei were resuspended in phosphate-buffered saline containing 0.4% Nonidet P-40 and frozen at −80°C for 30 min. The cytoplasmic and nuclear fractions were then solubilized in disruption buffer. Samples were subjected to electrophoresis and Western blot analysis with antibodies against p65 (Santa Cruz Biotechnology), GRP78 (glucose-regulated protein 78) (BD Transduction Laboratories), and histone H3 (Cell Signaling).
Quantitative real-time PCR.
Total RNAs from mock-infected or virus-infected DCs were extracted by using the RNeasy kit (Qiagen Inc.). Equal amounts of RNA from each sample were used to synthesize cDNA as suggested by the manufacturer (Invitrogen). cDNAs were then subjected to real-time PCR analysis for ICP27, UL30, and UL44 with specific primers (19). Real-time PCR analysis was performed with the SYBR green system, and all data are presented as relative expression units after normalization to 18S rRNA.
RESULTS
HSV γ134.5 suppresses dendritic cell maturation by lipopolysaccharide.
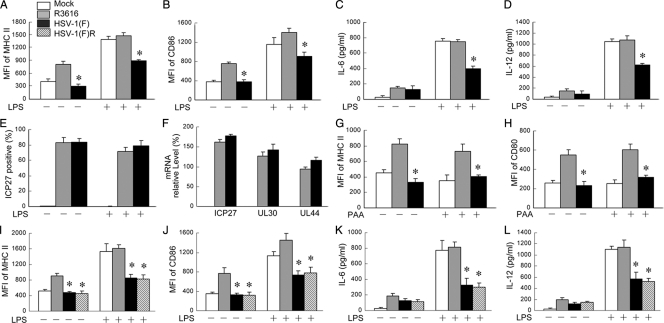
We sought to investigate the function of HSV γ134.5 in TLR-mediated DC maturation. As TLR4 recognizes HSV (38), immature CD11c+ DCs, mocked infected or infected with viruses, were treated with lipopolysaccharide (LPS), which is a TLR4 agonist that induces DC maturation. Cells were then subjected to flow cytometry analysis. As illustrated in Fig. 1 A and B, mock-infected immature DCs exhibited basal levels of major histocompatibility complex (MHC) class II and CD86. Infection with the γ134.5 null mutant R3616, but not wild-type HSV-1(F), stimulated the expression of MHCII and CD86. LPS increased levels of MHCII and CD86 in mock- or R3616-infected DCs. However, this was drastically suppressed in DCs infected with HSV-1(F). A similar response pattern was seen for the production IL-6 and IL-12 as determined by ELISA (Fig. 1C and D). Under these conditions, both HSV-1(F) and R3616 infected DCs equally well (Fig. 1E). Quantitative real-time PCR analysis revealed no significant difference in the expression of immediately-early (ICP27), early (UL30), and late (UL44) genes for HSV-1(F) and R3616 (Fig. 1F). Furthermore, treatment with phosphonoacetic acid (PAA), an inhibitor of HSV DNA replication, did not alter the effects of HSV-1(F) and R3616 on DCs (Fig. 1G and H). Additional analysis with HSV-1(F)R showed that the restoration of wild-type γ134.5 in this recombinant virus resulted in the inhibition of MHCII, CD86, IL-6, and IL-12 expressions (Fig. 1I to L). Collectively, these results suggest that γ134.5 inhibits TLR4-induced DC maturation in HSV infection.
FIG. 1.
HSV γ134.5 is required to suppress LPS-induced dendritic cell maturation in virus infection. CD11c+ dendritic cells were mock infected or infected with viruses (2 PFU/cell). At 12 h after infection, the cells were stimulated with LPS (500 ng/ml; Sigma) for an additional 12 h. (A and B) The cells were stained with PE-labeled antibodies against MHCII (A) and CD86 (B) and subjected to FACS analysis. (C and D) In parallel, cell supernatants were collected to measure the production of IL-6 (C) and IL-12 (D) by ELISA. (E) The viral infectivity of DCs was determined by examining ICP27 expression as described in Materials and Methods. (F) Viral gene expression in DCs was determined 20 h after infection by quantitative real-time RT-PCR. Data are presented as relative expression percentages after normalization to 18S rRNA. (G and H) Effect of viral DNA replication inhibitors on DC maturation. Immature DCs were infected with HSV-1(F) or R3616 in the absence or presence of PAA (400 μg/ml; Sigma). Cells were stained for the expression of MHCII and CD86. As an additional control for the γ134.5 null mutant R3616, a repair virus, HSV-1(F)R, and wild-type HSV-1(F) were used to infect DCs as described above (A to D). (I to L) Levels of expression of MHCII (I), CD86 (J), IL-6 (K), and IL-12 (L) were determined. The data are representative of three independent experiments with standard deviations. Asterisks denote statistical differences (P < 0.05) between different treatment groups. MFI, mean fluorescence intensity.
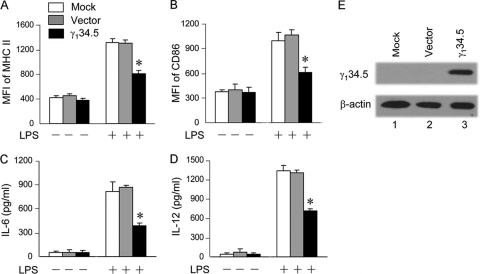
Because HSV is a complex DNA virus, we asked whether γ134.5 works in the absence of any other HSV proteins. Specifically, immature DCs transduced with a retroviral vector or γ134.5 were examined in response to LPS stimulation. As shown in Fig. 2 A and B, when left untreated, cells displayed low levels of MHCII and CD86 expression. Upon LPS stimulation, mock- or vector-transduced cells expressed significantly higher levels of costimulatory molecules than did wild-type γ134.5-transduced cells. Similarly, LPS induced IL-6 and IL-12 expression in mock- or vector-transduced cells. These phenotypes were suppressed in cells transduced with wild-type γ134.5, which correlated with γ134.5 expression (Fig. 2C, D, and E). Therefore, HSV γ134.5 plays a direct role in suppressing DC maturation.
FIG. 2.
HSV γ134.5 inhibits LPS-induced dendritic cell maturation. Immature CD11c+ dendritic cells were transduced with a retroviral vector or γ134.5. At 5 days after transduction, GFP-positive DCs were isolated by FACS analysis and treated with or without LPS (500 ng/ml) for 12 h. (A and B) Cells were stained with PE-labeled antibodies against MHCII (A) and CD86 (B) and subjected to FACS analysis. (C and D) Cell supernatants were collected to measure the production of IL-6 (C) and IL-12 (D) by ELISA. (E) Protein expression. Lysates of cells were subjected to Western blot analysis with antibodies against β-actin (Sigma) and γ134.5 (43), respectively. The data are representative of three independent experiments with standard deviations. Asterisks denote statistical differences (P < 0.05) between different treatment groups.
HSV γ134.5 inhibits p65/RelA phosphorylation and nuclear translocation in dendritic cells.
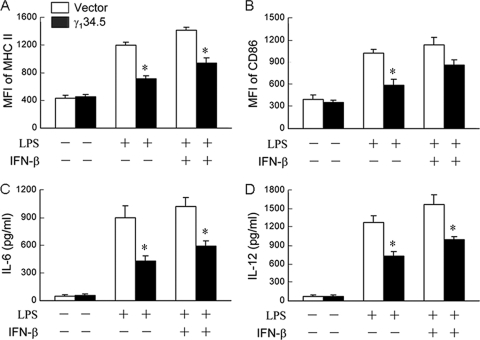
DC maturation is linked to type I IFN production (34). Because γ134.5 inhibits type I IFN induction (18, 43), we asked whether exogenous type I IFN was able to relieve the inhibitory effect of γ134.5. Data presented in Fig. 3 show that γ134.5 suppressed the LPS-stimulated expression of MHC class II, CD86, IL-6, and IL-12 compared to control cells. The addition of IFN-β moderately or marginally relieved the inhibitory effect of γ134.5. This was more evident with respect to IL-6 and IL-12 expression. We reasoned that γ134.5 might regulate the expression of MHC class II, CD86, IL-6, and IL-12 through a component independently of type I IFN.
FIG. 3.
Effects of exogenous IFN-β on γ134.5 activity. Immature CD11c+ dendritic cells were mock treated or transduced with a retroviral vector or γ134.5. GFP-positive cells were isolated and treated with IFN-β (800 U/ml; PBL laboratories) overnight. (A and B) Cells were then stimulated with LPS (500 ng/ml; Sigma) for 12 h and stained with PE-labeled antibodies against MHC class II (A) and CD86 (B). (C and D) Cell supernatants were used to determine the production of IL-6 (C) and IL-12 (D) by ELISA. The data are representative of three independent experiments with standard deviations. Asterisks denote statistical differences (P < 0.05) between different treatment groups.
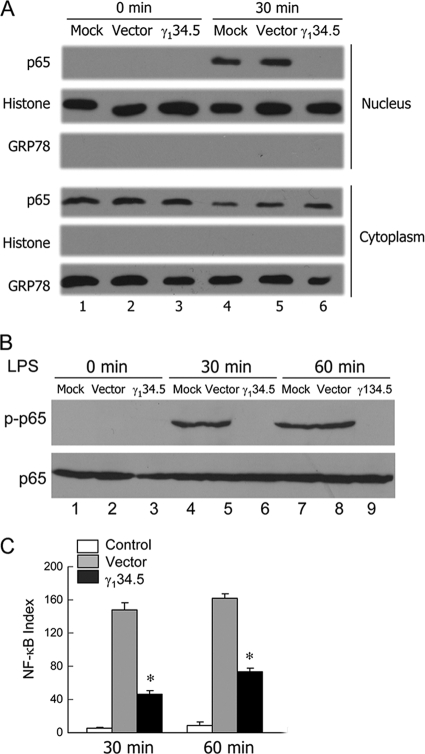
In addition to IRF3, which induces type I IFN, NF-κB is critical for DC maturation (34), where it positively regulates the expression of costimulatory molecules and cytokines. Accordingly, we evaluated the impact of γ134.5 on NF-κB activation. As illustrated in Fig. 4 A, p65/RelA was located primarily in the cytoplasm fraction in unstimulated cells. Upon LPS stimulation, p65/RelA appeared in the nuclear fraction in mock- or vector-transduced DCs. In stark contrast, its nuclear translocation was blocked in DCs transduced with γ134.5. In correlation, p65/RelA phosphorylation was inhibited by γ134.5, although levels of p65 expression were comparable in all cells (Fig. 4B). p65/RelA phosphorylation was further confirmed with ELISA (Fig. 4C). Thus, when expressed in DCs, HSV γ134.5 is able to suppress NF-κB activation by the TLR4 pathway.
FIG. 4.
The γ134.5 protein blocks NF-κB activation in dendritic cells. (A) Cell fractionation analysis. Immature CD11c+ dendritic cells, transduced with a retroviral vector or wild-type γ134.5, were stimulated with LPS (500 ng/ml). The cytoplasmic and nuclear fractions were then subjected to Western blot analysis with antibodies against p65/RelA (Santa Cruz Biotech), GRP78 (BD Transduction Laboratories), and histone H3 (Cell Signaling), respectively. (B) Cells were treated as described above (A), and cells lysates were processed for Western blot analysis with antibodies against phosphorylated p65/RelA (p-p65) and p65/RelA (Santa Cruz Biotech). (C) Lysates of cells were also collected to quantify p65 phosphorylation by ELISA (Cell Signaling). Results are expressed as fold activation with standard deviations among triplicate samples. The data are representative of three independent experiments.
HSV γ134.5 blocks NF-κB activation by associating with IκB kinase.
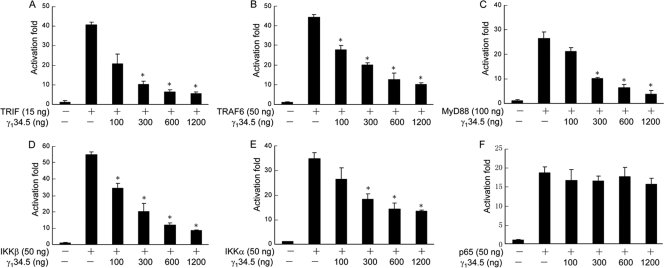
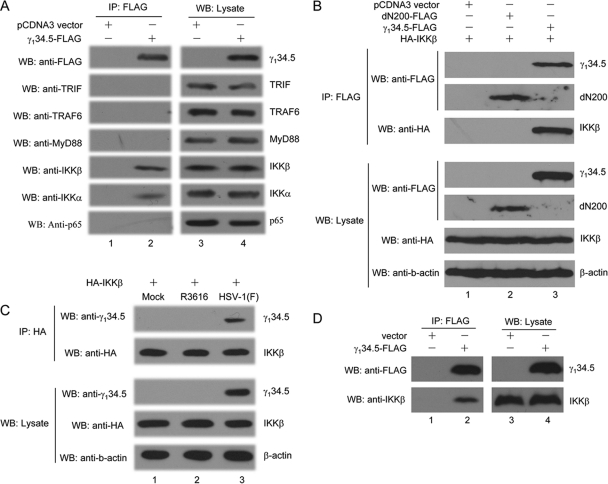
In response to LPS, TLR4 relays the signal to several adaptors and kinases, leading to NF-κB activation (41). To assess at which step γ134.5 exerted its activity, we carried out luciferase reporter assays. As indicated in Fig. 5, when expressed in 293T cells, TRIF, TRAF6, MyD88, IKKβ, IKKα, and p65/RelA activated the NF-κB reporter. Although the coexpression of γ134.5 inhibited NF-κB activation by TRIF, TRAF6, MyD88, IKKβ, and IKKα in a dose-dependent manner (Fig. 5A to E), it had no effect on p65/RelA (Fig. 5F), suggesting that γ134.5 works upstream of p65/RelA. To test the potential interaction of γ134.5 with a cellular component, we performed coimmunoprecipitation assays. As shown in Fig. 6 A, when expressed in HeLa cells, HSV γ134.5 associated with endogenous IKKα and IKKβ. Under this condition, it exhibited no interaction with TRIF, TRAF6, MyD88, or p65/RelA, indicating that γ134.5 specifically targets the IκB kinase complex. IKKα and IKKβ share significant amino acid identity, and their domain structures are similar, with an amino-terminal kinase, a leucine zipper, and helix-loop-helix motifs (12). We further analyzed IKKβ because of its essential role in the classical NF-κB pathway that regulates inflammatory cytokines and costimulatory molecules. As illustrated in Fig. 6B, when ectopically expressed in 293T cells, γ134.5 specifically associated with IKKβ but not with a control protein, dN200 (a mutant Ebola virus VP35). Similarly, γ134.5 associated with IKKβ in virus-infected cells (Fig. 6C). Indeed, when transduced in DCs, γ134.5 associated with endogenous IKKβ (Fig. 6D). We conclude from these experiments that HSV γ134.5 inhibits NF-κB activation by targeting IκB kinase.
FIG. 5.
HSV γ134.5 inhibits NF-κB reporter activation. 293T cells were transfected with an empty vector or plasmids expressing TRIF (A), TRAF6 (B), MyD88 (C), IKKβ (D), IKKα (E), p65/RelA (F), and γ134.5 along with an NF-κB reporter gene expressing firefly luciferase by using Lipofectamine 2000 (Invitrogen). At 36 h posttransfection, cells were harvested for luciferase assays. Results are expressed as fold activation with standard deviations among triplicate samples. The data are representative of three independent experiments.
FIG. 6.
(A) The γ134.5 protein associates with endogenous IKKα/β. HeLa cells were transfected with FLAG-γ134.5 or an empty vector. At 36 h after transfection, lysates of cells were immunoprecipitated (IP) with anti-FLAG antibody. Samples from both cell lysates and immunoprecipitates were probed with antibodies against FLAG, TRIF (Cell Signaling), TRAF6 (Santa Cruz Biotech), MyD88 (Santa Cruz Biotech), IKKβ (Cell Signaling), IKKα (Cell Signaling), and p65/RelA (Santa Cruz Biotech). (B) Interaction of γ134.5 and IKKβ. 293T cells were cotransfected with FLAG-γ134.5 along with an empty vector, HA-IKKβ, or FLAG-dN200 (a mutant of Ebola virus VP35). At 36 h after transfection, lysates of cells were immunoprecipitated with anti-FLAG antibody. Samples were probed with antibodies against FLAG, HA, and β-actin. (C) HeLa cells were transfected with HA-IKKβ. Twenty-four hours after transfection, cells were mock infected or infected with viruses (5 PFU/cell). At 10 h after infection, lysates of cells were immunoprecipitated with anti-HA antibody (Santa Cruz Biotech). Samples from both cell lysates and immunoprecipitates were analyzed with antibodies against HA, γ134.5, and β-actin. (D) The γ134.5 protein associates with endogenous IKKβ in DCs. CD11c+ DCs were transduced by FLAG-γ134.5 or an empty vector. Five days after transduction, GFP-positive cells were sorted and processed for immunoprecipitation with anti-FLAG antibody. Samples from both cell lysates and immunoprecipitates were subjected to Western blot (WB) analysis with antibodies against FLAG and IKKβ, respectively. The data are representative of three independent experiments.
HSV γ134.5 precludes IKKβ phosphorylation via protein phosphatase 1.
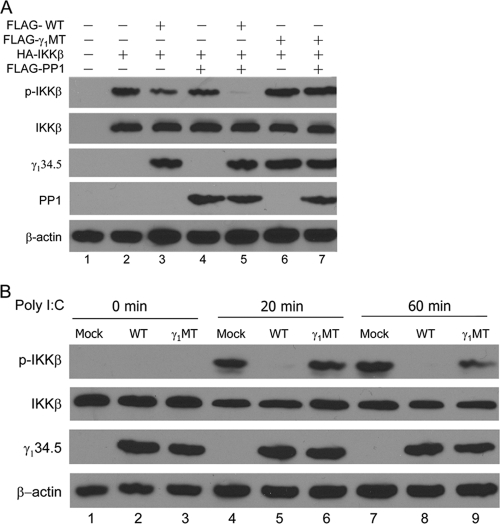
The association of γ134.5 with IKKα/β raised the possibility that it may inhibit IκB kinase activation. To address this, we examined the phosphorylation status of IKKβ in 293T cells overexpressing IKKβ, PP1, and wild-type (WT) γ134.5. Since γ134.5 is a virus-encoded protein phosphatase 1 regulator (13, 14), we also hypothesized that its PP1 site may be required to modulate IκB kinase. For this purpose, we included a γ134.5 mutant (γ1MT) with V193E and F195L substitutions, which disrupt its binding to PP1 (46). As shown by Western blot analysis (Fig. 7 A), these proteins were expressed at comparable levels after transfection. When ectopically expressed alone, IKKβ remained phosphorylated (Fig. 7A, lane 2). The addition of wild-type γ134.5 or PP1 reduced IKKβ phosphorylation slightly (lanes 3 and 4). When coexpressed with PP1 and wild-type γ134.5, IKKβ became completely unphosphorylated (lane 5). Nonetheless, IKKβ remained phosphorylated in cells expressing the γ134.5 mutant (γ1MT) (lanes 6 to 7). Without wild-type γ134.5, PP1 had little effect on IKKβ phosphorylation. Next, we examined endogenous IKKβ phosphorylation in HeLa cells that express TLR3. As illustrated in Fig. 7B, IKKβ was unphosphorylated in unstimulated cells (lanes 1 to 3). Poly(I:C) stimulation induced IKKβ phosphorylation in mock-transfected cells (Fig. 7B, lanes 4 and 7). The expression of wild-type γ134.5 precluded poly(I:C)-induced IKKβ phosphorylation (lanes 5 and 8). In contrast, the expression of γ1MT was unable to prevent IKKβ phosphorylation (lanes 6 and 9). These data suggest that the interaction of γ134.5 with protein phosphatase 1 inhibits IKKβ phosphorylation.
FIG. 7.
The γ134.5 protein mediates the dephosphorylation of IKKβ. (A) 293T cells were transfected with an empty vector, HA-IKKβ, or FLAG-PP1 along with FLAG-γ134.5 or FLAG-γ1MT, which bears V193E and F195L substitutions in the PP1 binding motif. At 36 h after transfection, lysates of cells were probed with antibodies against phosphorylated IKK-β, IKK-β, γ134.5, PP1, and β-actin. (B) The γ134.5 protein suppresses the phosphorylation of endogenous IKKβ. HeLa cells were transfected with FLAG-γ134.5 or FLAG-γ1MT. At 36 h after transfection, cells were left untreated or treated with poly(I:C) (20 μg/ml). Lysates of cells were then processed for Western blot analysis with antibodies against phosphorylated IKK-β, IKK-β, γ134.5, and β-actin. The data are representative of three independent experiments.
HSV γ134.5 forms a multiprotein complex which blocks NF-κB activation.
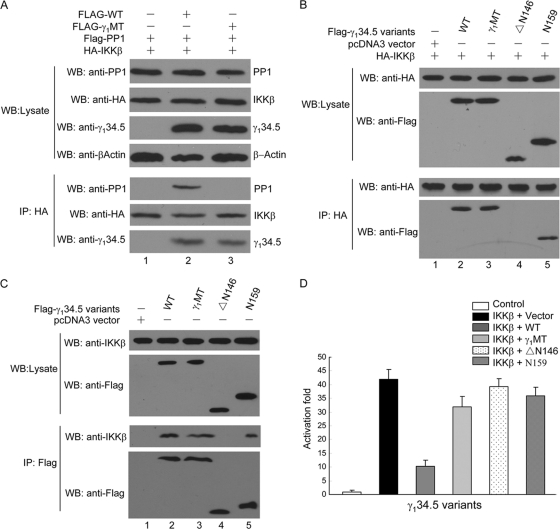
Based on the above-described analysis, we examined the nature of γ134.5, IKKβ, and PP1 interactions in a series of experiments. As indicated by the immunoprecipitation assay (Fig. 8 A), IKKβ coimmunoprecipitated with both wild-type γ134.5 and PP1 in transfected 293T cells (Fig. 8A, lane 2), where IKKβ, PP1, and γ134.5 variants were expressed at comparable levels (lanes 1 to 3). This indicates that IKKβ, PP1, and γ134.5 indeed formed a complex. Intriguingly, in the absence of γ134.5, IKKβ did not associate with PP1 (Fig. 8A, lane 1). Hence, HSV γ134.5 serves as a bridge for IKKβ and PP1. Interestingly, γ1MT, which cannot bind to PP1, retained its ability to interact with IKKβ, suggesting that the interaction of γ134.5 with IKKβ is independent of PP1 (Fig. 8A, lane 3). To define the domain required to interact with IKKβ, we tested additional γ134.5 mutants. As shown in Fig. 8B, like wild-type γ134.5, γ1MT associated with IKKβ (lanes 2 and 3). Moreover, the γ134.5 mutant N159, which lacked amino acids 160 to 263, was able to interact with IKKβ (lane 5). In contrast, the γ134.5 mutant ΔN146, which lacked amino acids 1 to 145, was unable to interact with IKKβ (lane 4). These interactions of γ134.5 variants with endogenous IKKβ were also observed for DCs (Fig. 8C). Thus, the amino terminus of γ134.5 is sufficient to associate with IKKβ. To establish a functional link between γ134.5, IKKβ, and PP1, we examined γ134.5 variants in reporter assays (Fig. 8D). When expressed, IKKβ induced NF-κB promoter activation by 40-fold. The expression of wild-type γ134.5 drastically inhibited its activation. However, the coexpression of N159, ΔN146, or γ1MT failed to suppress NF-κB promoter activation by IKKβ. Hence, both the amino- and carboxyl-terminal domains are required to inhibit NF-κB activation. These results suggest that γ134.5 forms a complex with IKKβ and PP1, where γ134.5 mediates IKKβ dephosphorylation by recruiting PP1.
FIG. 8.
(A) HSV γ134.5 mediates the formation of an IKK-γ134.5-PP1 complex. 293T cells were cotransfected with FLAG-γ134.5 or FLAG-γ1MT along with an empty vector, HA-IKKβ, or FLAG-PP1. At 36 h after transfection, lysates of cells were immunoprecipitated with anti-HA antibody. Samples were probed with antibodies against PP1, HA, or γ134.5 and β-actin. (B) Interaction of γ134.5 variants with IKKβ. 293T cells were cotransfected with HA-IKKβ and FLAG-γ134.5 variants as indicated. At 36 h after transfection, lysates were immunoprecipitated with anti-HA antibody. Proteins in the lysates and precipitates were analyzed by immunoblotting with anti-HA and anti-FLAG antibodies. (C) CD11c+ DCs were transduced with FLAG-γ134.5 or its variants. Five days after transduction, GFP-positive cells were sorted and processed for immunoprecipitation (IP) with anti-FLAG antibody. Samples from both cell lysates and immunoprecipitates were subjected to Western blot (WB) analysis with antibodies against FLAG and IKKβ, respectively. (D) Effect of γ134.5 variants on NF-κB promoter activation. 293T cells were cotransfected with an empty vector, HA-IKKβ (50 ng), FLAG-γ134.5 variants (800 ng), and an NF-κB luciferase reporter. At 36 h posttransfection, cells were harvested for luciferase assays. Results are expressed as fold activation with standard deviations among triplicate samples. The data are representative of three independent experiments.
IKKβ dephosphorylation by γ134.5 impairs dendritic cell activation.
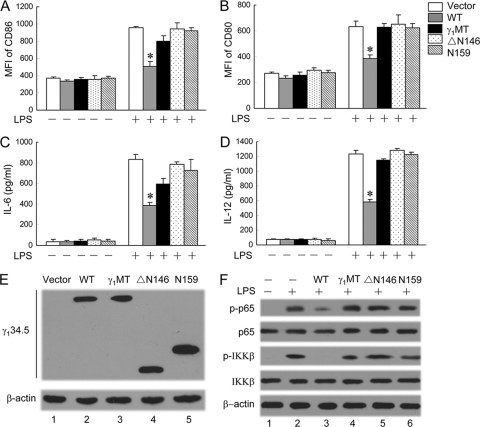
To determine whether IKKβ dephosphorylation by γ134.5 is linked to impaired DC maturation, we further tested γ134.5 variants in immature DCs. As shown in Fig. 9 A and B, all cells exhibited basal levels of CD80 and CD86 expression. The addition of LPS greatly stimulated the expression of these costimulatory molecules in cells transduced with a retroviral vector, N159, ΔN146, or γ1MT. This stimulation was suppressed in cells transduced with wild-type γ134.5. A similar phenotype was noted for IL-6 and IL-12 expressions (Fig. 9C and D). Western blot analysis showed that γ134.5 variants were expressed at comparable levels (Fig. 9E). To assess whether γ134.5 variants inhibited NF-κB activation in DCs, we measured the phosphorylation of p65 and IKKβ in dendritic cells. As indicated in Fig. 9F, wild-type γ134.5 precluded the phosphorylation of p65/RelA and IKKβ stimulated by LPS (lane 3), whereas N159, ΔN146, and γ1MT failed to do so (lanes 4 to 6). These phenotypes correlated with the ability of γ134.5 variants to suppress DC maturation. Thus, both IKKβ and PP1 binding domains of γ134.5 are indispensable. Taken together, these data demonstrate that the dephosphorylation of IκB kinase by γ134.5 impairs DC maturation.
FIG. 9.
Effects of γ134.5 variants on DC maturation. Immature CD11c+ dendritic cells, transduced with a retroviral vector or γ134.5 variants, were stimulated with LPS (500 ng/ml). (A and B) At 12 h after stimulation, cells were stained with PE-labeled antibodies against MHCII (A) and CD86 (B) and subjected to FACS analysis. (C and D) Cell media were collected to determine the levels of production of IL-6 (C) and IL-12 (D) by ELISA. (E) Expression of γ134.5 variants. Lysates of cells were subjected to Western analysis with anti-FLAG and anti-β-actin. (F) Effects of γ134.5 variants on the LPS-induced phosphorylation of IKKβ and p65/RelA. DCs transduced with a retroviral vector or γ134.5 variants were stimulated with LPS (500 ng/ml). At 20 min after stimulation, cell lysates were processed and probed with antibodies against phosphorylated p65/RelA, p65/RelA, phosphorylated IKKβ, IKKβ, and β-actin. The data are representative of three independent experiments with standard deviations. Asterisks denote statistical differences (P < 0.05) between different treatment groups.
DISCUSSION
HSV replicates in DCs and perturbs DC maturation, resulting in impaired T cell activation (19, 29, 32, 36). In this study, we provide evidence that HSV γ134.5 suppressed the induction of costimulatory molecules and inflammatory cytokines by TLR stimulation. When expressed, HSV γ134.5 recruited both cellular protein phosphatase 1 and IKK kinase. Accordingly, these proteins formed a complex that precluded the phosphorylation of IKKα/β and the subsequent activation of NF-κB. In this context, it is noteworthy that the HSV interaction with DCs determines the outcome of infection (1, 20, 40). Given that HSV γ134.5 is a virulence factor (4, 44), these results support the view that the interference of TLR or virus-mediated DC maturation by γ134.5 contributes to viral pathogenesis.
Our work indicates that HSV γ134.5 plays a direct role in the abrogation of TLR-mediated DC maturation. We previously noted that γ134.5 is required to inhibit DC maturation both in vivo and in vitro (18). In agreement with this observation, we found that γ134.5 also blocked DC maturation by TLR4 stimulation. Furthermore, when expressed alone, this viral factor was capable of suppressing the induction of MHC class II, CD86, IL-6, and IL-12 by LPS. Herein, these phenotypes are not due to a secondary effect by other HSV gene products. HSV γ134.5 associates with and inhibits TANK1 binding kinase 1 (13), an essential factor that activates interferon regulatory factor 3 and subsequent type I IFN expression. One possible reason why γ134.5 perturbs DC maturation is the block of type I IFN production. Our experimental data suggest that this model is not sufficient to reconcile the effect of γ134.5 on DCs. First, exogenous type I IFN marginally or moderately relieved the inhibitory effect by γ134.5. Second, when expressed in immature DCs, HSV γ134.5 blocked the phosphorylation and nuclear migration of p65/RelA in response to TLR4 activation. A logical explanation is that γ134.5 may work via an additional mechanism.
The IκB kinase complex sits at the center of innate immune pathways leading to the expression of cytokines and costimulatory molecules (12, 41). Our work reveals IκB kinase to be a novel cellular target of HSV γ134.5. When expressed in mammalian cells, γ134.5 inhibited NF-κB activation mediated by IKKα, IKKβ, TRIF, TRAF6, and MyD88 effectively. Although associated with IKKα or IKKβ, γ134.5 failed to interact with other components, including TRIF, TRAF6, MyD88, and p65/RelA. A specific interaction between γ134.5, IKKα, and IKKβ seems to be required to modulate NF-κB activation. The IκB kinase complex is activated in response to a variety of stimuli, including TLR signaling (12, 41). This requires the phosphorylation of either IKKα or IKKβ on two specific serine residues within the catalytic domain of each subunit (12). Importantly, γ134.5 inhibited the phosphorylation of IKKα/β mediated by TLR3 or TLR4 stimulation. Because IKKα/β sits at a converging point (12, 41), it is possible that γ134.5 may exert a broad impact on multiple TLR pathways leading to NF-κB activation. Additional work is needed to test this hypothesis.
HSV γ134.5 consists of 263 amino acids with large amino-terminal and carboxyl-terminal domains that are linked by triplet repeats of three amino acids (ATP) (4). It is well established that in response to PKR activation, γ134.5 recruits PP1 to prevent translation arrest (13, 14). Thus, one would assume a role of the γ134.5-PKR interaction in DC maturation. However, as DCs with a PKR deficiency mature normally in viral infection (16, 26), this model seems less likely to operate in HSV infection. What the present investigation suggests is that γ134.5 functions via a different pathway involving IκB kinase. In principle, γ134.5 may bind to the IκB kinase complex and impose a physical block to prevent its phosphorylation. Alternatively, γ134.5 may serve as a link to bridge IκB kinase and PP1 that dephosphorylates IκB kinase. Our data favor the latter possibility. We noted that when expressed alone, the amino terminus of γ134.5 interacted with IKKα/β but had no effect on IKKα/β phosphorylation. Although binding to PP1 (14), the carboxyl terminus itself exhibited no activity on IKKα/β phosphorylation. Furthermore, V193E and F195L substitutions abolished the interaction of γ134.5 with PP1 but not with IKKβ. Such mutations disrupted the ability of γ134.5 to preclude IKKβ phosphorylation. We postulate that upon HSV infection, γ134.5 recruits IKKα/β by the amino terminus and recruits PP1 by its carboxyl terminus, resulting in a multiprotein complex that prevents the activation of IKKβ, NF-κB, and DCs. Work is in progress to investigate details of the underlying mechanism.
HSV is a large DNA virus that replicates in DCs and perturbs their functions (19, 29, 32, 36). Previous studies demonstrated that the virion host shutoff protein functions to inhibit DC maturation (37). This is believed to result from its intrinsic RNase activity. Notably, the virion host shutoff protein blocks DC maturation by TLR-independent pathways of viral recognition (6). On the other hand, an immediate-early protein, ICP0, perturbs the function of mature DCs (23), where it mediates CD83 degradation via cellular proteasomes. Additionally, ICP0 as well as ICP27 inhibit NF-κB activation (8, 21, 28, 42). Our work reveals that γ134.5 functions to impair DC maturation by TLR4. These viral proteins possibly work in a coordinate manner, which creates a favorable environment for HSV infection. In this context, it is noteworthy that γ134.5 mediates IKKα/β dephosphorylation rather than a physical block of IKKα/β phosphorylation. This is probably related to the fact that HSV both activates and inhibits NF-κB (2, 8, 25, 28, 39, 42). While NF-κB activity is required for optimal HSV replication (11, 31), its activation is also antiviral in nature (8, 28). Thus, a delicate balance must be maintained during the complex HSV life cycle. This requires that NF-κB activity be tightly regulated. We speculate that during evolution, HSV has evolved sophisticated mechanisms to control NF-κB activity. In this regard, γ134.5 acts to regulate the level of NF-κB activation by a cycle on-off mechanism via the dephosphorylation of IKKα/β. This may serve as a strategy to downregulate host antiviral immunity.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI081711 and AI092230) and the National Natural Science Foundation of China (30670080 and 111).
We thank Mary Collins, Warner Greene, Bernard Roizman, Zuoming Sun, David Knipe, and Jurg Tschopp for providing valuable reagents.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Allan, R. S., et al. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science 301:1925-1928. [DOI] [PubMed] [Google Scholar]
- 2.Amici, C., et al. 2006. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 281:7110-7117. [DOI] [PubMed] [Google Scholar]
- 3.Burzyn, D., et al. 2004. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J. Virol. 78:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 5.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl. Acad. Sci. U. S. A. 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, C. R., et al. 2010. The virion host shut-off (vhs) protein blocks a TLR-independent pathway of herpes simplex type 1 recognition in human and mouse dendritic cells. PLoS One 5:e8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, A. L., F. Carbone, and T. B. Geijtenbeek. 2008. Langerhans cells and viral immunity. Eur. J. Immunol. 38:2377-2385. [DOI] [PubMed] [Google Scholar]
- 8.Daubeuf, S., et al. 2009. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood 113:3264-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 10.Feng, Z. D., M. Cerveny, Z. P. Yan, and H. B. 2007. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J. Virol. 81:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory, D., D. Hargett, D. Holmes, E. Money, and S. L. Bachenheimer. 2004. Efficient replication by herpes simplex virus type 1 involves activation of the IkappaB kinase-IkappaB-p65 pathway. J. Virol. 78:13582-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker, H., and M. Karin. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. [DOI] [PubMed] [Google Scholar]
- 13.He, B., M. Gross, and B. Roizman. 1998. The γ134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 14.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochrein, H., et al. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. U. S. A. 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda, K., et al. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. U. S. A. 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inaba, K., et al. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, H., et al. 2009. The γ134.5 protein of herpes simplex virus 1 is required to interfere with dendritic cell maturation during productive infection. J. Virol. 83:4984-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, C. A., et al. 2003. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J. Virol. 77:11139-11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassim, S. H., N. K. Rajasagi, X. Zhao, R. Chervenak, and S. R. Jennings. 2006. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J. Virol. 80:3985-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, J. C., et al. 2008. HSV-1 ICP27 suppresses NF-kappaB activity by stabilizing IkappaBalpha. FEBS Lett. 582:2371-2376. [DOI] [PubMed] [Google Scholar]
- 22.Krug, A., et al. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 23.Kummer, M., et al. 2007. Herpes simplex virus type 1 induces CD83 degradation in mature dendritic cells with immediate-early kinetics via the cellular proteasome. J. Virol. 81:6326-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurt-Jones, E. A., et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 25.Liu, X., K. Fitzgerald, E. Kurt-Jones, R. Finberg, and D. M. Knipe. 2008. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc. Natl. Acad. Sci. U. S. A. 105:11335-11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 27.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchjorsen, J., J. Siren, I. Julkunen, S. R. Paludan, and S. Matikainen. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 87:1099-1108. [DOI] [PubMed] [Google Scholar]
- 29.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orvedahl, A., et al. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23-35. [DOI] [PubMed] [Google Scholar]
- 31.Patel, A., et al. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 32.Pollara, G., et al. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J. Infect. Dis. 187:165-178. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen, S. B., et al. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 81:13315-13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis e Sousa, C. 2004. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin. Immunol. 16:27-34. [DOI] [PubMed] [Google Scholar]
- 35.Rowe, H. M., et al. 2009. Expression of vFLIP in a lentiviral vaccine vector activates NF-κB, matures dendritic cells, and increases CD8+ T-cell responses. J. Virol. 83:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 37.Samady, L., et al. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarangi, P. P., B. Kim, E. Kurt-Jones, and B. T. Rouse. 2007. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the Toll pathway in early inflammatory events in stromal keratitis. J. Virol. 81:11128-11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sciortino, M. T., et al. 2008. Involvement of HVEM receptor in activation of nuclear factor kappaB by herpes simplex virus 1 glycoprotein D. Cell. Microbiol. 10:2297-2311. [DOI] [PubMed] [Google Scholar]
- 40.Smith, C. M., et al. 2003. Conventional CD8 alpha+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J. Immunol. 170:4437-4440. [DOI] [PubMed] [Google Scholar]
- 41.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 42.van Lint, A. L., et al. 2010. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-κB signaling. J. Virol. 84:10802-10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the γ134.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitley, R. J., E. R. Kern, S. Chatterjee, J. Chou, and B. Roizman. 1993. Replication, establishment of latency, and induced reactivation of herpes simplex virus γ134.5 deletion mutants in rodent models. J. Clin. Invest. 91:2837-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, C., et al. 2008. A conserved domain of herpes simplex virus ICP34.5 regulates protein phosphatase complex in mammalian cells. FEBS Lett. 582:171-176. [DOI] [PubMed] [Google Scholar]
- 47.Zhao, X., et al. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]