Abstract
TEMPO-substituted pargyline analogues differentially inhibit recombinant human Monoamine Oxidase A (MAO A) and B (MAO B) in intact yeast mitochondria suggesting these membrane-bound enzymes are located on differing faces of the mitochondrial outer membrane (Upadhyay, A. and Edmondson, D.E., Biochemistry 48, 3928, 2009). This approach is extended to the recombinant rat enzymes and to rat liver mitochondria. The differential specificities exhibited for human MAO A and MAO B by the meta- and para-amido TEMPO pargylines are not as absolute with the rat enzymes. Similar patterns of reactivity are observed for rat MAO A and B in mitochondrial outer membrane preparations expressed in Pichia pastoris or isolated from rat liver. In intact yeast mitochondria, recombinant rat MAO B is inhibited by the pargyline analogue whereas MAO A activity shows no inhibition. Intact rat liver mitochondria exhibit an opposite inhibition pattern to that observed in yeast where MAO A is inhibited and MAO B activity is unaffected. Protease inactivation studies show specificity in that MAO A is sensitive to trypsin whereas MAO B is sensitive to β-chymotrypsin. In intact mitochondrial preparations, MAO A is readily inactivated in rat liver but not in yeast on trypsin treatment and MAO B is readily inactivated by β-chymotrypsin in yeast but not in rat liver. These data show MAO A is oriented on the cytosolic face and MAO B is situated on the surface facing the intermembrane space of the mitochondrial outer membrane in rat liver. The differential mitochondrial outer membrane topology of MAO A and MAO B is relevant to their inhibition by drugs designed to be cardio-protectants or neuro-protectants.
The known age-related increases in expression of Monoamine Oxidase B (MAO B)1 in neuronal tissue (1) and Monoamine Oxidase A (MAO A) in heart (2) have been implicated in neurological (3) and cardiovascular disorders (4). Design of highly specific reversible inhibitors for each enzyme that could serve as neuro-protectants and cardio-protectants has been and is currently receiving increased attention. It is known that MAO A and MAO B levels vary among different tissues (5). In all cases, both enzymes are dimeric (6) and found tightly bound to the outer membrane of the mitochondrion via C-terminal trans-membrane helices as well as undetermined membrane interactions with the core polypeptide chain (7, 8).
In spite of considerable information in the literature on the structures of MAO A (8, 9) and MAO B (7), their respective substrate and inhibitor specificities, and expression, there is little knowledge on the membrane topology of either enzyme. Early work to address this issue utilized polyclonal antibodies (10) and susceptibility to proteolysis (11). The results of these studies resulted in conflicting conclusions on the membrane orientations of MAO A and MAO B. Knowledge of the outer membrane topology of MAO A and MAO B is an important issue relative to selective inhibitor design. Although the mitochondrial outer membrane is classically thought to be permeable to molecules 6 kDa or lower (12), more recent work has demonstrated this permeability is highly controlled (13). Therefore, the assumption that outer membrane permeability would present no obstacles to MAO inhibitors (if they were required to traverse the outer membrane) to bind to the active site of either enzyme may not be valid. Alternatively, both enzymes may be oriented towards the cytosolic face of the outer membrane and therefore the issue of transport of inhibitors across the outer membrane becomes moot.
Previous published work from this laboratory (14) has shown that TEMPO-substituted pargyline (ParSL1–3, structures in Figure S1, Supplementary Materials) analogues exhibit differential reactivities with human MAO A and MAO B depending on whether the TEMPO moiety is in the meta (ParSL-3) or para (ParSL-2) amide linkages with the benzene ring whereas ParSL-1 inactivates either enzyme (14). It was also demonstrated that, in intact mitochondria isolated from the expression strain of Pichia pastoris, human MAO B is readily inactivated by a ParSL-1 whereas human MAO A is unreactive. Similar experiments with intact human placental mitochondria demonstrated MAO A is readily inactivated by ParSL-1. These results were interpreted to suggest that recombinant human MAO A is orientated on the surface facing the intermembrane space of the Pichia outer membrane and on the cytosolic face of human placental mitochondria, whereas recombinant human MAO B faces the cytosolic side of Pichia mitochondrial outer membrane.
These results provide the basis for the applicability of these pargyline analogues as well as proteolysis studies to probe the MAO topology in intact mitochondria. In this paper, we extend this approach to rat liver MAO A and MAO B. The rat is experimentally more accessible to carry out these studies since tissue samples are readily available. It is known that differences in inhibitor sensitivities exist between the rat and human enzymes (15, 16), therefore comparative inhibition studies are reported for purified and membrane bound forms of recombinant rat MAO A and MAO B and compared with those using rat liver membrane preparations. Proteolysis studies of rat MAO A and MAO B are also presented and compared with previous published studies (11). The results of the inhibition and proteolysis studies presented on recombinant and rat liver MAOA and B support the conclusion that rat liver MAO A is located on the cytosolic face of the mitochondrial outer membrane whereas MAO B is bound to the surface facing the intermembrane space. The significance of these findings is discussed with regard to MAO inhibitor design.
EXPERIMENTAL PROCEDURES
Materials
The detergents, β–octylglucopyranoside was obtained from Anatrace Inc. and reduced Triton X-100 was purchased from Fluka. Percoll was obtained from Amersham Biosciences. The TEMPO-substituted pargyline analogues (ParSL-1, ParSL-2, and ParSL-3) were synthesized by Dr. Anup Upadhyay in this laboratory as described previously (14). All other chemical used in this work were purchased from Sigma-Aldrich.
Expression and Purification of Rat MAO
Recombinant rat liver MAO A and MAO B were expressed and purified in Pichia pastoris strain KM71 as described previously (17, 18). The purified enzymes were stored in 50 mM potassium phosphate containing 50% (w/v) glycerol and 0.8% (w/v) β–octylglucopyranoside (pH 7.2) at −20°C. It should be noted that d-amphetamine, a reversible MAO A inhibitor used for stabilizing rat MAO A enzyme during purification, was removed prior to all kinetic measurements. All determinations of protein concentration were performed using the Biuret procedure.
Isolation of P. pastoris Mitochondria and Mitochondrial Outer Membranes
Intact mitochondria from P. pastoris cell paste were isolated by enzymatic disruption of the cell walls using zymolyase followed by homogenization and sequential centrifugations (19). Purified intact mitochondria were resuspended in 0.6 M mannitol, 10 mM Tris-HCl, pH 7.4, to a final concentration of 10 mg of protein/mL. Integrity of the isolated mitochondria was determined by measuring the rate of NADH oxidation following the rate of O2 uptake with an oxygen electrode relative to a control sample of disrupted mitochondria. The mitochondrial outer membrane (MOM) was isolated by osmotic shock and mild sonication of intact mitochondria followed by centrifugation in a 30–55% (w/v) sucrose density gradient in 10 mM Tris-HCl buffer (pH 7.4) at 20,000 rpm (Beckman SW-28 rotor) for 15 hours. MOM pellets were suspended in 10 mM Tris-HCl buffer containing 10% (v/v) glycerol at a protein concentration of 10 mg/mL and kept frozen in small aliquots at −80°C until used.
Isolation of Mitochondria from Rat Liver
Intact mitochondria from rat MAO liver were isolated by following a published method (20). Briefly, one Sprague-Dawley rat was sacrificed and the liver (20 grams) was perfused in 150 mM NaCl to remove any residual blood. The liver was then minced and homogenized (10% (w/v)) in ice-cold buffer containing 230 mM mannitol, 70 mM sucrose, 5 mM MOPS, 2 mM EGTA, 0.1% BSA, 1 mM PMSF, 10 mM NaF, 1 mM sodium pyrophosphate and 4 mM β-glycerophosphate, pH 7.4. Cell debris were removed by centrifuging twice at 600g for 5 minutes. The supernatant was collected and centrifuged at 10,300g for 10 minutes. The crude mitochondria were further purified by centrifugation (111,406g, 30 minutes) in 30% (w/v) Percoll in the above buffer. Intact mitochondria were broken after a 1:1 dilution in cold hypotonic buffer (20 mM sucrose, 5 mM MOPS, 1 mM MgCl2, 5 mM KH2PO4, 0.5 mM EGTA, pH 7.4) followed by vortexing for 5 minutes.
Measurement of Inhibition
MAO A activity in rat liver mitochondrial preparations using serotonin as substrate was assayed polarographically at 25°C in 50 mM potassium phosphate buffer (pH 7.5) by following the rate of O2 uptake. All other MAO activity assays were performed by monitoring the rate of product formation with time using a Perkin-Elmer Lambda 2 spectrophotometer at 25°C. Assays of purified rat MAO A and MAO B activity were conducted in 50 mM potassium phosphate buffer (pH 7.5) containing 0.5% (w/v) reduced Triton X-100 using kynuramine or benzylamine as substrates, respectively (21, 22). 3-(2-Aminomethyl)pyridine (3-AmMePy) was used as a MAO B-selective substrate (23) in the Amplex Red-peroxidase coupled assay in rat liver mitochondrial preparations. One Unit of catalytic activity is defined as the amount of enzyme catalyzing the formation of 1 μmole of product per minute.
Inhibition constants (Ki values) of purified rat MAO A and MAO B with the ParSL analogues were determined by measuring alterations in Km values for substrates in the presence of four different inhibitor concentrations. The rates of inactivation of MAO in purified enzymes, in intact/broken mitochondria or in MOM preparations were determined by measuring the loss of activity over time upon incubation with 1 mM ParSL analogues at room temperature.
Inhibition of MAO activity by protease treatment of recombinant MOM-bound rat MAO A and B and of rat liver mitochondria was carried out with either trypsin or β-chymotrypsin using a concentration ratio of 1:5 (w/w) protease to protein at room temperature. Samples were taken at various time periods, and protease activity was terminated by the addition of 1 mM PMSF (final concentration) and MAO activities were determined as described above.
RESULTS
Inhibition of Recombinant Rat MAO by Pargyline and Its TEMPO Analogues
To determine whether rat MAO A and MAO B exhibit similar specificities with the TEMPO-substituted pargyline analogues as observed with the human enzymes, the inhibition constants of ParSL-1, ParSL-2 and ParSL-3 for purified recombinant rat enzyme preparations were determined. All of the TEMPO-conjugated pargyline analogues function as competitive inhibitors of rat MAO A and B. As shown in Table 1, these pargyline analogues bind to rat MAO A with Ki values ranging from 65–165 μM and to rat MAO B with Ki values ranging from 32–250 μM. ParSL-1 and ParSL-2 inhibit human MAO B with Ki values of 22 and 15 μM, respectively with only ParSL-1 demonstrating binding to human MAO A (Ki=212 μM) (14). ParSL-3 weakly binds to human MAO A (Ki=268 μM) and exhibits no observable inhibition with human MAO B (14). The data demonstrate differences in behavior of the rat enzymes as compared with the human enzymes on interaction with these pargyline analogues. This differential behavior is also observed on comparisons of rates of inactivation of rat and human MAO A and MAO B.
Table 1.
Competitive Inhibition Constants for Inhibition of Purified Recombinant Rat MAO A and MAO B Activities by ParSL Analogues.
| Ki (μM) | ||
|---|---|---|
| Inhibitor | MAO A | MAO B |
| ParSL-1 | 65.6±4.7 | 32.2±2.3 |
| ParSL-2 | 165.9±13.2 | 83.8±4.0 |
| ParSL-3 | 125.0±7.4 | 251.3±14.2 |
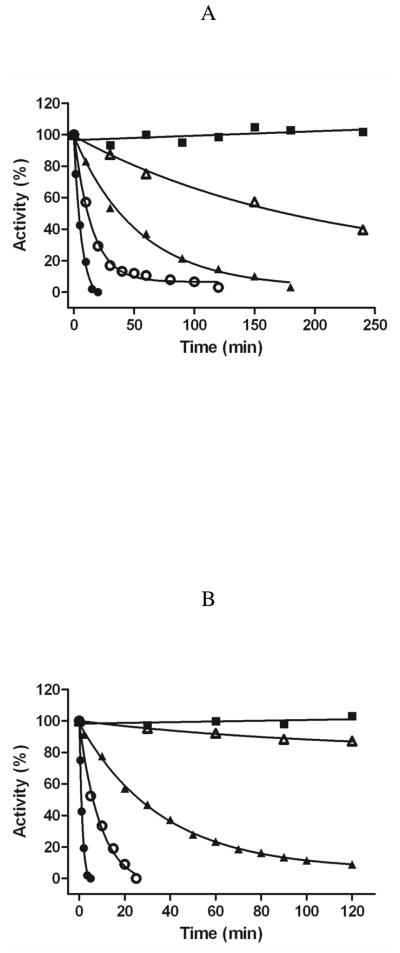
The results in Figure 1 and Table 2 show that purified and membrane bound rat MAO A are inactivated by ParSL-1 with 3.7-fold and 3-fold slower than the corresponding rates with rat MAO B. Comparable rates of inhibition are observed on inactivation of either enzyme with ParSL-2 and ParSL-3 although MAO B is only 50% inactivated by ParSL-3 while MAO A exhibits 13% residual activity on inactivation by ParSL-2. These kinetic results contrast to the behaviors exhibited by purified human enzymes (14) where no inhibition of MAO A is observed with ParSL-2 and MAO B is resistant to inactivation by ParSL-3. Thus, the rat enzymes do not exhibit the high level of specificity for these analogues as observed with the human enzymes. The apparent plateau of rat MAO B inactivation with ParSL-3 to a 50% level of activity also differs from the behavior observed with other analogues and with the human enzyme. Inactivation rates observed with recombinant MOM rat MAO A exhibit similar rates of inactivation as observed with the purified enzyme with the exception that inactivation by ParSL-2 plateaus at ~50% while other analogues totally inactivate the membrane bound form of the enzyme.
Figure 1.
Rates of inhibition of purified rat MAO A (A) and MOM-bound rat MAO A (B), purified rat MAO B (C), and MOM-bound rat MAO B (D) with 1 mM pargyline (●), ParSL-1 (▲), ParSL-2 (△) and ParSL-3 (○). The solid lines drawn are exponential fits to the data for a pseudo first order rate of decay. The control activities without inhibitor are shown by squares (■). A compilation of rate constant values for inhibition are shown in Table 2.
Table 2.
Apparent pseudo-first order rate constants for inhibition of recombinant rat MAO A and MAO B activities by pargyline and by TEMPO-pargyline analogues for both MOM-bound and detergent-purified forms.
| k (inactivation)(s−1) | ||||
|---|---|---|---|---|
| Inhibitor | MAO A (Purified) | MAO A (MOM) | MAO B (Purified) | MAO B (MOM) |
| Pargyline | 9.4 ±1.1 | 44.8±6.2 | too fast to measure | too fast to measure |
| ParSL-1 | 1.1±0.1 | 1.62±0.06 | 4.0±0.6 | 4.8±0.8 |
| ParSL-2 | 0.3±0.02 | 0.15±0.04 | 3.8±0.2 | 4.1±0.3 |
| ParSL-3 | 4.0±0.2 | 6.3±0.9 | 0.6±0.06 | 0.54±0.24 |
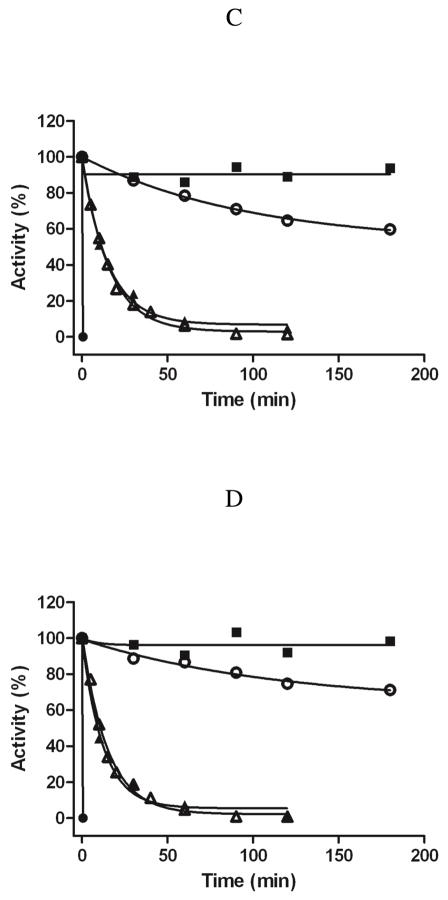
Incubations of intact yeast mitochondria containing the individually expressed recombinant enzymes demonstrate a differing pattern of inhibition than observed with the purified enzymes. Only about 20% inhibition of MAO A activity is observed when intact Pichia mitochondria are incubated with 1 mM ParSL-3 whereas essentially complete inhibition is observed with broken mitochondria or MOM (Figure 2A). In contrast, rat MAO B in intact yeast mitochondria is completely inhibited by 1 mM ParSL-2 as observed with broken mitochondria and MOM (Figure 2B).
Figure 2.
Comparison of the time dependence of rat MAO A inhibition by ParSL-3 (A) and rat MAO B inhibition by ParSL-2 (B) in intact mitochondria (■), in broken mitochondria (●) and in MOM (▲) of P. pastoris, respectively. ParSL-3 and ParSL-2 untreated intact mitochondria and broken mitochondria are shown with (□) and (○), respectively. The specific activities of rat MAO A and B MOM preparations are 12.4 mU/mg and 31.4 mU/mg, respectively.
Thus, recombinant rat MAO A and MAO B in Pichia exhibit similar reactivity patterns to the ParSL analogues as observed with the human enzymes in this yeast organelle (14). The data suggest that rat MAO A is unreactive to the TEMPO inhibitor due to its membrane orientation towards the intermembrane face of the MOM while rat MAO B (as is the case for the human enzyme) is oriented to the cytosolic face with unimpeded reactivity with this inhibitor. It should be noted that pargyline functions as an inhibitor with either enzyme in intact mitochondria which suggests the inability of ParSL-3 to inhibit MAO A is that it is unable to be transported across the mitochondrial outer membrane into the intermembrane space between outer and inner membranes.
MAO Inhibition in Intact Rat Liver Mitochondria
With this background of MAO reactivities in both purified and membrane bound forms of the recombinant system, we isolated intact mitochondria from rat liver to ask whether MAO A and MAO B exhibit behaviors consistent with orientations towards the cytosolic face of the MOM or whether they might face towards the intermembrane space. Since rat liver mitochondria contain both MAO A and MAO B in approximately equal levels (5), isozyme-specific substrates were used to selectively assay MAO A or MAO B. Serotonin oxidation was used for MAO A since its rate of oxidation by MAO B is much lower (5). Previous work in our laboratory has shown that 3-(2-aminomethyl)pyridine (3-AmMePy) is a good substrate for MAO B but is poorly oxidized by MAO A (23). The data in Figure 3A and 3C show that rat liver MAO A is readily inactivated on incubation with 1 mM ParSL-3 in both intact and broken mitochondria while only 20% of serotonin oxidase activity is inactivated by ParSL-2. This behavior with ParSL-2 is similar to that observed with recombinant yeast MOM MAO A (Figure 1B). In contrast, when intact mitochondria are assayed for MAO B (Figure 3B), neither ParSL-2 nor ParSL-3 incubation results in any observable inactivation. Disruption of the mitochondria results in the appearance of sensitivity of rat MAO B to ParSL-2 inhibition (Figure 3D) which was more effective than ParSL-3 (also observed with purified recombinant enzymes, Figure 1A and 1C).
Figure 3.
Comparison of the time dependence of rat MAO A and MAO B inhibition by ParSL-2 and ParSL-3 in intact and in broken rat liver mitochondria, respectively. (A) intact mitochondria, substrate: serotonin; (B) intact mitochondria, substrate: 3-AmMePy; (C) broken mitochondria, substrate: serotonin; (D) broken mitochondria, substrate: 3-AmMePy. ParSL-2 (or ParSL-3) untreated intact mitochondria and broken mitochondria are shown with empty squares (□). 1mM ParSL-2 and 1 mM ParSL-3 treated mitochondria (intact or broken) are shown with filled circles (●) and filled triangles (▲), respectively. The specific activity of rat liver mitochondria is 0.44 mU/mg (substrate: serotonin) and 1.56 mU/mg (substrate: 3-AmMePy), respectively.
Protease Inhibition as an Additional Probe of Membrane Orientation of Rat MAO A and MAO B
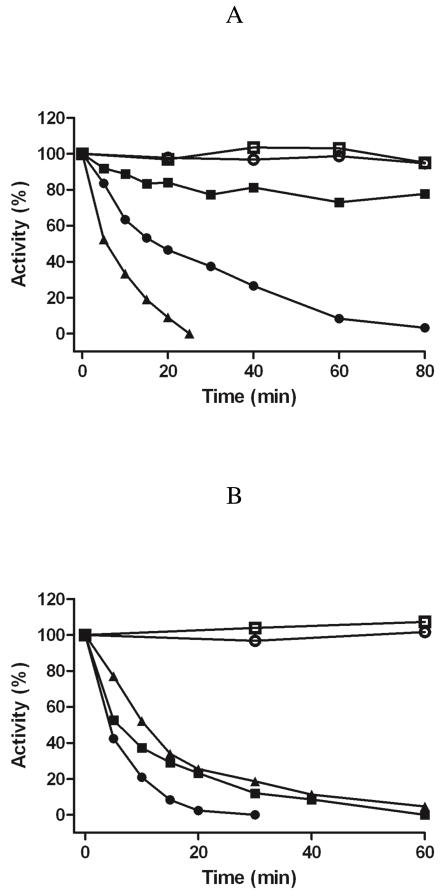
An additional probe of membrane orientation was used to compare with the inhibition data with the TEMPO-pargyline analogues presented above. Buckman et al. (11) demonstrated that human and rat MAO A activity was sensitive to treatment with trypsin. This observation is confirmed in this study using yeast MOM particles containing rat MAO A. When trypsin inactivation studies were performed with yeast MOM containing rat MAO B, no observable inactivation occurs within the time frame observed for MAO A. The data in Figure 4 show that yeast recombinant MOM-bound rat MAO A is sensitive to trypsin whereas β-chymotrypsin specifically inactivates rat MAO B. Therefore, although the two enzymes exhibit ~70% sequence identities and the human enzymes exhibit a high degree of structural identity, there is a marked difference in sensitivity to inactivation by proteolysis on trypsin and β-chymotrypsin treatment.
Figure 4.
Comparison of activity losses on proteolytic treatment of yeast recombinant MOM-bound rat MAO A and MAO B: trypsin-treated rat MAO A (●); β-chymotrypsin-treated rat MAO A (○); trypsin-treated rat MAO B (■); β-chymotrypsin-treated rat MAO B (□).
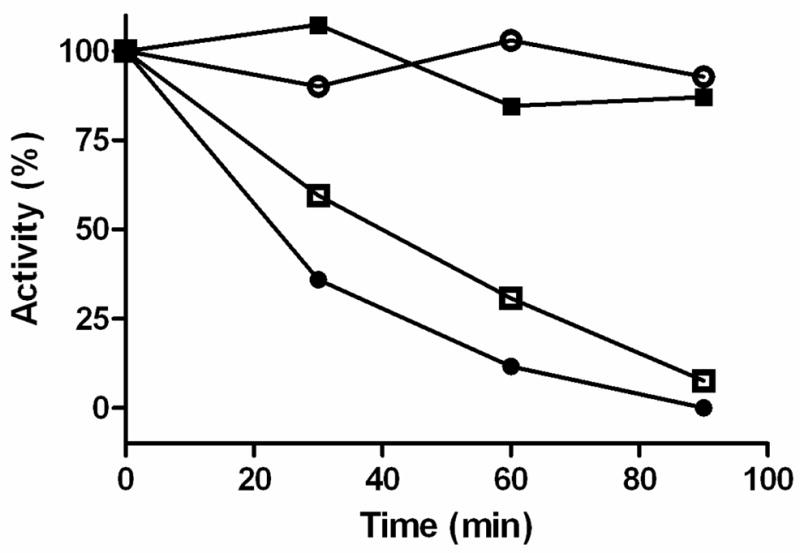
These differing reactivities to protease treatment were used to provide additional insights into the MOM topology of rat MAO A and MAO B in intact rat liver mitochondria. The data in Figure 5 shows that β-chymotrypsin treatment of rat liver mitochondria (estimated to be 70% intact by NADH oxidase activity) only results in 30% activity loss of MAO B activity In contrast, >90% of MAO A activity is lost on incubation of rat liver mitochondria with trypsin. These results suggest that, in rat liver mitochondria, MAO B is situated on the surface of the MOM facing the intermembrane space, while MAO A is situated facing the cytosolic face of the MOM. Thus, the protease inactivation results are in agreement with the conclusions reached from inhibition studies with the TEMPO-pargyline analogues described above.
Figure 5.
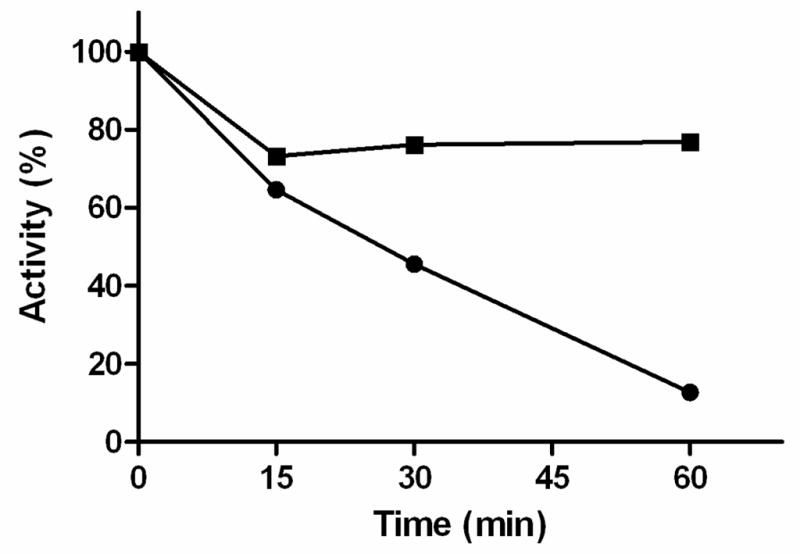
Comparison of activity losses on protease treatment of rat liver mitochondria: treated with trypsin using serotonin as substrate (●); treated with β-chymotrypsin using 3-AmMePy as substrate (■).
DISCUSSION
Comparison of TEMPO-Pargyline Reactivities of Rat and Human MAO A and MAO B
The absolute specificity of ParSL-2 for MAO B and ParSL-3 for MAO A is less strict with the rat enzymes than observed with the human recombinant enzymes human enzymes. Differences in inhibitor specificities and reactivities between rat and the human enzymes have been observed previously (15, 16) and this study provides an additional example. The differential reactivities observed previously with intact mitochondria isolated from yeast containing recombinant human enzymes and from human placenta (14) are also observed in this study using recombinant rat enzymes in the yeast organelles and in rat liver. Thus, the membrane bound human and rat enzymes exhibit similar sensitivities reflecting their topological orientation.
The Topology of Rat MAOs in Mitochondria from Different Sources
The mitochondrial outer membrane impermeability due to the polar nature of amide-linked-TEMPO group on these pargyline analogues has been suggested previously (14) and is also evident in this study with new insights into liver MAO B topology. The demonstrated reactivity of both MAO A and MAO B in intact mitochondria to pargyline but differential reactivity to TEMPO-pargyline analogues provides additional support for the inability of these analogues to traverse the mitochondrial outer membrane. The rat liver data support previous studies that MAO A is situated facing the cytosolic face of the MOM as found with intact human placental mitochondria. These results agree with the protease studies of Buckman et al. (11) which showed that serotonin oxidizing activity of rat liver mitochondria was sensitive to trypsin while phenylethylamine oxidizing activity was relatively less sensitive. Our trpysin inactivation studies confirm the results of Buckman et al (11) in that MAO A in intact rat liver mitochondria is situated on the cytosolic face. The recombinant rat enzyme in yeast mitochondria provides evidence for an opposite orientation in yeast (ie. MAO A is facing the intermembrane face).
An unexpected outcome of these studies is the demonstration that rat MAO B is situated facing the intermembrane face of rat liver mitochondria but cytosolic face of yeast mitochondria. Support for this conclusion comes from the differential sensitivities of rat MAO B to inactivation in intact mitochondria isolated from rat liver and from the yeast expression system. These conclusions are presented in a pictorial depiction as a cartoon in Figure 6.
Figure 6.
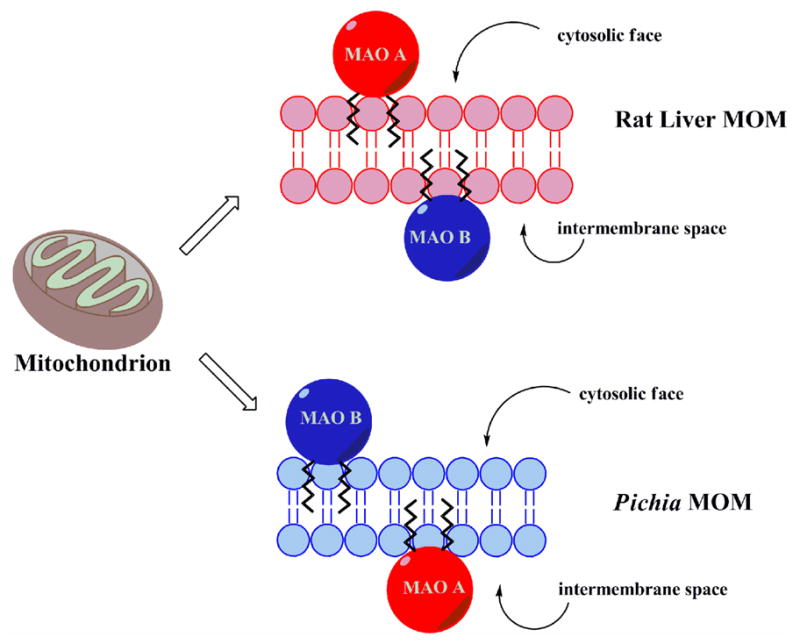
Depiction of topological orientations of rat MAO A and rat MAO B in rat liver MOM and in Pichia pastoris MOM.
The apparent opposite orientations found in Pichia mitochondrial preparations suggest that the lipid composition of yeast favors a differential orientation and therefore brings up a number of unanswered questions on how MAO A and MAO B are inserted in the MOM. Both enzymes have trans-membrane C-terminal helices that are involved in membrane binding. Recent reviews (13,24) suggest this post-translational insertion can occur via a “spontaneous” insertion which may or may not require chaperone assistance. If this type of insertion is operative, then MAO B may elicit an auxiliary mechanism in rat liver to promote its translocation to the intermembrane face of the MOM.
The finding of opposite orientations of MAO in rat liver also brings up the question of whether such a topological arrangement is tissue specific or is it general for mitochondria isolated from differing tissues. This remains an open question requiring further experimental investigation. This situation is also relevant to MAO B inhibitor design as neuroprotectants. Functional MAO B inhibitors may have to be readily transported into the intermembrane space in mitochondria assuming a generality of topology. If there is a tissue-specificity to topology, then an additional aspect of tissue specificity will have to be factored into MAO B-specific drug design.
Supplementary Material
Acknowledgments
The authors thank Ms. Milagros Aldeco for her technical assistance.
Footnotes
This work was supported by National Institute of Health Grant GM-29433 (DEE).
Abbreviations: MAO: Monoamine Oxidase; MOM: mitochondrial outer membrane; TEMPO: 2,2,6,6-tetramethylpiperidinyl-1-oxyl; ParSL: spin-labeled pargyline; 3- AmMePy: 3-(2-aminomethyl)pyridine.
Supporting Information Available: The structures of the TEMPO-substituted pargyline analogues used in this study are shown. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fowler JS, Logan J, Volkow ND, Wang GJ. Translational neuroimaging: positron emission tomography studies of monoamine oxidase. Mol Imaging Biol. 2005;7:377–387. doi: 10.1007/s11307-005-0016-1. [DOI] [PubMed] [Google Scholar]
- 2.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1460–1467. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- 3.Kumar MJ, Nicholls DG, Andersen JK. Oxidative alpha-ketoglutarate dehydrogenase inhibition via subtle elevations in monoamine oxidase B levels results in loss of spare respiratory capacity: implications for Parkinson’s disease. J Biol Chem. 2003;278:46432–46439. doi: 10.1074/jbc.M306378200. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circul. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 5.Weyler W, Hsu YP, Breakefield XO. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther. 1990;47:391–417. doi: 10.1016/0163-7258(90)90064-9. [DOI] [PubMed] [Google Scholar]
- 6.Upadhyay AK, Borbat PP, Wang J, Freed JH, Edmondson DE. Determination of the oligomeric states of human and rat monoamine oxidases in the outer mitochondrial membrane and octyl beta-D-glucopyranoside micelles using pulsed dipolar electron spin resonance spectroscopy. Biochemistry. 2008;47:1554–1566. doi: 10.1021/bi7021377. [DOI] [PubMed] [Google Scholar]
- 7.Binda C, Hubalek F, Li M, Edmondson DE, Mattevi A. Crystal structure of human monoamine oxidase B, a drug target enzyme monotopically inserted into the mitochondrial outer membrane. FEBS Lett. 2004;564:225–228. doi: 10.1016/S0014-5793(04)00209-1. [DOI] [PubMed] [Google Scholar]
- 8.Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-angstrom resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci USA. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Colibus L, Li M, Binda C, Lustig A, Edmondson DE, Mattevi A. Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B. Proc Natl Acad Sci USA. 2005;102:12684–12689. doi: 10.1073/pnas.0505975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell SM, Davey J, Mayer RJ. The vectorial orientation of human monoamine oxidase in the mitochondrial outer membrane. Biochem J. 1979;181:7–14. doi: 10.1042/bj1810007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckman TD, Sutphin MS, Eiduson S. Proteases as probes of mitochondrial monoamine oxidase topography in situ. Mol Pharmacol. 1984;25:165–170. [PubMed] [Google Scholar]
- 12.Pfaff E, Klingenb M, Ritt E, Vogell W. Correlation of Unspecific Permeable Mitochondrial Spaces with Intermembrane Spaces. Eur J Biochem. 1968;5:222–232. doi: 10.1111/j.1432-1033.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol. 2010;11:655–667. doi: 10.1038/nrm2959. [DOI] [PubMed] [Google Scholar]
- 14.Upadhyay AK, Edmondson DE. Development of spin-labeled pargyline analogues as specific inhibitors of human monoamine oxidases A and B. Biochemistry. 2009;48:3928–3935. doi: 10.1021/bi9002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novaroli L, Daina A, Favre E, Bravo J, Carotti A, Leonetti F, Catto M, Carrupt PA, Reist M. Impact of species-dependent differences on screening, design, and development of MAO B inhibitors. J Med Chem. 2006;49:6264–6272. doi: 10.1021/jm060441e. [DOI] [PubMed] [Google Scholar]
- 16.Fierro A, Osorio-Olivares M, Cassels BK, Edmondson DE, Sepulveda-Boza S, Reyes-Parada M. Human and rat monoamine oxidase-A are differentially inhibited by (S)-4-alkylthioamphetamine derivatives: Insights from molecular modeling studies. Bioorgan Med Chem. 2007;15:5198–5206. doi: 10.1016/j.bmc.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Edmondson DE. High-level expression and purification of rat monoamine oxidase A (MAO A) in Pichia pastoris: Comparison with human MAO A. Protein Expr Purif. 2010;70:211–217. doi: 10.1016/j.pep.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upadhyay AK, Edmondson DE. Characterization of detergent purified recombinant rat liver monoamine oxidase B expressed in Pichia pastoris. Protein Expr Purif. 2008;59:349–356. doi: 10.1016/j.pep.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daum G, Bohni PC, Schatz G. Import of Proteins into Mitochondria - Cytochrome-B2 and Cytochrome-C Peroxidase Are Located in the Intermembrane Space of Yeast Mitochondria. J Biol Chem. 1982;257:3028–3033. [PubMed] [Google Scholar]
- 20.Hoppel CL, Kerner J, Turkaly P, Turkaly J, Tandler B. The malonyl-CoA-sensitive form of carnitine palmitoyltransferase is not localized exclusively in the outer membrane of rat liver mitochondria. J Biol Chem. 1998;273:23495–23503. doi: 10.1074/jbc.273.36.23495. [DOI] [PubMed] [Google Scholar]
- 21.Walker MC, Edmondson DE. Structure-activity relationships in the oxidation of benzylamine analogues by bovine liver mitochondrial monoamine oxidase B. Biochemistry. 1994;33:7088–7098. doi: 10.1021/bi00189a011. [DOI] [PubMed] [Google Scholar]
- 22.Miller JR, Edmondson DE. Structure-activity relationships in the oxidation of para-substituted benzylamine analogues by recombinant human liver monoamine oxidase A. Biochemistry. 1999;38:13670–13683. doi: 10.1021/bi990920y. [DOI] [PubMed] [Google Scholar]
- 23.Li M. PhD Dissertation. Emory University; 2005. [Google Scholar]
- 24.Borgese N, Brambillasca S, Colombo S. How tails guide tail-anchored proteins to their destinations. Curr Opin Cell Biol. 2007;19:368–375. doi: 10.1016/j.ceb.2007.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.