Abstract
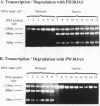
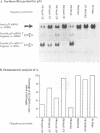
We have previously demonstrated, in vitro, that phosphodiester and phosphorothioate antisense oligodeoxynucleotides could direct ribonuclease H to cleave non-target RNA sites and that chimeric methylphosphonodiester/phosphodiester analogue structures were substantially more specific. In this report we show that such chimeric molecules can promote point mutation-specific scission of target mRNA by both Escherichia coli and human RNases H in vitro. Intact human leukaemia cells 'biochemically microinjected' with antisense effectors demonstrated efficient suppression of target mRNA expression. It was noted that the chimeric methylphosphonodiester/phosphodiester structures showed single base discrimination, whereas neither the phosphodiester nor phosphorothioate compounds were as stringent. Finally, we show that the antisense effects obtained in intact cells were due to endogenous RNase H activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry E. L., Gesek F. A., Friedman P. A. Introduction of antisense oligonucleotides into cells by permeabilization with streptolysin O. Biotechniques. 1993 Dec;15(6):1016-8, 1020. [PubMed] [Google Scholar]
- Bertrand E., Fromont-Racine M., Pictet R., Grange T. Visualization of the interaction of a regulatory protein with RNA in vivo. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3496–3500. doi: 10.1073/pnas.90.8.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E., Pictet R., Grange T. Can hammerhead ribozymes be efficient tools to inactivate gene function? Nucleic Acids Res. 1994 Feb 11;22(3):293–300. doi: 10.1093/nar/22.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke S. T. Therapeutic applications of oligonucleotides. Annu Rev Pharmacol Toxicol. 1992;32:329–376. doi: 10.1146/annurev.pa.32.040192.001553. [DOI] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G., Leonetti J. P., Milhaud P., Mechti N., Lebleu B. Antisense inhibitors of HIV: problems and perspectives. Antiviral Res. 1992 Apr;17(4):279–287. doi: 10.1016/0166-3542(92)90023-x. [DOI] [PubMed] [Google Scholar]
- Eder P. S., Walder R. Y., Walder J. A. Substrate specificity of human RNase H1 and its role in excision repair of ribose residues misincorporated in DNA. Biochimie. 1993;75(1-2):123–126. doi: 10.1016/0300-9084(93)90033-o. [DOI] [PubMed] [Google Scholar]
- Giles R. V., Spiller D. G., Tidd D. M. Chimeric oligodeoxynucleotide analogues: enhanced cell uptake of structures which direct ribonuclease H with high specificity. Anticancer Drug Des. 1993 Feb;8(1):33–51. [PubMed] [Google Scholar]
- Giles R. V., Tidd D. M. Enhanced RNase H activity with methylphosphonodiester/phosphodiester chimeric antisense oligodeoxynucleotides. Anticancer Drug Des. 1992 Feb;7(1):37–48. [PubMed] [Google Scholar]
- Giles R. V., Tidd D. M. Increased specificity for antisense oligodeoxynucleotide targeting of RNA cleavage by RNase H using chimeric methylphosphonodiester/phosphodiester structures. Nucleic Acids Res. 1992 Feb 25;20(4):763–770. doi: 10.1093/nar/20.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard G., François J. C., Duroux I., Asseline U., Chassignol M., Nguyen T., Hélène C., Saison-Behmoaras T. Photochemically and chemically activatable antisense oligonucleotides: comparison of their reactivities towards DNA and RNA targets. Nucleic Acids Res. 1994 Nov 11;22(22):4789–4795. doi: 10.1093/nar/22.22.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrouy B., Blonski C., Boiziau C., Stuer M., Moreau S., Shire D., Toulmé J. J. RNase H-mediated inhibition of translation by antisense oligodeoxyribonucleotides: use of backbone modification to improve specificity. Gene. 1992 Nov 16;121(2):189–194. doi: 10.1016/0378-1119(92)90121-5. [DOI] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. C., Bement W. M., Dersch M. A., Dworkin-Rastl E., Dworkin M. B., Capco D. G. Nonspecific effects of oligodeoxynucleotide injection in Xenopus oocytes: a reevaluation of previous D7 mRNA ablation experiments. Development. 1990 Nov;110(3):769–779. doi: 10.1242/dev.110.3.769. [DOI] [PubMed] [Google Scholar]
- Spiller D. G., Tidd D. M. The uptake kinetics of chimeric oligodeoxynucleotide analogues in human leukaemia MOLT-4 cells. Anticancer Drug Des. 1992 Apr;7(2):115–129. [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Krieg A. M. Problems in interpretation of data derived from in vitro and in vivo use of antisense oligodeoxynucleotides. Antisense Res Dev. 1994 Summer;4(2):67–69. doi: 10.1089/ard.1994.4.67. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey A., Oates D., Banks L., Crawford L., Crook T. Anti-sense phosphorothioate oligonucleotides have both specific and non-specific effects on cells containing human papillomavirus type 16. Nucleic Acids Res. 1991 Aug 11;19(15):4109–4114. doi: 10.1093/nar/19.15.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M. A potential role for antisense oligonucleotide analogues in the development of oncogene targeted cancer chemotherapy. Anticancer Res. 1990 Sep-Oct;10(5A):1169–1182. [PubMed] [Google Scholar]
- Tidd D. M. Methylphosphonodiester/phosphodiester chimeric oligodeoxynucleotides. Biochem Soc Trans. 1992 Nov;20(4):746–749. doi: 10.1042/bst0200746. [DOI] [PubMed] [Google Scholar]
- Tidd D. M., Warenius H. M. Partial protection of oncogene, anti-sense oligodeoxynucleotides against serum nuclease degradation using terminal methylphosphonate groups. Br J Cancer. 1989 Sep;60(3):343–350. doi: 10.1038/bjc.1989.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994 Nov 24;372(6504):333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- Woolf T. M., Melton D. A., Jennings C. G. Specificity of antisense oligonucleotides in vivo. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7305–7309. doi: 10.1073/pnas.89.16.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. M., Bradbury D. A., Russell N. H. Wild-type p53 is required for apoptosis induced by growth factor deprivation in factor-dependent leukaemic cells. Br J Cancer. 1994 Mar;69(3):468–472. doi: 10.1038/bjc.1994.85. [DOI] [PMC free article] [PubMed] [Google Scholar]