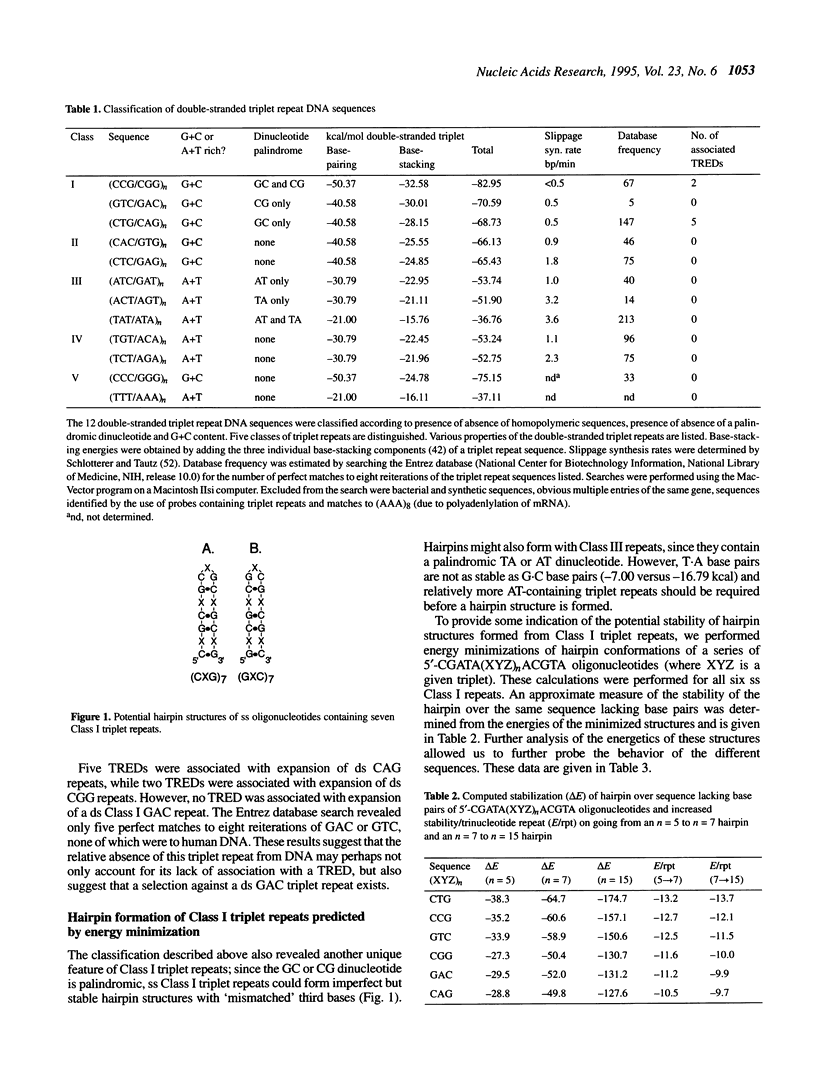
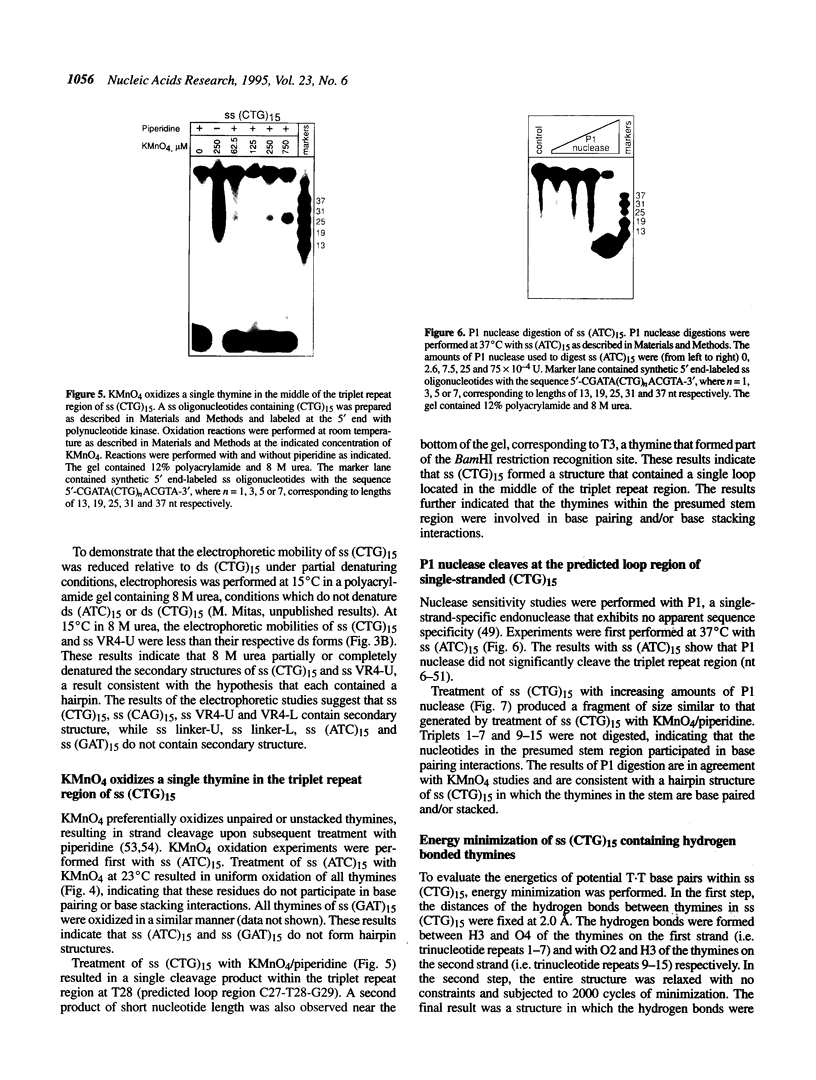
Abstract
Although triplet repeat DNA sequences are scattered throughout the human genome, their biological function remains obscure. To aid in correlating potential structures of these nucleic acids with their function, we propose their classification based on the presence or absence of a palindromic dinucleotide within the triplet, the G + C content, and the presence or absence of a homopolymer. Five classes of double-stranded (ds) triplet repeats are distinguished. Class I repeats, which are defined by the presence of a GC or CG palindrome, have the lowest base stacking energies, exhibit the lowest rates of slippage synthesis [Schlötterer and Tautz (1992) Nucleic Acids Res., 20, 211] and are uniquely associated with triplet repeat expansion diseases. The six single-stranded (ss) triplet repeats within Class I also have the potential to form hairpin structures, as determined by energy minimization. To explore the possibility of hairpin formation by ss Class I triplet repeats, studies were performed with a ss oligonucleotide containing 15 prototypic CTG repeats [ss (CTG)15]. Electrophoretic, P1 nuclease and KMnO4 oxidation data demonstrate that ss (CTG)15 forms a hairpin containing base paired and/or stacked thymines in the stem. Potential functions of hairpins containing Class I triplet repeats are discussed with respect to protein translation and mRNA splicing. Further, potential roles of hairpin structures in triplet repeat expansion events are discussed.
Full text
PDF







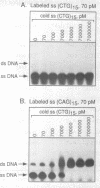
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balagurumoorthy P., Brahmachari S. K., Mohanty D., Bansal M., Sasisekharan V. Hairpin and parallel quartet structures for telomeric sequences. Nucleic Acids Res. 1992 Aug 11;20(15):4061–4067. doi: 10.1093/nar/20.15.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. V., Hirst M. C., Nakahori Y., MacKinnon R. N., Roche A., Flint T. J., Jacobs P. A., Tommerup N., Tranebjaerg L., Froster-Iskenius U. Physical mapping across the fragile X: hypermethylation and clinical expression of the fragile X syndrome. Cell. 1991 Feb 22;64(4):861–866. doi: 10.1016/0092-8674(91)90514-y. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Pizzuti A., Fu Y. H., Fenwick R. G., Jr, Nelson D. L. Triplet repeat mutations in human disease. Science. 1992 May 8;256(5058):784–789. doi: 10.1126/science.1589758. [DOI] [PubMed] [Google Scholar]
- Chen L., Frankel A. D. An RNA-binding peptide from bovine immunodeficiency virus Tat protein recognizes an unusual RNA structure. Biochemistry. 1994 Mar 8;33(9):2708–2715. doi: 10.1021/bi00175a046. [DOI] [PubMed] [Google Scholar]
- Collick A., Jeffreys A. J. Detection of a novel minisatellite-specific DNA-binding protein. Nucleic Acids Res. 1990 Feb 11;18(3):625–629. doi: 10.1093/nar/18.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas J. F. Estimation of microsatellite mutation rates in recombinant inbred strains of mouse. Mamm Genome. 1992;3(8):452–456. doi: 10.1007/BF00356155. [DOI] [PubMed] [Google Scholar]
- Dix D. J., Lin P. N., McKenzie A. R., Walden W. E., Theil E. C. The influence of the base-paired flanking region on structure and function of the ferritin mRNA iron regulatory element. J Mol Biol. 1993 May 20;231(2):230–240. doi: 10.1006/jmbi.1993.1278. [DOI] [PubMed] [Google Scholar]
- Dolinnaya N. G., Braswell E. H., Fossella J. A., Klump H., Fresco J. R. Molecular and thermodynamic properties of d(A(+)-G)10, a single-stranded nucleic acid helix without paired or stacked bases. Biochemistry. 1993 Sep 28;32(38):10263–10270. doi: 10.1021/bi00089a049. [DOI] [PubMed] [Google Scholar]
- Dolinnaya N. G., Fresco J. R. Single-stranded nucleic acid helical secondary structure stabilized by ionic bonds: d(A(+)-G)10. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9242–9246. doi: 10.1073/pnas.89.19.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Goguel V., Rosbash M. Splice site choice and splicing efficiency are positively influenced by pre-mRNA intramolecular base pairing in yeast. Cell. 1993 Mar 26;72(6):893–901. doi: 10.1016/0092-8674(93)90578-e. [DOI] [PubMed] [Google Scholar]
- Goguel V., Wang Y., Rosbash M. Short artificial hairpins sequester splicing signals and inhibit yeast pre-mRNA splicing. Mol Cell Biol. 1993 Nov;13(11):6841–6848. doi: 10.1128/mcb.13.11.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatsu H., Ukita T. The selective degradation of pyrimidines in nucleic acids by permanganate oxidation. Biochem Biophys Res Commun. 1967 Nov 30;29(4):556–561. doi: 10.1016/0006-291x(67)90521-9. [DOI] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C. Homocopolymer sequences in the spacer of a sea urchin histone gene repeat are sensitive to S1 nuclease. Nature. 1982 Feb 25;295(5851):714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., BERTSCH L. L., JACKSON J. F., KHORANA H. G. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID, XVI. OLIGONUCLEOTIDES AS TEMPLATES AND THE MECHANISM OF THEIR REPLICATION. Proc Natl Acad Sci U S A. 1964 Feb;51:315–323. doi: 10.1073/pnas.51.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Cash F., Eshleman S. S. Translation inhibition by an mRNA coding region secondary structure is determined by its proximity to the AUG initiation codon. J Mol Biol. 1992 Aug 5;226(3):609–621. doi: 10.1016/0022-2836(92)90619-u. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Martin J. B. Molecular genetics of neurological diseases. Science. 1993 Oct 29;262(5134):674–676. doi: 10.1126/science.8235586. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCarthy J. G., Rich A. Detection of an unusual distortion in A-tract DNA using KMnO4: effect of temperature and distamycin on the altered conformation. Nucleic Acids Res. 1991 Jun 25;19(12):3421–3429. doi: 10.1093/nar/19.12.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S. Triplet repeats strike again. Nat Genet. 1994 Jan;6(1):3–4. doi: 10.1038/ng0194-3. [DOI] [PubMed] [Google Scholar]
- Morrison P. J. Trinucleotide repeat repeat repeat. Lancet. 1993 Aug 14;342(8868):385–386. doi: 10.1016/0140-6736(93)92809-8. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Warren S. T. Trinucleotide repeat instability: when and where? Nat Genet. 1993 Jun;4(2):107–108. doi: 10.1038/ng0693-107. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Pause A., Méthot N., Svitkin Y., Merrick W. C., Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994 Mar 1;13(5):1205–1215. doi: 10.1002/j.1460-2075.1994.tb06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. M. Advance directives, proxies, and the practice of surgery. Am J Surg. 1992 Mar;163(3):277–82. doi: 10.1016/0002-9610(92)90001-8. [DOI] [PubMed] [Google Scholar]
- Pierce J. C., Kong D., Masker W. The effect of the length of direct repeats and the presence of palindromes on deletion between directly repeated DNA sequences in bacteriophage T7. Nucleic Acids Res. 1991 Jul 25;19(14):3901–3905. doi: 10.1093/nar/19.14.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Yu S., Sutherland G. R. Fragile X syndrome unstable element, p(CCG)n, and other simple tandem repeat sequences are binding sites for specific nuclear proteins. Hum Mol Genet. 1993 Sep;2(9):1429–1435. doi: 10.1093/hmg/2.9.1429. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Sutherland G. R. Dynamic mutations: a new class of mutations causing human disease. Cell. 1992 Sep 4;70(5):709–712. doi: 10.1016/0092-8674(92)90302-s. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlötterer C., Tautz D. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992 Jan 25;20(2):211–215. doi: 10.1093/nar/20.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Zischler H., Epplen J. T. (CAC)5, a very informative oligonucleotide probe for DNA fingerprinting. Nucleic Acids Res. 1988 Jun 10;16(11):5196–5196. doi: 10.1093/nar/16.11.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Wells R. D. DNA structure, mutations, and human genetic disease. Curr Opin Biotechnol. 1992 Dec;3(6):612–622. doi: 10.1016/0958-1669(92)90005-4. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Zheng G. X., Brankamp R. G., Allen K. N. On the deletion of inverted repeated DNA in Escherichia coli: effects of length, thermal stability, and cruciform formation in vivo. Genetics. 1991 Dec;129(4):991–1005. doi: 10.1093/genetics/129.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992 Sep;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Tarleton J. C., Saul R. A. Molecular genetic advances in fragile X syndrome. J Pediatr. 1993 Feb;122(2):169–185. doi: 10.1016/s0022-3476(06)80110-1. [DOI] [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989 Aug 25;17(16):6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984 May 25;12(10):4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Trick M., Dover G. A. Cryptic simplicity in DNA is a major source of genetic variation. Nature. 1986 Aug 14;322(6080):652–656. doi: 10.1038/322652a0. [DOI] [PubMed] [Google Scholar]
- Theil E. C. The IRE (iron regulatory element) family: structures which regulate mRNA translation or stability. Biofactors. 1993 May;4(2):87–93. [PubMed] [Google Scholar]
- Trinh T. Q., Sinden R. R. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature. 1991 Aug 8;352(6335):544–547. doi: 10.1038/352544a0. [DOI] [PubMed] [Google Scholar]
- Trinh T. Q., Sinden R. R. The influence of primary and secondary DNA structure in deletion and duplication between direct repeats in Escherichia coli. Genetics. 1993 Jun;134(2):409–422. doi: 10.1093/genetics/134.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. J., Elder J. F., Jr, Laughlin T. F., Davis W. P. Genetic variation in clonal vertebrates detected by simple-sequence DNA fingerprinting. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5653–5657. doi: 10.1073/pnas.87.15.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega Laso M. R., Zhu D., Sagliocco F., Brown A. J., Tuite M. F., McCarthy J. E. Inhibition of translational initiation in the yeast Saccharomyces cerevisiae as a function of the stability and position of hairpin structures in the mRNA leader. J Biol Chem. 1993 Mar 25;268(9):6453–6462. [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Ohtsuka E., Khorana H. G. Studies on polynucleotides. L. Synthetic deoxyribopolynucleotides as templates for the DNA polymerase of Escherichia coli: a new double-stranded DNA-like polymer containing repeating dinucleotide sequences. J Mol Biol. 1965 Nov;14(1):221–237. doi: 10.1016/s0022-2836(65)80242-x. [DOI] [PubMed] [Google Scholar]
- Wohlrab F. Enzyme probes in vitro. Methods Enzymol. 1992;212:294–301. doi: 10.1016/0076-6879(92)12018-l. [DOI] [PubMed] [Google Scholar]
- Wrogemann K., Biancalana V., Devys D., Imbert G., Trottier Y., Mandel J. L. Microsatellites and disease: a new paradigm. EXS. 1993;67:141–152. doi: 10.1007/978-3-0348-8583-6_13. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Nomoto S., Mishima Y., Kominami R. A 35-kDa protein binding to a cytosine-rich strand of hypervariable minisatellite DNA. J Biol Chem. 1992 Jun 15;267(17):12311–12316. [PubMed] [Google Scholar]
- Yoon H., Miller S. P., Pabich E. K., Donahue T. F. SSL1, a suppressor of a HIS4 5'-UTR stem-loop mutation, is essential for translation initiation and affects UV resistance in yeast. Genes Dev. 1992 Dec;6(12B):2463–2477. doi: 10.1101/gad.6.12b.2463. [DOI] [PubMed] [Google Scholar]
- van Gelder C. W., Gunderson S. I., Jansen E. J., Boelens W. C., Polycarpou-Schwarz M., Mattaj I. W., van Venrooij W. J. A complex secondary structure in U1A pre-mRNA that binds two molecules of U1A protein is required for regulation of polyadenylation. EMBO J. 1993 Dec 15;12(13):5191–5200. doi: 10.1002/j.1460-2075.1993.tb06214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]