Abstract
Objective:
To characterize the neuropathologic features of neuromyelitis optica (NMO) at the medullary floor of the fourth ventricle and area postrema. Aquaporin-4 (AQP4) autoimmunity targets this region, resulting in intractable nausea associated with vomiting or hiccups in NMO.
Methods:
This neuropathologic study was performed on archival brainstem tissue from 15 patients with NMO, 5 patients with multiple sclerosis (MS), and 8 neurologically normal subjects. Logistic regression was used to evaluate whether the presence of lesions at this level increased the odds of a patient with NMO having an episode of nausea/vomiting.
Results:
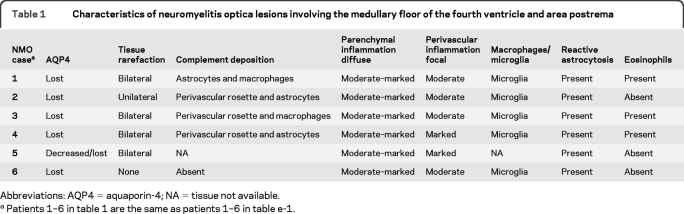
Six patients with NMO (40%), but no patients with MS or normal controls, exhibited unilateral or bilateral lesions involving the area postrema and the medullary floor of the fourth ventricle. These lesions were characterized by tissue rarefaction, blood vessel thickening, no obvious neuronal or axonal pathology, and preservation of myelin in the subependymal medullary tegmentum. AQP4 immunoreactivity was lost or markedly reduced in all 6 cases, with moderate to marked perivascular and parenchymal lymphocytic inflammatory infiltrates, prominent microglial activation, and in 3 cases, eosinophils. Complement deposition in astrocytes, macrophages, and/or perivascularly, and a prominent astroglial reaction were also present. The odds of nausea/vomiting being documented clinically was 16-fold greater in NMO cases with area postrema lesions (95% confidence interval 1.43–437, p = 0.02).
Conclusions:
These neuropathologic findings suggest the area postrema may be a selective target of the disease process in NMO, and are compatible with clinical reports of nausea and vomiting preceding episodes of optic neuritis and transverse myelitis or being the heralding symptom of NMO.
Neuromyelitis optica (NMO) is a CNS inflammatory demyelinating autoimmune disease characterized by relapsing attacks that target the optic nerves and spinal cord,1,2 as well as aquaporin-4 (AQP4)–enriched periventricular brain regions.3,4 The circulating disease-specific autoantibody marker of NMO, NMO–immunoglobulin G (IgG), binds specifically to the AQP4 water channel concentrated in astrocytic foot processes.5–7
Clinical, serologic, CSF, neuroimaging,1,3,4 and neuropathologic criteria5,8 distinguish NMO from multiple sclerosis (MS). Characteristic symptoms of NMO medullary involvement include intractable, but reversible, nausea that may be associated with vomiting/hiccups.9–11 These symptoms may accompany or precede NMO relapses.10,11 Patients who present with intractable vomiting as the first and isolated symptom of NMO are usually evaluated by gastroenterologists and neurologic evaluation is often not pursued.9
Area postrema, the emetic reflex center,12 is comprised of 2 symmetric structures at the floor of the rhomboid fossa. Like other circumventricular organs (CVO), it consists of loose tissue containing glia and neurons, has a thin ependymal cover, and is penetrated by convoluted capillaries that lack tight endothelial junctions forming a permeable blood–brain barrier (BBB).12,13 Area postrema is connected to hypothalamic and brainstem nuclei regulating fluid balance, osmoregulation, immunomodulation, and other important physiologic systems.12,14
Like other periventricular areas, the medullary floor of the fourth ventricle and area postrema express AQP4 abundantly and are thus preferential targets for NMO lesions.3,4,8,15 Expectedly, clinical manifestations of NMO include intractable nausea/vomiting/hiccups. This study is the first to thoroughly characterize the neuropathology of NMO lesions involving the area postrema and the medullary floor of the fourth ventricle. These lesions presumably represent the pathologic substrate for the intractable but reversible nausea and vomiting that occur in NMO.
METHODS
Archival material.
To characterize the NMO neuropathology at the medullary floor of the fourth ventricle and area postrema, we analyzed microscopic transverse sections of the caudal medulla in archival autopsy tissue from 15 clinically and pathologically confirmed NMO cases, 5 MS control cases (4 acute and 1 chronic), and 8 neurologically normal controls. Autopsies were performed at Mayo Clinic, Rochester, MN, or submitted from other institutions for diagnostic purposes between 1958 and 2009. This study was approved by the Mayo Clinic Institutional Review Board. Clinical follow-up blinded to pathologic outcome was obtained via review of medical records. All 15 cases included in the pathologic NMO cohort met Wingerchuk criteria for NMO or NMO spectrum disorder (NMOSD),1 and exhibited NMO-characteristic histopathologic optic or spinal lesions.5,8 The characteristics of this cohort are listed in table e-1 on the Neurology® Web site at www.neurology.org. Median age was 45 years at symptom onset (range 12–74) and 48.5 years at death (range 16–79). Median disease duration was 36 months (range 8–108). NMO-IgG serostatus was known in 6 cases (positive in 5, including all 3 NMOSD cases).
Neuropathologic evaluation and immunohistochemistry.
Specimens were fixed in 10%–15% formalin and embedded in paraffin. Sections were stained with hematoxylin & eosin (H&E) to demonstrate morphology of tissues and cells and Luxol fast blue/periodic acid–Schiff to demonstrate myelin and myelin degradation products. Immunohistochemistry was performed using an avidin-biotin or alkaline phosphatase/anti-alkaline phosphatase technique without modification and primary antibodies specific for AQP4, myelin proteolipid protein, glial fibrillary acidic protein (GFAP), neurofilament, all T (CD3), cytotoxic T (CD8), and B (CD20) lymphocytes, plasma cells (CD138), macrophages/microglia (KiM1P), and activated complement (C9neo) (table e-2).8
Inflammatory infiltrates were characterized with respect to their presence or absence, character (diffuse parenchymal/focal perivascular), and severity (mild, moderate, marked) and graded on the basis of inflammatory cell cuff thickness as follows: absent, mild (few vessels with 1 partial or complete cuff), or moderate to marked (many vessels with 1 cuff or few vessels with 2 cuffs, or few to many vessels having >3 cuffs).
Statistical analysis.
We used Wilson confidence intervals (CI) to estimate proportions and 95% CIs and logistic regression to evaluate whether the presence of lesions at the level of the medullary floor of the fourth ventricle and area postrema increased the odds of a patient with NMO having an episode with nausea/vomiting. Confidence intervals for the odds ratio (OR) of nausea/vomiting given the presence vs absence of NMO lesions at this level were calculated using the profile likelihood method. The statistical analysis was performed using R software version 2.8.1.
RESULTS
Neuropathologic findings at the level of the medullary floor of the fourth ventricle and area postrema in NMO.
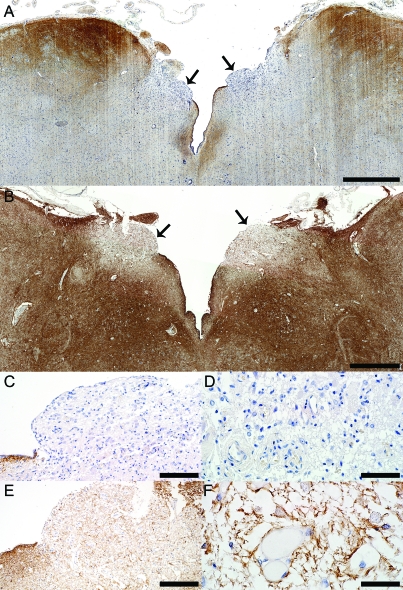
Six of the 15 clinically and pathologically confirmed NMO/NMOSD cases included in our study (40%) (figure 1, A, C, D, and figure e-1, A–C), but no control cases (MS or normal) (figure 1, B, E, F; figure e-1, D and E; and figure 3, D and E), showed loss of or reduced AQP4 immunoreactivity in the floor of the fourth ventricle or area postrema compared to baseline normal expression. No specimen had evidence of viral or autoimmune encephalitis, ischemic stroke, Balo lesions, or other superimposed pathology that could explain the focal loss of AQP4 at this level.8,16,17 Table 1 lists the pathologic characteristics of NMO lesions in this region. Most lesions selectively involved lateral aspects of the ventricular floor including the area postrema bilaterally, and extended ventrally into the medullary tegmentum (figure 1, A, C, D, and figure e-1, A–C). In only a single case, AQP4 loss also extended into the median aspect of the ventricular floor around the obex.8 Lesions were confined to the medullary floor of the fourth ventricle and area postrema and did not represent rostral extensions of cervical myelitis or dorsal extensions of ventral medullary lesions. Only one patient with area postrema AQP4 loss (patient 1 in table 1) showed the presence of a median ventral medullary lesion that extended dorsally to the level of the retro-olivary sulcus, but did not reach the ventricular floor. No other NMO medulla with AQP4 loss at the level of the ventricular floor and area postrema demonstrated additional medullary lesions (table 1). As previously reported, AQP4 immunoreactivity in control (normal and MS) and nonlesional NMO medullae was most intense in subependymal regions at the floor of the fourth ventricle, and was localized to astrocytic end-feet abutting blood vessels and neurons, and to basolateral membranes of ependymal cells (figure 1, A, C, D, and figure e-1, A–C).8 AQP4 immunoreactivity was clearly evident in the area postrema in MS and healthy control tissues, as well as in nonlesional NMO medullae (manifested by AQP4+ and GFAP+ astrocytic end-feet surrounding the basolateral endothelial membranes and neurons), but was less intense than in the remainder of the medullary ventricular floor (figure 1, B, E, F; figure 2, C and D; figure e-1, D and E; and figure 3, D and E). By contrast, area postrema in NMO lesioned tissues was devoid of AQP4 (figure 1, A, C, D, and figure e-1, A–C).
Figure 1. Aquaporin-4 (AQP4) is selectively lost in area postrema in neuromyelitis optica (NMO).
(A) The area postrema (arrows) in NMO patient 1 (patient 1 in table 1 and table e-1) who presented a clinically documented episode of nausea and intractable vomiting shows a characteristic selective loss of AQP4 (AQP4, scale bar = 1 mm). (B) In comparison, AQP4 is preserved in the area postrema (arrows) from a control patient with acute multiple sclerosis (Ctrl-MS) (AQP4, scale bar = 1 mm). (C) Higher magnification of the NMO area postrema (right arrow in A) shows a selective loss of AQP4, while subependymal immunoreactivity of AQP4 is preserved (AQP4, scale bar = 100 μm). (D) Higher magnification of the NMO area postrema (right arrow in A) shows the loss of the characteristic rim/rosette AQP4 distribution around blood vessels (AQP4, scale bar = 50 μm). (E) Higher magnification of area postrema (left arrow in B) shows preservation of AQP4 in Ctrl-MS (AQP4, scale bar = 200 μm). (F) Higher magnification of Ctrl-MS area postrema (left arrow in B) shows the preservation of AQP4 rim distribution around blood vessels (AQP4, scale bar = 50 μm).
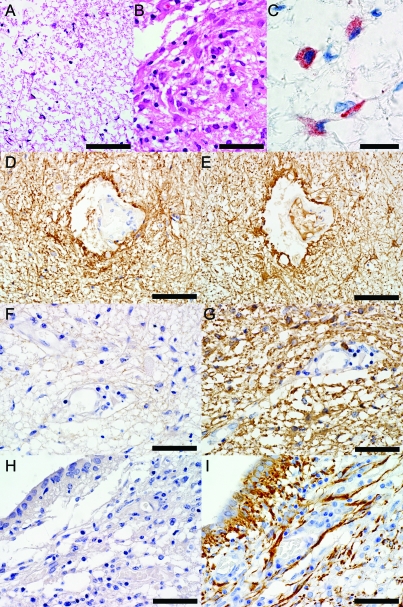
Figure 3. Astrocytic changes in neuromyelitis optica (NMO) area postrema lesions.
(A) Astrogliosis in an NMO area of aquaporin-4 (AQP4) loss and tissue rarefaction in the ventricular floor with uninucleated small reactive astrocytes with elongated, thin processes (hematoxylin & eosin [H&E], scale bar = 75 μm). (B) Astrogliosis in NMO area postrema with uninucleated small reactive astrocytes (H&E, scale bar = 50 μm). (C) Complement deposition in reactive astrocytes is localized within intracytoplasmic vacuoles (C9neo, scale bar = 20 μm). (D) Normal distribution of AQP4 in medulla from a neurologically normal control patient (Ctrl-N) (AQP4, scale bar = 75 μm). (E) Normal glial fibrillary acidic protein immunoreactivity in Ctrl-N medulla (GFAP, scale bar = 75 μm). (F) AQP4 is lost in NMO area postrema (AQP4, scale bar = 50 μm), but (G) GFAP immunoreactive astrocytes extending foot processes surrounding the endothelial cells are still present in the lesion (GFAP, scale bar = 50 μm). (H) AQP4 is lost in NMO medullary floor of the fourth ventricle (AQP4, scale bar = 50 μm), but (I) GFAP immunoreactive astrocytes extending foot processes surrounding the ependymal cells are still present in the lesion (GFAP, scale bar = 50 μm).
Table 1.
Characteristics of neuromyelitis optica lesions involving the medullary floor of the fourth ventricle and area postrema
Abbreviations: AQP4 = aquaporin-4; NA = tissue not available.
Patients 1–6 in table 1 are the same as patients 1–6 in table e-1.
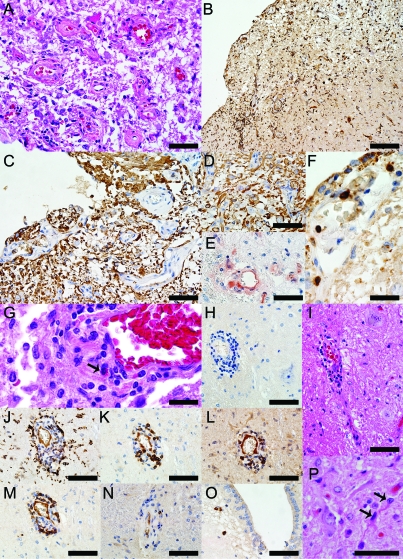
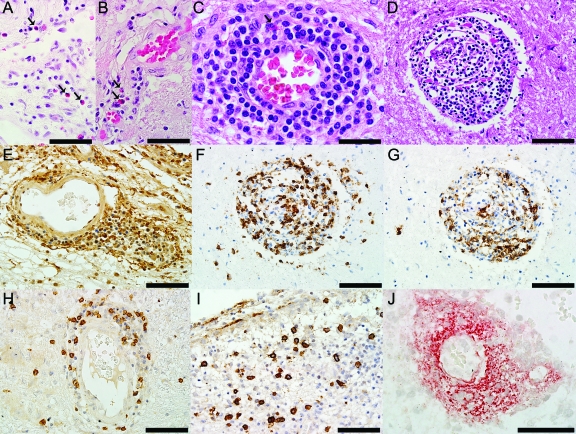
Figure 2. Neuropathologic findings in the medullary floor of the fourth ventricle and area postrema of the neuromyelitis optica (NMO) patient 1 illustrated in figure 1, A, C, and D.
(A) Thickening of the blood vessels in area postrema (hematoxylin & eosin [H&E], scale bar = 50 μm). (B) Profound activation of macrophages/microglia (KiM1P, scale bar = 100 μm). (C) Glial fibrillary acidic protein (GFAP) immunoreactive astrocytes extending foot processes that surrounded the endothelial cells are still present throughout these lesions (GFAP, scale bar = 50 μm). (D) GFAP immunoreactive astrocytes and preservation of neurons in the NMO area postrema (GFAP, scale bar = 50 μm). (E) Complement deposition in perivascular macrophages and astrocytes (C9neo, scale bar = 50 μm). (F) CD3+ T lymphocytes are present in the subependyma of the floor of the fourth ventricle (CD3, scale bar = 20 μm). (G) Eosinophils are components of the inflammatory infiltrates (H&E, scale bar = 20 μm). (H) Moderate perivascular inflammation in an area of aquaporin-4 (AQP4) loss (AQP4, scale bar = 50 μm). (I) Neurons are preserved around the blood vessel shown in H (H&E, scale bar = 50 μm). (J) Macrophages/microglia (KiM1P, scale bar = 50 μm), (K) CD3+ T lymphocytes (CD3, scale bar = 50 μm), (L) CD8+ T lymphocytes (CD8, scale bar = 50 μm), (M) B lymphocytes (CD20, scale bar = 50 μm), (N) Plasma cells (CD138, scale bar = 50 μm) are components of the perivascular inflammatory infiltrates. (O) Parenchymal plasma cells located in the subependyma of the floor of the fourth ventricle (CD138, scale bar = 50 μm). (P) Uninucleated, small, reactive astrocytes with thin, elongated processes (H&E, scale bar = 50 μm).
In 5 of 6 cases, AQP4 loss areas exhibited tissue rarefaction or vacuolation and blood vessel wall thickening (figure 2A). Three cases exhibited perivascular complement deposition (figures 2E and 4J). Complement products were also found in macrophages and reactive distended astrocytes (figures 2E and 3C). No acute neuronal pathology was found. Because area postrema contains unmyelinated axons, the presence or absence of demyelination at this site could not be assessed. However, as previously reported,8 myelin and axons were preserved in areas of AQP4 loss extending from the floor of the fourth ventricle into the dorsal medullary tegmentum and myelin-laden macrophages were rarely observed. All 6 cases with focally lost or reduced AQP4 contained moderate to marked parenchymal and perivascular inflammation. The inflammatory cells were identified as T lymphocytes (CD3+ [figure 2, F and K, and figure 4F] and CD8+ [figures 2L and 4H]), B lymphocytes (figures 2M and 4G), and plasma cells (figure 2, N and O, and figure 4I). No control medullae showed perivascular inflammation and nonlesioned NMO medullae showed only mild perivascular inflammation. All 6 cases exhibited prominent activation of parenchymal microglia and perivascular macrophages (figure 2, B and J, and figure 4E), and in 3 cases the inflammatory infiltrates contained eosinophils (figure 2G and figure 4, A–C).
Figure 4. Components of the inflammatory infiltrates in neuromyelitis optica (NMO) lesions.
(A) Parenchymal eosinophils (hematoxylin & eosin [H&E], scale bar = 50 μm) and (B, C) perivascular eosinophils (H&E, B scale bar = 50 μm, C scale bar = 30 μm) are components of the parenchymal and perivascular inflammatory infiltrates. (D) Marked perivascular inflammatory infiltrate (H&E, scale bar = 75 μm) composed of (E) macrophages/microglia (KiM1P, scale bar = 75 μm), (F) CD3+ T lymphocytes (CD3, scale bar = 75 μm), (G) B lymphocytes (CD20, scale bar = 75 μm), and (H) CD8+ T lymphocytes (CD8, scale bar = 75 μm). (I) Parenchymal plasma cells located in the subependyma of the medullary floor of the fourth ventricle (CD138, scale bar = 75 μm). (J) NMO perivascular deposition of complement in a “rosette” pattern (C9Neo, scale bar = 50 μm).
Despite AQP4 loss (figure 1, C and D, and figure 3, F and I), these lesions uniformly retained GFAP immunoreactive astrocytic foot processes (figure 2, C and D, and figure 3, G and I). Astroglial reaction was prominent: some were binucleated and many had multiple, elongated, thin processes (figure 2P and figure 3, A and B).
Clinical episodes of nausea and vomiting.
Blinded retrospective review of the patients' clinical histories revealed documentation of nausea/vomiting episodes in 4 of the 6 patients with area postrema lesions (table e-1). In 3 patients, the episode occurred within 2 months of death. In one patient, it occurred as a relapse during established disease. Clinical information was limited in the other 2 cases. Among the 9 patients without lesions at this level, only one had a documented episode of nausea/vomiting as initial disease presentation. Based on a logistic regression model with pathologic involvement of the medullary floor of the fourth ventricle and area postrema as the predictor and nausea/vomiting as the event, the odds of nausea/vomiting symptoms being present were an estimated 16 times greater with presence vs absence of lesions at this level (95% CI 1.43–437; p = 0.02).
DISCUSSION
In contrast to MS, intractable nausea/vomiting/hiccups is a characteristic symptom of NMO often preceding optic neuritis or transverse myelitis10,11 and may occur as an isolated clinical manifestation of early disease.9 Our study demonstrates pathologic abnormalities in the caudal medullae at the floor of the fourth ventricle and area postrema in 40% of patients with NMO. This frequency corresponds to the incidence of documented clinical episodes of intractable nausea/vomiting/hiccups in NMO.10,11 The occurrence of nausea/vomiting was overrepresented among patients with NMO with lesions in this region (67%) compared to patients with NMO without lesions (11%). The presence of these lesions increased the odds of nausea/vomiting 16 times (95% CI 1.43–437, p = 0.02). Thus, despite selection, ascertainment, and other biases inherent in neuropathologic studies, it would be unlikely that the effect was smaller than a 43% increase in the odds and it could be higher than a 16-fold increase.
Selective loss of AQP4 was the unifying neuropathologic characteristic of all NMO lesions found in the medullary floor of the fourth ventricle and area postrema. This was accompanied by tissue rarefaction, inflammation, variable complement deposition, and nonlytic alterations in GFAP-positive reactive astrocytes. As previously reported, lesions at this level were nondestructive with relative neuronal, axonal, and myelin preservation.8
All 6 NMO cases exhibited AQP4 loss from the area postrema and lateral regions of the medullary ventricular floor. Only one case also had midline involvement.8 These histopathologic findings suggest that area postrema may be a preferential and selective target of the NMO disease process, and are compatible with clinical reports of nausea/vomiting preceding episodes of optic neuritis and transverse myelitis10,11 and even representing the heralding disease symptom.9 MRI also had demonstrated isolated involvement of the area postrema in NMO.9 Pathologic involvement of the area postrema independently of the fourth ventricle floor further supports the involvement of this region as a potential site of initial NMO-IgG entry into the CNS. In common with other CVO, the area postrema lacks tight endothelial cell junctions,12,13 and neural cells in this region are readily accessible to circulating IgG.18 Furthermore, the area postrema is one of the most vascularized brain regions. Its plasma flow and surface area/permeability ratio are higher than adjacent medullary regions. Slowing of blood flow by specialized pericapillary “pools” of interstitial fluid in the area postrema increases the exposure of local neurons and glia to bloodborne constituents.13 The endothelial permeation of NMO-IgG and its delayed clearance may summate to maintain an exceptionally high concentration around astrocytic end-feet at this site, thus enhancing the vulnerability of area postrema to AQP4-IgG–induced pathophysiology. The selective bilateral involvement of the area postrema in 5/6 NMO cases suggests that IgG targeting AQP4 at this site might be sufficient to initiate the NMO disease process without requiring antigen-specific T-cells to breach the BBB. This suggestion is supported by the reported passive transfer of “essential hypernatremia” to mice by an IV injected human autoantibody targeting the sodium-level sensing Nax channels expressed in astrocytes, ependymal cells, and pituicytes in the CVO.19,20
Previous reports of NMO pathology emphasized astrocyte necrosis and GFAP loss secondary to the complement-mediated lysis that follows AQP4-IgG binding.21–23 By contrast, area postrema lesions were characterized by a prominent nonlytic reaction of GFAP-positive astrocytes. The medullary ventricular floor and area postrema contained an abundance of uninucleated and binucleated reactive astrocytes, some having multiple, elongated, thin processes. Despite AQP4 loss, they retained GFAP-positive foot processes, suggesting that complement-mediated cytotoxicity in this region is minimized by rapid and persistent downregulation of AQP4.24 The appearance of the astrocytes was reminiscent of cultured astrocytes in which AQP4 reduction by RNAi treatment was accompanied by transformation from polygonal to elongated morphology, interpreted as a cell strategy to increase surface/volume ratio to maintain water flux in the absence of AQP4.25
The nonlytic lesions containing intact GFAP-positive reactive astrocytes that we identified in the medullary floor of the fourth ventricle and area postrema contrast with the destructive demyelinating NMO lesions found in the same patients' spinal cords or optic nerves.5,8 It is conceivable that perivascular astrocytes in the area postrema may have unique properties. Regional differences in complement-regulatory protein expression are known to determine vulnerability to autoantibody-induced complement-mediated damage in different regions of the nervous system.26 Complement-regulatory proteins in the plasmalemma of area postrema astrocytes may therefore render them resistant to lysis by complement.27 Additional studies are needed to confirm if this is the case for NMO. In the absence of astrocytic lysis, IgG-mediated cross-linking of AQP4 in the area postrema may trigger reactive astrocytosis. In Trypanosoma brucei infection,19 another immune-mediated CVO disorder, reactive astrocytes in the area postrema respond as innate immune effectors, producing chemokines and cytokines that recruit and activate eosinophils, macrophages, and lymphocytes. Indeed we have observed in NMO analogous recruitment of perivascular macrophages, eosinophils, and pronounced activation of parenchymal microglia. Factors released from reactive astrocytes in the area postrema can activate neighboring cells to further skew and amplify the immune response, enhance BBB permeability, and attract granulocytes and monocytes from the systemic circulation.27 Products of early-cleaved complement components, as well as cytokines and chemokines produced by the reactive astrocytes, would also activate resident microglia and dendritic cells.27,28
Inflammatory cytokine signaling from area postrema microglia, macrophages, and dendritic cells spreads to microglia in adjacent brainstem nuclei as a volume transmission signal. Diffusion, relayed by intermediate molecules, propagates the signal throughout the brain parenchyma by recruiting adjacent microglia.29 Because the ependymal lining of the area postrema does not form a tight blood–CSF barrier,30 cytokines released therein can access the CSF to influence distant brain structures.31 CSF is also another potential pathway for NMO-IgG to spread from the area postrema to distant brain regions.32 Additionally, immune cells in area postrema are known to activate area postrema sensory neurons.28 Projections from area postrema to adjacent brainstem and hypothalamic nuclei are responsible for the emetic reflex but also for the sympathetic nervous system and hypothalamo-pituitary-adrenal axis activation which may modulate the cytokine-induced sickness behavior, immune response, or brain water homeostasis.12,14,20
It remains unclear why symptomatic pathophysiology at the level of the medullary floor of the fourth ventricle and area postrema is not a universal NMO pathologic hallmark. A plausible explanation is that the reversibility of these inflammatory nondestructive medullary lesions may reflect a transient functional impairment of the astrocyte to mediate water flux that is rapidly compensated in AQP4-enriched regions.8 This is supported by clinical data showing that patients with NMO can recover spontaneously from intractable episodes of nausea/vomiting9–11 and by imaging data describing rapid resolution of such NMO medullary lesions.3,4,9,10 Furthermore, AQP4 and excitatory amino acid transporter 2 (EAAT2) exist in astrocytic membranes as a macromolecular complex and binding of NMO-IgG to AQP4 downregulates both AQP4 and EAAT2 resulting in impaired glutamate homeostasis.21 However, whereas EAAT2 is heavily expressed in the central spinal cord, the area postrema lacks EAAT2.21,33 The concomitant loss of both AQP4 and EAAT2 in the spinal cord in the context of NMO attacks, resulting in glutamate-mediated tissue injury, might account for the occurrence of NMO destructive central spinal cord lesions.8,21 The fact that the area postrema is not dependent on EAAT2 to regulate glutamate homeostasis could also explain why area postrema NMO lesions are nondestructive as well as clinically and radiographically reversible.
Why is nausea/vomiting not a universal NMO symptom? First, nausea/vomiting are overlooked in the context of NMO, especially as the initial isolated symptoms.9 Second, other CVO could serve as initial points of attack. Hypothalamic involvement has been described in NMO.34 An initial episode at the level of the organum vasculosum of lama terminalis would present with fever episodes reflecting NMO-IgG binding–induced cytokine action at the level of hypothalamus35 or with hypothermia in the more advanced settings of destructive NMO hypothalamic lesions.34,36 Febrile episodes have been described preceding NMO exacerbations and even as initial presenting NMO symptoms11 but they have been attributed to viral infections. Third, autoantibodies must enter or be locally synthesized in sufficient concentrations to be pathogenic in the CNS. Nausea/vomiting has been documented in some patients at the peak of their NMO-IgG titer.11 However, when the NMO-IgG serum concentration is not high enough to cause glial activation and neuronal impairment in the CVO, astrocytes and endothelial cells could be affected by proinflammatory cytokines released in response to bacterial or viral infections37 and, in such instances, NMO-IgG would enter the CNS through a diffusely disrupted BBB.
We have documented unique neuropathologic features of NMO lesions at the level of the medullary floor of the fourth ventricle and area postrema. These lesions are distinct from NMO lesions in other CNS regions by being inflammatory, nondestructive, and nondemyelinating and likely represent the pathologic substrate for the intractable but reversible nausea/vomiting that sometimes occur in NMO. Our observation that area postrema astrocytes appear to resist complement-mediated lysis suggests that IgG-induced crosslinking of AQP4 may activate an orchestrated astrocytic inflammatory response that can be propagated through the CNS astrocytic network, thus enhancing BBB permeability, NMO-IgG entry in other brain regions, and recruitment and activation of leukocytes that further exacerbate and propagate the disease pathology. The novel characteristics that we have documented in NMO lesions involving the medullary floor of the fourth ventricle and area postrema further distinguish NMO from MS pathologically.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Hans Lassmann, Centre for Brain Research, Medical University of Vienna, Vienna, Austria, and members of his laboratory for performing the C9neo immunohistochemistry stain, and Patricia Ziemer, Mayo Clinic, for technical assistance. The Thomas Willis Oxford Brain Collection provided 2 of the cases described in this report.
Editorial, page 1202
Supplemental data at www.neurology.org
- AQP4
- aquaporin-4
- BBB
- blood–brain barrier
- CI
- confidence interval
- CVO
- circumventricular organs
- EAAT2
- excitatory amino acid transporter 2
- GFAP
- glial fibrillary acidic protein
- H&E
- hematoxylin & eosin
- IgG
- immunoglobulin G
- MS
- multiple sclerosis
- NMO
- neuromyelitis optica
- NMOSD
- neuromyelitis optica spectrum disorder
- OR
- odds ratio
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by S.D. Weigand.
DISCLOSURE
Dr. Popescu reports no disclosures. Dr. Lennon is a named investor on a patent application filed by the Mayo Foundation for Medical Education and Research that relates to the NMO antigen and its application to the diagnosis of NMO; may accrue revenue for a patent re: Aquaporin-4 associated antibodies for diagnosis of neuromyelitis optica; and receives research support from the NIH and the Guthy Jackson Charitable Foundation. Dr. Parisi serves on scientific advisory boards for the US Government Defense Health Board and the Subcommittee for Laboratory Services and Pathology; serves as a Section Editor for Neurology®; receives royalties from the publication of Principles & Practice of Neuropathology, 2nd ed. (Oxford University Press, 2003); and receives research support from the NIH. Dr. Howe, S.D. Weigand, and Dr. Cabrera-Gómez report no disclosures. Dr. Newell receives/has received research support from the NIH. Dr. Mandler reports no disclosures. Dr. Pittock may accrue revenue for patents re: Aquaporin-4 associated antibodies for diagnosis of neuromyelitis optica and aquaporin-4 autoantibody as a cancer marker; and has received research support from Alexion Pharmaceuticals, Inc. and the Guthy Jackson Charitable Foundation. Dr. Weinshenker serves on data safety monitoring boards for Novartis and Biogen Idec; serves on the editorial boards of the Canadian Journal of Neurological Sciences and the Turkish Journal of Neurology; has received research support from Genzyme Corporation and the Guthy-Jackson Charitable Foundation; and receives license royalties from RSR Ltd. for a patent re: Aquaporin-4 associated antibodies for diagnosis of neuromyelitis optica. Dr. Lucchinetti may accrue revenue for a patent re: Aquaporin-4 associated antibodies for diagnosis of neuromyelitis optica; receives royalties from the publication of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); and receives research support from the NIH, the Guthy Jackson Charitable Foundation (PI), and the National MS Society.
REFERENCES
- 1. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 3. Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390–396 [DOI] [PubMed] [Google Scholar]
- 4. Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–968 [DOI] [PubMed] [Google Scholar]
- 5. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112 [DOI] [PubMed] [Google Scholar]
- 7. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007;130:1194–1205 [DOI] [PubMed] [Google Scholar]
- 9. Apiwattanakul M, Popescu BF, Matiello M, et al. Intractable vomiting as the initial presentation of NMO. Ann Neurol 2010;68:757–761 [DOI] [PubMed] [Google Scholar]
- 10. Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 2005;65:1479–1482 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi T, Miyazawa I, Misu T, et al. Intractable hiccup and nausea in neuromyelitis optica with anti-aquaporin-4 antibody: a herald of acute exacerbations. J Neurol Neurosurg Psychiatry 2008;79:1075–1078 [DOI] [PubMed] [Google Scholar]
- 12. Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev 2007;56:119–147 [DOI] [PubMed] [Google Scholar]
- 13. Gross PM. Morphology and physiology of capillary systems in subregions of the subfornical organ and area postrema. Can J Physiol Pharmacol 1991;69:1010–1025 [DOI] [PubMed] [Google Scholar]
- 14. Price CJ, Hoyda TD, Ferguson AV. The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist 2008;14:182–194 [DOI] [PubMed] [Google Scholar]
- 15. Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci USA 1995;92:4328–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma R, Fischer MT, Bauer J, et al. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol 2010;120:223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsuoka T, Suzuki SO, Iwaki T, Tabira T, Ordinario AT, Kira JI. Aquaporin-4 astrocytopathy in Balo's disease. Acta Neuropathol Epub 2010 Aug 3 [DOI] [PubMed] [Google Scholar]
- 18. Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol 1993;120:245–263 [DOI] [PubMed] [Google Scholar]
- 19. Quan N, Mhlanga JD, Whiteside MB, McCoy AN, Kristensson K, Herkenham M. Chronic overexpression of proinflammatory cytokines and histopathology in the brains of rats infected with Trypanosoma brucei. J Comp Neurol 1999;414:114–130 [DOI] [PubMed] [Google Scholar]
- 20. Hiyama TY, Matsuda S, Fujikawa A, et al. Autoimmunity to the sodium-level sensor in the brain causes essential hypernatremia. Neuron 2010;66:508–522 [DOI] [PubMed] [Google Scholar]
- 21. Hinson SR, Roemer SF, Lucchinetti CF, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med 2008;205:2473–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lassmann H. Experimental models of neuromyelitis optica. Rinsho Shinkeigaku 2009;49:900–901 [DOI] [PubMed] [Google Scholar]
- 23. Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 2007;130:1224–1234 [DOI] [PubMed] [Google Scholar]
- 24. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007;69:2221–2231 [DOI] [PubMed] [Google Scholar]
- 25. Nicchia GP, Frigeri A, Liuzzi GM, Svelto M. Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. Faseb J 2003;17:1508–1510 [DOI] [PubMed] [Google Scholar]
- 26. Tang H, Brimijoin S. Complement regulatory proteins and selective vulnerability of neurons to lysis on exposure to acetylcholinesterase antibody. J Neuroimmunol 2001;115:53–63 [DOI] [PubMed] [Google Scholar]
- 27. Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol 2007;28:138–145 [DOI] [PubMed] [Google Scholar]
- 28. Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience 2006;140:1415–1434 [DOI] [PubMed] [Google Scholar]
- 29. Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience 1999;89:535–548 [DOI] [PubMed] [Google Scholar]
- 30. Gotow T, Hashimoto PH. Fine structure of the ependyma and intercellular junctions in the area postrema of the rat. Cell Tissue Res 1979;201:207–225 [DOI] [PubMed] [Google Scholar]
- 31. Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci 1991;48:PL117–121 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 2007;130:1235–1243 [DOI] [PubMed] [Google Scholar]
- 33. Berger UV, Hediger MA. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J Comp Neurol 2000;421:385–399 [DOI] [PubMed] [Google Scholar]
- 34. Viegas S, Weir A, Esiri M, et al. Symptomatic, radiological and pathological involvement of the hypothalamus in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2009;80:679–682 [DOI] [PubMed] [Google Scholar]
- 35. Blatteis CM, Sehic E, Li S. Pyrogen sensing and signaling: old views and new concepts. Clin Infect Dis 2000;31 (suppl 5):S168–S177 [DOI] [PubMed] [Google Scholar]
- 36. Poppe AY, Lapierre Y, Melancon D, et al. Neuromyelitis optica with hypothalamic involvement. Mult Scler 2005;11:617–621 [DOI] [PubMed] [Google Scholar]
- 37. Sellner J, Hemmer B, Muhlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (Devic) syndromes. J Autoimmun 2010;34:371–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.