Abstract
This paper presents the Adaptive Calibration Model (ACM), an evolutionary-developmental theory of individual differences in the functioning of the stress response system. The stress response system has three main biological functions: (1) to coordinate the organism’s allostatic response to physical and psychosocial challenges; (2) to encode and filter information about the organism’s social and physical environment, mediating the organism’s openness to environmental inputs; and (3) to regulate the organism’s physiology and behavior in a broad range of fitness-relevant areas including defensive behaviors, competitive risk-taking, learning, attachment, affiliation and reproductive functioning. The information encoded by the system during development feeds back on the long-term calibration of the system itself, resulting in adaptive patterns of responsivity and individual differences in behavior. Drawing on evolutionary life history theory, we build a model of the development of stress responsivity across life stages, describe four prototypical responsivity patterns, and discuss the emergence and meaning of sex differences. The ACM extends the theory of biological sensitivity to context (BSC) and provides an integrative framework for future research in the field.
Keywords: Adaptation, allostasis, biological sensitivity to context, cortisol, developmental switch point, evolution, gender, life history strategies, plasticity, reactivity, sex differences, stress
1. Introduction
The stress response system (SRS) is an ancient biological mechanism, fine-tuned by natural selection and crucially involved in a wide range of adaptive functions in humans as well as other animals. The basic structure of the SRS is highly conserved across species (Nesse et al., 2007); however, the SRS exhibits a striking amount of individual variation in its working parameters (e.g., baseline activation, hormone levels) and in its responsivity to external events (e.g., the magnitude of cortisol response, or the balance between sympathetic and parasympathetic activation). In turn, individual differences in stress responsivity consistently relate to differences in psychological functioning, social relations, and in the risk for mental and physical disorders. The SRS is highly plastic, especially in response to early experience (e.g., Boyce and Ellis, 2005; Gunnar et al., 2009a; Levine, 2005; Parker et al., 2006); at the same time, widespread allelic variation exists in many genes that can affect SRS functioning (e.g., Alexander et al., 2009, 2011; Ouellet-Morin et al., 2008; Propper et al., 2008; Wüst et al., 2004). Understanding individual differences in stress responsivity – their causes, effects, and developmental processes leading to different patterns of responsivity – has become a major research focus in neuroscience, psychology, and medicine (e.g., Cameron et al., 2005; Ellis et al., 2006; Gunnar et al., 2009a; Korte et al., 2005). To date, however, a unifying theoretical framework for the study of individual differences in SRS functioning is still lacking.
In this paper we advance an evolutionary model of the development of stress responsivity in humans: the Adaptive Calibration Model (ACM). Our aim is to develop a biologically rigorous framework to understand the meaning of individual differences in responsivity and describe the developmental trajectories leading to such differences. The ACM is an extension of the theory of biological sensitivity to context (BSC; Boyce and Ellis, 2005; Ellis et al., 2005; Ellis and Boyce, 2008); to our knowledge, the ACM is the first theory of stress development to take full advantage of the tools of modern evolutionary and developmental biology. In particular, our model draws extensively on life history theory (Ellis et al., 2009; Roff, 2002), sexual selection and parental investment theory (Trivers, 1972; Kokko and Jennions, 2008), and the theory of developmental plasticity (West-Eberhard, 2003).
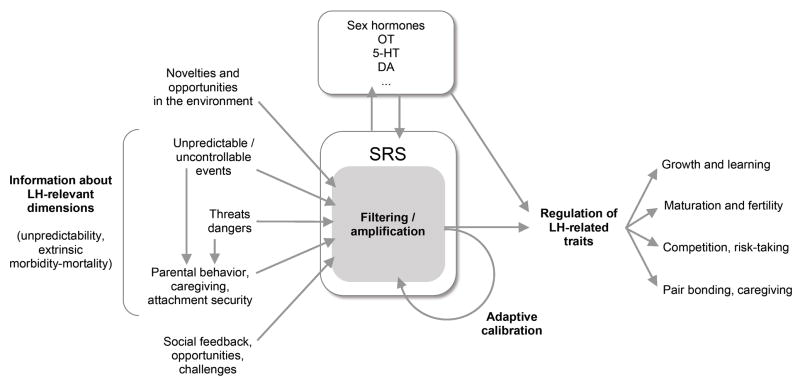
The ACM postulates that individual differences in stress responsivity are largely (though not exclusively) the result of conditional adaptation – the evolved ability of an organism to modify its developmental trajectory (and the resulting phenotype) to match the local conditions of the social and physical environment. Individual variation in responsivity is primarily seen as the result of adaptive mechanisms, rather than the outcome of pathological or dysfunctional processes. In the framework developed here, the SRS has three main biological functions: (1) to coordinate the organism’s allostatic response to physical and psychosocial challenges; (2) to encode and filter information about the organism’s social and physical environment, mediating the organism’s openness to environmental inputs; and (3) to regulate the organism’s physiology and behavior in a broad range of fitness-relevant areas including growth, competitive risk-taking, learning, attachment, affiliation, and reproductive functioning. All these traits and behaviors can be seen as components of the organism’s life history (LH) strategy, a biological construct describing the developmental schedule of an organism and its allocation of time and energy to different fitness-promoting activities, such as mating and parenting (Figure 1).
Figure 1.
Conceptual structure of the Adaptive Calibration Model. SRS: stress response system; LH: life history; OT: oxytocin; 5-HT: serotonin, DA: dopamine.
The central concept of the ACM is that information encoded by the SRS in the course of development feeds back on the long-term calibration of the system itself, resulting in adaptive patterns of responsivity and individual differences in life history-related behavior (the curved arrow in Figure 1). In other words, the SRS acts as an integrative mechanism, mediating the development of alternative LH strategies that are adaptive in different environmental conditions (or at least have been adaptive during the course of human evolution). In this paper we describe the evolutionary logic of adaptive calibration and employ that logic to model a number of developmental trajectories – from birth to adulthood – that lead to stable patterns of individual differences in responsivity to environmental threats and opportunities.
1.1. Conditional adaptation: A new paradigm for individual differences in stress responsivity
Developmental psychologists frequently consider the effects of life experience on development but rarely consider how these effects have been structured by natural selection. Despite this oversight, the burgeoning field of evolutionary-developmental biology has exciting and profound implications for the study of human development (see especially West-Eberhard, 2003). Over the last two decades, theory and research in the field has come to acknowledge that, in most species, single “best” strategies for survival and reproduction are unlikely to evolve. This is because the “best” strategy varies as a function of the physical, economic, and socioemotional parameters of one’s environment (Crawford and Anderson, 1989), and thus a strategy that promotes success in some environmental contexts may lead to failure in others (Belsky et al., 1991; Ellis and Boyce, 2008; Meaney, 2010). This adaptationist perspective challenges the prevailing notion (e.g., Beauchaine et al., 2007; Cicchetti and Rogosch, 2001; El-Sheikh et al., 2009; Evans and Kim, 2007) that childhood exposures to stress and adversity routinely derail normal development (i.e., induce dysregulated biological and behavioral functioning). Rather, both stressful and supportive environments have been part of the human experience throughout our evolutionary history, and thus our developmental systems have been shaped by natural selection to respond adaptively to a range of different contexts. When people encounter stressful environments, this does not so much disturb their development as direct or regulate it toward strategies that are adaptive under stressful conditions; conversely, when people encounter well-resourced and supportive environments, it directs or regulates development toward strategies that are adaptive in that context (Ellis et al., 2011a, 2011b; Flinn, 2006).
Consider the extensive experimental work conducted by Michael Meaney and colleagues, showing that relatively low quality maternal care in the rat (i.e., low levels of maternal licking and grooming) alters pups’ stress physiology and brain morphology. Although such changes may seem disadvantageous (i.e., higher corticosterone levels, shorter dendritic branch lengths, and lower spine density in hippocampal neurons), they actually enhance learning and memory processes under stressful conditions (Bagot et al., 2009; Champagne et al., 2008). Moreover, such physiological and morphological changes mediate the effects of maternal behavior on central features of defensive and reproductive strategies: behavior under threat, open-field exploration, pubertal development, sexual behavior, and parenting (Cameron et al., 2005, 2008a, 2008b). In the rodent model presented by Meaney and colleagues, then, variations in stress physiology and brain morphology apparently represent strategic – that is, functional – ways of developing under different rearing conditions (Meaney, 2010).
A central premise of the ACM is that human children likewise have evolved to function competently – to survive and ultimately reproduce – in a variety of contexts; thus, our default assumption is that alternative patterns of stress responsivity and related variation in life history-related behavior, both in response to stressful and supportive environmental conditions (within the range encountered over human evolution), constitute adaptive developmental variation. Along these lines, an evolutionary-developmental perspective emphasizes conditional adaptation: “evolved mechanisms that detect and respond to specific features of childhood environments, features that have proven reliable over evolutionary time in predicting the nature of the social and physical world into which children will mature, and entrain developmental pathways that reliably matched those features during a species’ natural selective history” (Boyce and Ellis, 2005, p. 290; for a comprehensive treatment of conditional adaptation, see West-Eberhard, 2003). Conditional adaptation, which is closely related to the concept of a predictive adaptive response (e.g., Gluckman et al., 2007), is guided by both external environmental factors (e.g., predation pressures, quality of parental investment, seasonal change, diet) and indicators of the individual’s status or relative competitive abilities in the population (e.g., age, body size, health, history of wins and losses in agonistic encounters).
1.2. Biological sensitivity to context
Boyce and Ellis (2005) have proposed a conditional adaptation model of developmental variation in the human SRS. This model articulated the precepts and rationale for a new claim about the nature of relations between early life experience and stress responsivity. Boyce and Ellis (2005) contend that heightened responsivity may reflect, not simply exaggerated arousal under challenge, but rather a form of enhanced, neurobiologically-mediated sensitivity to context, or biological sensitivity to context (BSC).
The concept of BSC has its early roots in a 1995 Psychosomatic Medicine report by Boyce and colleagues (1995b), presenting two studies of naturally occurring environmental adversities and stress reactivity as predictors of respiratory illnesses in 3–5 year old children. Results revealed, first, that children showing low cardiovascular or immune reactivity to stressors had approximately equal rates of respiratory illnesses in both low and high adversity settings. Second, and consistent with the prevailing diathesis-stress model, highly biologically responsive children exposed to high adversity child care settings or home environments had substantially higher illness incidences than all other groups of children. The third, and unexpected, finding was that highly responsive children living in lower adversity conditions – i.e., more supportive child care or family settings – had the lowest illness rates, significantly lower than even low responsive children in comparable settings.
These data suggested that children differed in their susceptibility to environmental influence in a “for better and for worse” manner (Belsky et al., 2007), with more biologically responsive children experiencing unusually poor outcomes in high-stress, unsupportive social conditions but flourishing under low-stress, nurturing, and predictable conditions. Further, the initial Boyce et al. (1995b) research, together with subsequent work (Boyce and Ellis, 2005), identified candidate physiological mechanisms of environmental susceptibility – autonomic, adrenocortical, or immune reactivity to psychosocial stressors – and proposed that psychobiologic reactivity moderated the effects of early environmental exposures on physical and mental health outcomes in a bivalent manner. More responsive children displayed heightened sensitivity to both positive and negative environmental influences and thus were given the shorthand designation of orchid children, signifying their special susceptibility to both highly stressful and highly nurturing environments. Children low in responsivity, on the other hand, were designated as dandelion children, reflecting their relatively high capacity for survival in species-typical circumstances of all varieties (Boyce and Ellis, 2005).
Although the findings of Boyce et al. (1995b) stimulated a provisional interpretation of how environmental exposures and psychobiologic responsivity worked together in regulating children’s mental and physical health, conspicuously missing was a broader, more heuristic theoretical framework in which these findings could be interpreted and explained. Boyce and Ellis’s (2005; see also Ellis et al., 2005, 2006; Ellis and Boyce, 2008) BSC theory was an effort to provide such an evolutionary functional analysis, advancing as it did two key propositions. The first involved a new hypothesis about the function of the SRS and the second a novel evolutionary hypothesis about its developmental calibration. Each is considered here, in turn.
With respect to the function of the SRS, it was clear that biological reactivity to stressors comprises a complex, integrated system of central neural and peripheral neuroendocrine responses designed to prepare the organism for challenge or threat. On the other hand, according to BSC theory, the components of the “stress response” system also function to increase susceptibility to resources and support in the environment. This dual function signifies the need to conceptualize stress responsivity more broadly as BSC, which Boyce and Ellis (2005) defined as neurobiological susceptibility to both cost-inflicting and benefit-conferring features of the environment and operationalized as an endophenotypic property indexed by heightened responsivity in one or more components of the SRS.
Highly responsive children experience either the best or the worst of psychiatric and biomedical outcomes within the populations from which they are drawn. Under conditions of adversity, such children sustain higher rates of disease, behavioral problems, and injuries than their more normatively reactive peers. By contrast, such highly responsive children in low-stress, protective social environments experience substantially lower rates of physical and mental health problems than their less reactive counterparts (Boyce, 1996; Boyce et al., 1995b; Bubier et al., 2009; Ellis et al., 2011b; Essex et al., 2011; Obradović et al., 2010, 2011; Quas et al., 2004). BSC theory therefore posits that individual differences in the magnitude of biological stress responses function to regulate openness or susceptibility to environmental influences, ranging from harmful to protective. Complementing this perspective, Jay Belsky’s theory of differential susceptibility also postulates that children, due to variation in temperament and genetics, differ in their susceptibility to both the adverse effects of risk-promoting early environments and the beneficial effects of development-enhancing rearing conditions (Belsky, 1997, 2005; Belsky and Pluess, 2009).
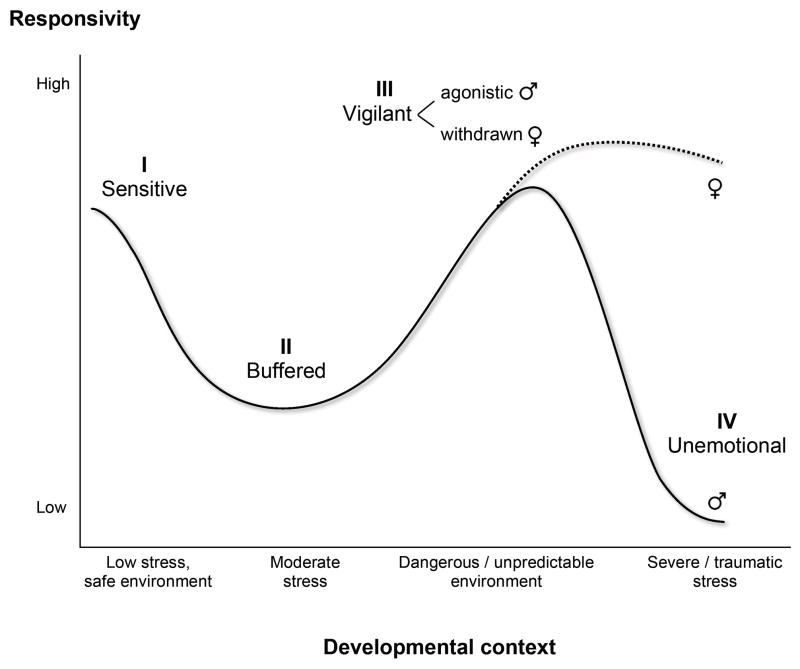
The second evolutionary proposition of BSC, concerning developmental calibration of the SRS, draws on the concept of conditional adaptation. BSC theory proposed that humans have evolved developmental mechanisms for detecting and internally encoding information about levels of support versus adversity in early childhood environments; this information is then used to calibrate activation thresholds and response magnitudes within stress response systems to match those environments. Given past evidence that early trauma can increase stress responsivity and newer evidence that responsivity can enhance developmental functioning in highly supportive settings, Boyce and Ellis (2005) postulated a curvilinear, U-shaped relation between early support-adversity and the magnitude of biological response dispositions. Specifically, Boyce and Ellis hypothesized that: a) exposure to acutely stressful childhood environments up-regulates BSC, increasing the capacity and tendency of individuals to detect and respond to environmental dangers and threats; b) exposure to especially supportive childhood environments also up-regulates BSC, increasing susceptibility to social resources and ambient support; and (c) by contrast, exposure to childhood environments that are not extreme in either direction down-regulates BSC. Because development of heightened BSC has associated fitness costs (e.g., increased rates of mental and physical disorders; reviewed in Boyce and Ellis, 2005), enhanced neurobiological susceptibility to the environment is unlikely to be adaptive in the large majority of children who grow up in normative environments. Instead, low to normative levels of BSC should produce the best fitness outcomes in such contexts, buffering individuals against the chronic stressors encountered in a world that is neither highly threatening nor consistently safe. Exploratory analyses in two studies offered confirmatory evidence that the lowest prevalences of highly responsive phenotypes were found in conditions of moderate stress and that both tails of the support-adversity distribution were associated with higher proportions of responsive children (Ellis et al., 2005; see also Gunnar et al., 2009a; Hagan et al., 2010). Converging findings of a curvilinear relationship between early stress and later responsivity have also been reported in recent studies of mice (Macrì et al., 2007, 2009).
1.2.1. Extending the BSC theory
The ACM extends and refines the original BSC theory in a number of ways. First of all, we explicitly connect the concept of sensitivity to context to the broader evolutionary framework of LH theory. Second, we discuss the adaptive meaning and developmental origin of sex differences in responsivity, a crucial aspect that was missing from the initial formulation of the BSC theory. Third, we attempt to model the trajectories leading to individual differences in a more fine-grained way, by discussing the development of stress responsivity at different life stages and identifying a number of “switch points” when plasticity is preferentially expressed. Finally, we refine the BSC construct of stress responsivity by considering distinct roles for the three main components of the SRS (the parasympathetic and sympathetic systems and the hypothalamic-pituitary-adrenal axis). This allows us to model four prototypical responsivity patterns, each reflecting the coordinated activity of the SRS components.
1.3. Overview of the paper
The paper is organized as follows. Section 2 introduces the basics of LH theory and describes the role of LH strategies in the organization of behavior. Section 3 presents the evolutionary view of human development as a sequence of stages and switch points, and discusses the main switch points in the development of LH strategies. Section 4 is a synthetic review of the biological functions fulfilled by the SRS – the coordination of the allostatic response, the encoding/filtering of environmental information, and the regulation of life history-relevant traits. In sections 5 and 6 we present the Adaptive Calibration Model, describe four prototypical patterns of stress responsivity, and explore their developmental trajectories across life stages. We conclude with a theoretical integration and a discussion of current limitations and future directions.
2. Life history theory
Life history theory is a branch of evolutionary biology dealing with the way organisms allocate time and energy to the various activities that comprise their life cycle (see Ellis et al., 2009; Hill, 1993; Kaplan and Gangestad, 2005; McNamara and Houston, 1996; Penke, 2010; Roff, 2002). All organisms live in a world of limited resources; the energy that can be extracted from the environment in a given amount of time, for example, is intrinsically limited. Time itself is also a limited good; the time spent by an organism looking for mates cannot be used to search for food or care for extant offspring. Since all these activities contribute to an organism’s evolutionary fitness, devoting time and energy to one will typically involve both benefits and costs, thus engendering trade-offs between different fitness components. For example, there is a trade-off between bodily growth and reproduction because both require substantial energetic investment, and thus producing offspring reduces somatic growth. Childhood is an expression of this trade-off – the initial phase of an organism’s life cycle is usually non-reproductive and characterized instead by a fast growth rate. As Ellis et al. (2009, p. 208) have stated: “Each trade-off constitutes a decision node in allocation of resources, and each decision node influences the next decision node (opening up some options, foreclosing others) in an unending chain over the life course.” Natural selection strongly favors organisms that are able to schedule development and activities in a manner that optimizes this chain of resource allocation decisions. LH theory concerns optimal allocation of time and energy toward competing life functions – bodily maintenance, growth, and reproduction – over the life cycle.
2.1. Life history strategies
LH theory employs formal modeling to solve the complex optimization problem of how – and when – to allocate limited resources to gain the maximum reproductive success. LH strategies1 are adaptive solutions to a number of simultaneous fitness trade-offs. The most basic trade-offs are between somatic effort (i.e., growth, body maintenance, and learning) and reproductive effort; and, within reproductive effort, between mating (i.e., finding and attracting mates, conceiving offspring) and parenting (i.e., investing resources in already conceived offspring). From another perspective, the critical decisions involved in a LH strategy can be summarized by the trade-offs between current and future reproduction, and between quality and quantity of offspring (see Ellis et al., 2009). Is the organism going to reproduce as soon as it can, or to wait longer, in order to accumulate resources that can then increase offspring “quality” and reproductive success – and thereby the parent’s own inclusive fitness? The more time spent waiting, the more resources (e.g., energy reserves, but also skills and social status) could become available, but the risk of dying before reproducing will increase as well. And is the organism going to put all of its reproductive effort into increasing the number of offspring, or will it channel resources and parenting effort into increasing the quality and long-term prospects of a few, selected descendants?
One of the most important implications of LH theory is that no strategy can be optimal in every situation; more specifically, the optimal (i.e., fitness-maximizing) strategy for a given organism depends on its ecology and on a series of factors such as resource availability, mortality and environmental uncertainty. Indeed, organisms usually embody mechanisms that allow them to fine-tune their life histories according to the environmental cues they encounter during development. Within the same species, different individuals can find themselves in dramatically different environmental conditions, which may call for adjustment in the way strategic trade-offs are resolved. For this reason, LH traits and strategies tend not to be genetically fixed, but rather evolve to show developmental plasticity (Belsky et al., 1991; Ellis et al., 2006; West-Eberhard, 2003). Developing organisms assess their local environments and adjust their strategic allocation choices, following evolved rules that maximize expected fitness in different ecological conditions. To the extent they result from evolved mechanisms of plasticity, individual differences in LH strategies are examples of conditional adaptation (section 1).
2.1.1. Sex differences in life history strategies
In sexual species, the two sexes predictably differ on LH-related dimensions; they thus can be expected to employ somewhat different strategies in response to the same cues in the environment. In most species, males tend to engage in higher mating effort and lower parental effort than females (Geary, 2002; Kokko and Jennions, 2008; Trivers, 1972). In addition, males usually undergo stronger sexual selection (i.e., their reproductive success is more variable) and tend to mature more slowly in order to gain the competitive abilities and qualities needed for successful competition for mates. Sexual asymmetries in LH strategies can be attenuated in species with monogamous mating systems and when both parents contribute to offspring care. Compared with other mammals, humans show an unusual degree of paternal investment; we are clearly adapted for the possibility of monogamous, long-term relationships. However, human paternal care is highly variable and facultative (e.g., Geary, 2005; Quinlan, 2008), and strict monogamy is rarely found (Marlowe 2000, 2003). The reproductive success of men is more variable than that of women, especially in societies characterized by polygyny or serial monogamy (Brown et al., 2009). Human mating is best characterized as strategically flexible (Gangestad and Simpson, 2000), with a widely documented tendency for men to engage in higher mating effort than women (e.g., Schmitt, 2005).
As a result of these biological differences, the various components of LH strategies do not typically carry the same weight for men and women. The current versus future reproduction tradeoff is more pressing for women: women’s reproductive rate is limited by the long duration of gestation and the conspicuous energetic investment of pregnancy and lactation, and their window for successful reproduction necessarily ends with menopause. In contrast, men can potentially sire many offspring in a very short time, as well as for a more extensive period of their lives. Men’s crucial trade-off is the mating versus parenting one: the payoffs of high mating effort are potentially much larger for males, who can benefit directly from having access to a large number of partners; women can have only one child at a time (twin pregnancies aside), and thus benefit relatively less from mating with multiple partners.
2.2. Determinants of life history strategies
2.2.1. Environmental factors
The key dimensions of the environment that affect the development of LH strategies are resource availability, extrinsic morbidity-mortality, and unpredictability, as signaled by observable cues. Because successful conversion of energy harvested from the environment into reproduction is the central task faced by all organisms, obtaining an adequate supply of food is and always has been a fundamental adaptive problem. Consequently, energetic conditions – caloric intake, energy expenditures, and related health conditions – set a baseline for many developmental processes, including development of LH strategies. Drawing on LH theory, various evolutionary biologists and psychologists (e.g., MacDonald, 1999; Ellison, 2001; Surbey, 1998) have argued that energetic stress (i.e., malnutrition, low energy intake, negative energy balance, and associated internal stressors such as disease) cause the developing person to shift toward a slower LH strategy. This translates into development of a more energy-sparing phenotype: slower growth, delayed sexual maturation, low gonadal steroid production, small adult body size, and low fecundity. Developmental responses to resource scarcity, therefore, include trade-offs favoring maintenance over growth, future over current reproduction (late age at first birth) and offspring quality over quantity (low offspring number). Along these lines, monogamous marriage and father-present social systems are more likely to be found among hunter-gatherers inhabiting harsh physical environments where biparental care (male provisioning) is substantial and important for offspring survival and reproductive success (Draper and Harpending, 1988; Geary, 2000; Kaplan and Lancaster, 2003: Table 7–1; Marlowe, 2003).
Development of fast LH strategies depends on adequate bioenergetic resources (low resource scarcity/energetic stress) to support growth and development. Once this energetic threshold is crossed, other environmental conditions (i.e., extrinsic morbidity-mortality, unpredictability) become salient determinants of LH strategy (Ellis et al., 2009). Extrinsic morbidity-mortality constitutes external sources of disability and death that are relatively insensitive to the adaptive decisions of the organism. When environmental factors cause high levels of extrinsic morbidity-mortality, even prime-age adults suffer relatively high levels of disability and death. According to LH theory, environmental cues indicating high levels of extrinsic morbidity-mortality cause individuals to develop faster LH strategies (Belsky et al., 1991; Chisholm, 1993, 1999; Ellis et al., 2009; Pennington and Harpending, 1988; Quinlan, 2007). Faster strategies in this context – a context that devalues future reproduction – function to reduce the risk of disability or death prior to reproduction. Accordingly, exposure to environmental cues indicating extrinsic morbidity-mortality (i.e., observable cues that reliably covaried with morbidity-mortality risks during our evolutionary history, such as exposures to violence, dangerous ecological conditions, or harsh childrearing practices) should shift LH strategies toward current reproduction by maturing and starting mating early (Belsky et al., 1991), even at a cost for one’s future reproductive potential. Moreover, high extrinsic morbidity-mortality means that investing in parental care has quickly diminishing returns (by definition, parental effort beyond a basic level cannot shield offspring against extrinsic morbidity-mortality). Thus, high extrinsic morbidity-mortality favors quantity versus quality of offspring.
In addition to the effects of levels of extrinsic morbidity-mortality, variation in extrinsic morbidity-mortality over time and space – environmental unpredictability – also regulates development of LH strategies (Ellis et al., 2009). In environments that fluctuate unpredictably (e.g., changing randomly between Conditions A and B, so that exposure by parents or their young offspring to Condition A does not reliably forecast whether offspring will mature into Condition A or B), long-term investment in a development of a slow LH strategy does not optimize fitness; all of the energy invested in the future would be wasted if the individual matures into an environment where life expectancy is short. Instead, given adequate bioenergetic resources to support growth and development, the psychobiological mechanisms regulating LH strategies should detect and respond to proximal cues to environmental unpredictability (e.g., stochastic changes in ecological context, geography, economic conditions, family composition, parental behavior) by entraining faster LH strategies. Because levels of and variability in extrinsic morbidity-mortality are distinct, developmental exposures to each of these environmental factors should uniquely contribute to variation in LH strategy (Ellis et al., 2009).
2.2.2. Genetic factors and gene-environment interactions
In heterogeneous environments, adaptive developmental plasticity can match the organism’s phenotype to its developmental context in a manner that maximizes the organism’s expected fitness. This is only possible, however, if the relevant dimensions of the environment can be detected and predicted with some reliability, and if the organism is equipped with biological mechanisms capable of responding appropriately to environmental cues. While adaptive plasticity is widespread, it may not always be the best option. For example, if the cost of maintaining the mechanisms that regulate plasticity is high, or if there are no reliable cues in the environment on which to base the organism’s strategy, natural selection may favor fixed alternative phenotypes (i.e., specialists) in heterogeneous environments. Such alternative phenotypes may be implemented through a stochastic developmental switch (as in the case of bet-hedging; see Philippi and Seger, 1989) or be based on DNA sequence variation (polymorphisms). Natural selection tends to maintain genetic variation when there are multiple ecological niches in the environment, and individuals are able to select the niche that best fits their genotype (and expressed phenotype; Wilson, 1994); or when temporally changing environments produce fluctuating selection pressures that are stronger than any unidirectional selection pressure, and alternative genotypes (and their expressed phenotypes) have approximately the same fitness when averaged across the fluctuating selection regimes (Penke et al., 2007); or when the fitness of alternative genotypes (and their expressed phenotypes) varies depending on their frequency in the population (Maynard Smith, 1998).
A crucial question is, to what degree should LH strategies be developmentally contingent and plastic, rather than canalized and more strictly determined by genotype? The answer is not simple; indeed, what is typically found in organisms is a mixture of the two. Theoretical models suggest that one should often expect a balance between genetic and environmental determination of phenotypic individual differences. At the individual level, a model by Leimar and colleagues (2006) indicates that, in a broad range of conditions, plasticity switches should evolve so as to integrate both genetic and environmental information in phenotypic determination. Indeed, all the LH traits studied so far in humans show at least moderate heritability (e.g., Figueredo et al., 2004; Kirk et al., 2001; Pettay et al., 2005; Rodgers et al., 2001). At the population level, the opportunity for habitat choice plus heterogeneous environmental conditions can maintain a diverse population composed of both “specialists” (fixed phenotypes) and “generalists” (plastic phenotypes), as shown by Wilson and Yoshimura (1994). In a similar vein, differential susceptibility theory (Belsky, 1997, 2005) maintains that, because the cues driving the development of conditional phenotypes are not completely reliable, children vary in their susceptibility to rearing influences (section 1). Such differential susceptibility underlies pervasive person-by-environment interactions, whereby individuals with given genotypes or phenotypes show higher sensitivity to environmentally-induced effects on development (see Belsky, 1997, 2005; Belsky and Pluess, 2009; Boyce and Ellis, 2005; Ellis et al., 2011a).
2.3. Life history strategies and the organization of behavior
When interpreted in a narrow sense, LH strategies refer mainly to growth- and reproduction-related traits such as maturation timing, age at first reproduction, fertility, and number of sexual partners. However, it is easy to see that the choice of a specific strategy will affect a much broader range of traits and behaviors (Belsky et al., 1991; Figueredo et al., 2004, 2006; Wolf et al., 2007). Imagine an organism that, following cues of extrinsic morbidity-mortality and unpredictability, adopts a strategy characterized by early reproduction and high mating effort. To succeed, the organism needs to outcompete same-sex conspecifics and be chosen by members of the other sex. Especially for males, this is likely to involve status-seeking behavior, plus considerable investment in traits and displays that the other sex finds attractive in short-term mates; in humans, these may involve verbal and creative displays, competitive sports, humor, and so on (Jackson and Ellis, 2009; Locke and Bogin, 2006; Miller, 2000). The cues of environmental risk that drive the choice of the strategy will also prompt higher risk-taking in other domains (e.g., exploration, fighting, dangerous sexual displays), preference for immediate over delayed rewards, and impulsivity (Chisholm, 1999; Daly and Wilson, 2005). A recent model by Wolf and colleagues (2007) formally showed that individual differences in present- versus future–oriented strategies can be expected to result in consistent individual differences in risk-related traits, such as boldness and aggression. Impulsivity and competitive attitudes, in turn, should decrease the willingness to engage in long-term cooperation and to behave altruistically (Belsky et al., 1991; Curry et al., 2008); and since the behaviors associated with a fast strategy predictably increase the organism’s expected morbidity-mortality, the very fact of having adopted the strategy may act as a “self-produced cue” of increased hazard, leading to self-reinforcing feedback on behavior.
In summary, LH strategies play a powerful role in the organization of behavior. Traits and behaviors that covary along LH dimensions form a broad, integrated cluster which includes exploration/learning styles, mating and sexual strategies, pair-bonding, parenting styles, status- and dominance-seeking, risk-taking, impulsivity, aggression, cooperation, and altruism. Correlations within this cluster have been documented both in humans (e.g., Del Giudice, 2009a; Figueredo et al., 2006; Kruger et al., 2008) and other animals (e.g., Dingemanse and Réale, 2005; Korte et al., 2005). For example, Figueredo and colleagues were able to identify a heritable, general factor accounting for a large proportion of variance in psychological traits including security of attachment to romantic partners, mating style, impulsivity, and altruism (Figueredo et al., 2004, 2005, 2006).
Life history strategies organize individual differences across multiple domains, from physical growth and maturation to social, sexual, and parental behavior. This requires the evolution of physiological mechanisms capable of coordinating the development of LH-related traits in an integrated, adaptive fashion. In this paper we argue that the SRS is such an integrative mechanism. As we will show in section 4, the SRS contributes to the regulation of a wide range of LH-related traits – from sexual maturation and fertility to risk-taking and parenting styles. Even more importantly, the SRS is well suited to make use of environmental information to entrain the development of alternative strategies.
3. Stages and switch points in human development
Adaptive scheduling of developmental tasks requires tight coordination between physiology and behavior, and the emergence of life stages is an effective solution to this problem. For example, if an organism has to grow to a certain size and accumulate a minimum quantity of resources before it can engage in successful reproduction, it will need to: (1) promote bodily growth; (2) keep the neuroendocrine machinery devoted to mating shut off, or at least reduce its activity; (3) intensify behaviors related to resource seeking and acquisition; and (4) track body size and resource level to decide when to begin to look for mates. These multiple goals can be achieved by putting all the relevant metabolic and behavioral processes under the control of a single switch mechanism, so that the organism will first specialize in growth and resource acquisition and then in mating and reproduction. In this way, the organism avoids interference and competition for time and metabolic resources between different developmental tasks. Although LH trade-offs do not always lead to mutually exclusive choices (e.g., parenting and searching for new mates may coexist up to a point), the competition between different fitness-related activities encourages developmental specialization. Then once life stages begin to evolve, one can expect them to become increasingly more specialized as traits that work well together cluster with one another.
At the molecular level, the selective expression of different traits is permitted by expression of different sets of genes; thus, transitions between life stages involve the turning on and off of co-expressed genetic networks. The suite of traits expressed in a given stage fulfills the definition of evolutionary modules: Switch-controlled sub-units of the phenotype that display coordinated expression as a unit; are internally integrated (recurrence together in time or space of the same elements, indicating a common source of regulation); are temporally or spatially discrete relative to other systems; display stereotypy of form and location across individuals of the same species; and are semidissociable (able to be deleted or reexpressed as a unit) (West-Eberhard, 2003). Modules are highly integrated by pleiotropic effects of the underlying genes2 and relatively isolated from other such sets by a paucity of pleiotropic effects (Wagner and Altenberg, 1996; Wagner et al., 2005).
Transitions between life stages are often under hormonal control (Adkins-Regan, 2005; Heyland et al., 2005). The widely distributed effects of hormonal signaling systems allows them to act as crucial nodes in complex regulatory networks, coordinating the expression of traits in the whole organism (Dufty et al., 2002; Heyland et al., 2005). Working through both peripheral and central nervous system pathways, hormonal regulation of between-stage transitions allows for remarkable plasticity, making these transitions sensitive to social and environmental cues. More generally, it is becoming increasingly clear that neuroendocrine systems are crucially involved in the expression of developmental plasticity in most species, integrating and “interpreting” environmental variation and adaptively shaping the development of the whole organism (Dufty et al., 2002; Kaplan and Gangestad, 2005; Nijhout, 2003; Ricklefs and Wikelski, 2002).
3.1. Developmental switch points
West-Eberhard (2003) has modeled the role of switch-controlled modular systems in development. Her model can be usefully applied both to sequential changes in biologically distinct life stages (e.g., the pubertal transition) and to development of alternative LH strategies. Transitions between stages are coordinated by developmental switches that operate through hormonal mechanisms. Most critically, developmental plasticity is regulated through developmental switches and, thus, enhanced at life stage transitions.
To illustrate the concept of a developmental switch, we focus on puberty: the transition from the pre-reproductive phase of the human lifespan, when energy is primarily allocated toward physical and psychological growth, to the reproductive phase of the lifespan, when energy is allocated toward transforming the developing adolescent into a reproductively competent individual (see Schlegel, 1995; Weisfeld and Janisse, 2005). This transition is marked by puberty-specific physical and psychological changes that function to promote reproductive competence (e.g., greater height and muscle mass in adolescent boys aids in face-to-face competition with rivals; widening of the pelvis and accumulation subcutaneous fat in adolescent girls promotes successful pregnancy and lactation; increasing attraction – and reaction – to romantic, sexual, and peer contexts helps adolescents break into the breeding pool). The pubertal transition, like other developmental switch points, is a sensitive period for developmental change (Forbes and Dahl, 2010; Nelson et al., 2005), with increased susceptibility to genetic and environmental influences.
Perhaps the most striking feature of the pubertal transition is its variation. Some individuals complete pubertal development in primary school while others are still relatively undeveloped when they start high school. This variation begins with individual difference in maturation of the reproductive axis – when and how fast puberty occurs – and then, in a linked set of resource allocation decisions that characterize development of LH strategies, feeds forward to many other reproductive characteristics. For example, girls who experience early pubertal development, compared with their later maturing peers, tend to have higher levels of serum estradiol and lower sex hormone binding globulin concentrations that persist through 20–30 years of age; have shorter periods of adolescent sub-fertility (the time between menarche and attainment of fertile menstrual cycles); experience earlier ages at first sexual intercourse, first pregnancy, and first childbirth; and tend to be heavier and carry more body fat in adolescence and early adulthood (reviewed in Ellis, 2004; see also St. George et al., 1994; van Lenthe et al., 1996).
How do genes and environments affect this variation? Developmental change is coordinated by regulatory switch mechanisms, which serve as transducers (mediators) of genetic, environmental, and structural influences on phenotypic variation. These switch mechanisms control developmental switch points: “A point in time when some element of phenotype changes from a default state, action, or pathway to an alternative one – it is activated, deactivated, altered, or moved” (West-Eberhard, 2003, p. 67). This can involve a discrete structural change or a change in the rates of a process. Genetic and environmental inputs interact with extant phenotypic qualities to determine the functioning of regulatory switch mechanisms and influence their thresholds. Once a threshold is passed (i.e., the switch occurs), the regulatory mechanism coordinates the expression and use of gene products and environmental elements that mediate the species-typical transition to the new phenotypic stage as well as individually differentiated pathways within that stage.
For example, consider the key event in the pubertal transition – gonadarche (maturation of the gonads) – and its regulatory functions. Gonadarche begins at approximately 9 or 10 years of age in girls and soon thereafter in boys; it involves a change from low-level, irregular secretion of gonadotropin-releasing hormone (GnRH) to a pattern of distinct pulses. At gonadarche, the GnRH surge markedly increases pulsatile secretion of luteinizing hormone and follicle-stimulating hormone, leading to a cascade of events, including gonadal maturation, increased production of steroid hormones, growth acceleration, weight gain, development of secondary sexual characteristics, and so forth (see Ebling, 2005; Grumbach and Styne, 2003; Plant and Barker-Gibb, 2004).
A switch point is controlled by a condition-sensitive, quantitatively variable regulatory mechanism with a given activation threshold. Appropriate pulsatile secretion of GnRH is the threshold that must be passed for gonadarche to occur. In terms of pubertal development, gonadarche can be thought of as a master switch, with subsequent decision points working as subordinate switches in a developmental sequence. The neurotransmitter and neuromodulatory systems that control the GnRH secretory network are a major locus of operations for both environmental and genetic influences on not only the awakening of the hypothalamic–pituitary–gonadal (HPG) axis (gonadarche) but also regulating its functioning after gonadarche occurs. Environmental factors, such as nutrition and exercise, predation threats, or family stress and support, can potentially influence the reproductive axis at every developmental switch point (reviewed in Ellis, 2004). In addition, a large number of genes influence the maturation and functioning of the HPG axis. For example, seventeen different single-gene mutations have been associated with delayed or absent puberty in humans (Herbison, 2007).
Most critically, regulatory switch mechanisms provide a common locus of operations for genetic and environmental influences on phenotypic development; that is, these mechanisms are the vehicle through which gene-gene, environment-environment, and gene-environment interactions occur. These inputs structure the operation of regulatory switch mechanisms (e.g., determine levels of pulsatile release of GnRH) and may affect the threshold necessary for a developmental switch to occur and/or the organism’s ability to cross that threshold (West-Eberhard, 2003; Ellis, in press).
3.2. Human juvenility
Human development can be segmented in a number of biologically distinct stages separated by transitional periods: a prenatal stage (conception to birth), infancy (birth to about 2 years), childhood (about 3 to 6 years), juvenility or middle childhood (about 7 to 11 years), adolescence (about 12 to 17 years), and adulthood (Bogin, 1997, 1999). Each is characterized by a specific pattern of growth, physiology and behavior, and fulfills a distinct set of biological functions. Infancy, childhood and adolescence are often discussed in relation to the development of the SRS (Adam et al., 2007; Gunnar and Donzella, 2002; Gunnar and Vazquez, 2006; Gunnar et al., 2009c; Shirtcliff and Ruttle, in press). More recently, there has also been a surge of interest in the prenatal stage as a critical period in stress development (e.g., Bergman et al., 2007; Gutteling et al., 2004, 2005; Poggi Davis et al., 2010). Much less attention has been devoted to the developmental role of juvenility and to the neuroendocrine changes that take place in the passage from early to middle childhood. Juvenility, however, has an important place in our model, and in sections 5 and 6 we argue that it represent a critical transition period in the development of individual and sex differences in responsivity. In order to give the reader the necessary background, we now present an overview of human juvenility and its psychological and neurobiological correlates.
Juvenility is defined as a pre-reproductive stage in which the youngster is independent from parents for survival, but still sexually immature. Human juvenility is a biological label for what is usually called “middle childhood”. Children become relatively able to gather food and protect themselves from predators at about 6–7 years of age. In most societies, children rely on parental provision and protection until adolescence or young adulthood (Lancaster and Kaplan, 2009); however, a typical 7-year-old child has some chances of surviving on his/her own if forced to do so, and is able to make a significant contribution to the household economy when ecological conditions allow (Bogin, 1999; Kramer, 2002, 2005).
By age seven, gross motor development is complete, walking efficiency is comparable to that of adults, and the brain has virtually stopped growing in weight. The juvenile growth pattern of humans is marked by a slight acceleration at the beginning of this stage (the “mid-growth spurt”), followed by a deceleration that brings juvenility to the slowest growth rate from birth. Permanent teeth begin to erupt, allowing the child to eat adult-type food (see Bogin, 1999; Kramer, 2005). In the psychological domain, the beginning of juvenility witnesses dramatic increases in self-control, emotional and attentional regulation, and executive functions in general, which collectively go under the label of “the 5- to 7-years shift” (Best et al., 2009; Sameroff and Haith, 1996).
Whatever the original selective pressures driving the evolution of juvenility (see Janson and van Schaik, 1993; Kaplan et al., 2000; Pereira and Fairbanks, 1993; Joffe, 1997), evolutionary theorists agree that one of the key functions of this delayed growth phase is learning. Once equipped with the basic motor and cognitive toolkit, human juveniles engage in active learning and experimentation in all domains: technical skills (e.g., hunting, gathering, manufacturing), parenting, social role, and culturally transmitted abilities in general (Bogin, 1999; Campbell, 2006; Lancy and Grove, in press). In primates, the duration of juvenility correlates with social group size, suggesting that social learning is indeed a crucial function of this life stage (Joffe, 1997).
Another crucial characteristic of juvenility is the growing importance of peers in children’s interpersonal relations. Children begin to form stable groups with clearly defined hierarchies, and engage in intense social competition3 for status and dominance within their group. The outcomes of social competition can carry over well into adulthood; longitudinal studies of dominance and peer acceptance, for example, suggest that ranks acquired in childhood may be relatively stable over many years (reviewed in Weisfeld, 1999). The first romantic/sexual attractions also appear in middle childhood (Herdt and McClintock, 2000; McClintock and Herdt, 1996). As a consequence, children receive important feedback about their desirability as a group member and a mate, and more generally about their competitive abilities in the social world.
From an evolutionary standpoint, the above implies that sexual selection (natural selection through mate choice and/or same-sex competition for mates) begins to operate already in middle childhood. One would then expect increased sex differentiation of behavior in middle childhood – and there is abundant evidence that this is the case. Sex differences in aggression, play activities, and language use intensify or peak in middle childhood. For example, there is a peak in fighting and rough-and tumble play (especially boys), play parenting (usually girls), and sex segregation between groups of boys and girls (Geary, 2010). Boys also engage in more locomotor and exploratory play (Smith, 2005). At the same time, sex differences in aggression become larger, with girls showing substantially less physical aggression and slightly more relational aggression than boys (Pellegrini and Archer, 2005). Recently, it has been proposed that attachment styles undergo a sex-specific reorganization in middle childhood, with insecure boys shifting towards avoidance and insecure girls toward ambivalence (Del Giudice, 2009a; Del Giudice and Belsky, 2010a). As concerns LH trade-offs, the juvenile stage is largely devoted to somatic effort through the acquisition of knowledge and skills; however, social competition can also be seen as a form of anticipatory mating effort, thus underlining the complex functional role played by juvenility.
The activation/reorganization of the motivational systems mediating social competition and attachment in juvenility is reflected in the age distribution of psychiatric diagnoses. The overall prevalence of psychiatric diagnoses peaks at 9–10 years, then declines and rises again around age 14 (Costello et al., 2003; Kessler et al., 2005). The middle childhood peak is mainly due to an increase in aggressive and disruptive behavior, impulsivity, and anxiety (including attachment-related anxiety). Several anxiety- and aggression-related syndromes have a typical age-at-onset range that includes (or is even limited to) middle childhood: specific and social phobias, separation anxiety disorders, oppositional-defiant disorder (ODD), conduct disorder (CD), and attention deficit-hyperactivity disorder (ADHD).
3.2.1. Adrenarche and the juvenile transition
The transition from childhood to juvenility is marked by the event of “adrenal puberty” or adrenarche (Auchus and Rainey, 2004; Ibáñez et al., 2000). At about 6–8 years, with little difference between the sexes but substantial individual variation in timing, the cortex of the adrenal glands begins to secrete increasing quantities of androgens, mainly dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS). The sequence of physiological events leading to the initiation of adrenarche remains largely unknown and may consist of a gradual maturational process (Gell et al., 1998; Palmert et al., 2001).
Adrenarche has a minor impact on bodily development; the effects of adrenal androgens include the initial appearance of pubic and axillary hair, increased oiliness of the skin and hair, changes in body odor, subtle voice changes and a small, temporary acceleration of skeletal growth. Adrenal androgens, however, can have stronger effects on brain functioning and maturation. DHEA and DHEAS modulate the action of GABA, and the action of DHEAS at sex-steroid receptors may reduce fear and anxiety in social interactions and improve memory by modulating neural activity in the amygdala and the hippocampus. DHEAS may also increase neural plasticity thanks to its role as a synaptic modulator (see Campbell, 2006; Simon and Lu, 2006; Wolf and Kirschbaum, 1999). In addition to their direct actions, both DHEA and DHEAS can be converted to testosterone and/or estrogen in several organs – including the brain (Labrie et al., 2001, 2005). The indirect action of adrenal androgens may account for much of their behavioral effects in middle childhood and links them to the intensification of sex differences observed during this developmental stage. In juveniles, adrenal steroids can produce behavioral effects in specific domains regulated by “adult” sex hormones while having minimal effects on physical maturation, thus shifting development on sexually differentiated pathways even before full reproductive maturity.
Based on the mechanism of adrenarche, the passage from early childhood to juvenility has the quality of a discrete psychobiological event. Del Giudice and colleagues (2009) proposed the label juvenile transition to emphasize the shift in developmental function and the accompanying endocrine and neurobiological changes (see also Campbell, 2006). They further theorized that the juvenile transition works as one of the main switch points in the development of individual LH strategies.
3.3. The development of life history strategies
The concept of development as a sequence of switch points can be used to sketch a general developmental model of human LH strategies. Del Giudice and Belsky (2010b) proposed that LH strategies develop in a sequential, multi-stage fashion, whereby early experiences shift development on alternative pathways with the possibility of later “revision.” A sequential process of assessment-adjustment provides the best compromise between early commitment to a strategy (with the benefit of having time to develop the appropriate skills) and finely tuned tracking of changes in ecological and social conditions. This process is marked by a number of developmental switch points when plasticity is preferentially expressed and development is directed (or re-directed) along alternative pathways. At developmental switch points, genotypic variation is integrated with information from the environment, and the result of this integration shapes strategy choice.
What are the main switch points in the development of human LH strategies? To begin with, some preliminary strategy-setting may occur even before birth. For example, energetic conditions experienced in utero affect subsequent metabolism (e.g., Worthman and Kuzara, 2005); likewise, prenatal exposure to maternal stress hormones is linked to temperamental reactivity and stress physiology, likely through epigenetic regulation of gene expression (de Weerth et al., 2003; Gutteling et al., 2005; Möhler et al., 2006; O’Connor et al., 2005; Poggi Davis et al., 2010). Then, during the first years of life, the child can sample the environment to gather information about the local ecology (including extrinsic morbidity-mortality, unpredictability, and resource availability); such environmental sampling can take place both directly and via the mediation of parental behavior (Belsky et al., 1991; Chisholm, 1993, 1996). With the juvenile transition, this information could be translated into nascent behavioral strategies through the neurobiological mediation of adrenarche. First, adrenal androgens can contribute to the phenotypic expression of a suite of LH-related traits, including attachment, competitive risk-taking, aggression, and the first manifestation of sexuality. For example, there is a correlation between levels of DHEAS and the severity of disruptive behaviors in middle childhood (van Goozen et al., 1998, 2000, 2007). Second, adrenal androgens can regulate development in a sex-specific way through activation of sexually differentiated brain structures; for example, fast LH strategies are expected to prompt an increase in high-risk, physically aggressive dominance-seeking in boys more than in girls (section 2). Third, adrenal androgens can mediate conditional adaptation by interacting with the action of other neuroendocrine systems (such as the SRS). This is consistent with the results of a longitudinal study by Ellis and Essex (2007), who found that familial stress experienced in childhood anticipates adrenarche in both boys and girls. There is also evidence that early insecure attachment anticipates the onset of sexualized behavior in juveniles (Sroufe et al., 1993) and promotes early puberty (Belsky et al, 2010). Fourth, activation of sex hormone pathways can reveal individual variation at the genetic level – for example in conversion enzymes, hormone receptors, or hormone response elements – thus contributing to the development of individual differences in LH-related traits (for extended discussion see Del Giudice et al., 2009). Finally, juvenility provides an assessment period before the actual onset of mating and reproduction; such an assessment period may be crucial for appraising the likely success of a chosen strategy, prompting strategic revision in case the strategy is unsuccessful or does not match the child’s social environment (see Del Giudice, 2009b; Del Giudice et al., 2009; Del Giudice and Belsky, 2010b).
The next switch point is provided by puberty, when individuals first enter the arena of actual mating and reproduction (section 3.1). There are a suite of puberty-specific physical, emotional, motivational, and cognitive changes that constitute a re-orientation of social behavior (Forbes and Dahl, 2010; Nelson et al., 2005) and support the transition from the pre-reproductive to the reproductive phase of the human life cycle. Both sexual promiscuity and the intensity of sexual competition peak in adolescence and early adulthood, when most people have not yet found a stable partner and the mating market is maximally open. This time of heightened promiscuity and competition may help young people determine their own status and attractiveness, refine their mate preferences, and practice mate attraction strategies (Weisfeld, 1999; Weisfeld and Coleman, 2005). In this context, both risk-taking and sex differences in risk-taking peak in adolescence and early adulthood (e.g., Kruger and Nesse, 2006; Wilson et al., 2002). Jackson and Ellis (2009) proposed that, especially for males, the social status acquired in adolescence should be a critical factor affecting the development of LH strategies. Consistent with this claim is evidence that the degree of agonistic stress experienced in early adolescence affects the choice of mating strategies in adulthood (Davis and Werre, 2007), although the effects of social competition on LH strategy development probably start already in juvenility.
LH trade-offs extend well beyond puberty, and it seems likely that other switch points can be found across the life course. For example, menopause most certainly represents a fundamental switch point for women, and there seems to be a tendency for men around the world to increase their parental effort when approaching middle age (Winking et al., 2007). Other factors may also contribute to strategic adjustment during adult life, even without qualifying as identifiable switch points. An event of special significance may be represented by the birth of one’s first child: not only does it signal (some degree of) reproductive success, but it is known to affect hormonal functioning in both sexes (e.g., Storey et al., 2000) and could thus directly interact with the endocrine systems that regulate the expression of LH strategies. Dramatic changes in social dominance (especially for men) and in social support (especially for women) may also act as triggers for recalibrating one’s strategy in response to changing opportunities in the environment.
4. The stress response system: Organization and function
In this section we review the neurobiology of the stress response system and provide an integrative overview of its biological functions. The SRS comprises three anatomically distinct neuroendocrine circuits: the sympathetic (SNS) and parasympathetic (PNS) branches of the autonomic nervous system and the hypothalamic-pituitary-adrenal axis (HPA). The activity of these circuits is integrated and cross-regulated, so that they can be considered as components of a single functional system despite their anatomical and physiological diversity (e.g., Adam et al., 2007; Boyce and Ellis, 2005; Ellis et al., 2006; Habib et al., 2001; Porges, 1995; Schlotz et al., 2008).
The biological function of the SRS is threefold (see Figure 1). First, the SRS coordinates the organism’s physiological and behavioral response to environmental threats and opportunities. This includes any event that may have important (i.e., fitness-relevant) consequences for the organism and requires the organism to modify its current state in order to be dealt with effectively (section 4.1). In addition to threats and dangers, environmental opportunities may be represented by unexpected or novel events, and even highly pleasurable situations (e.g., signs of sexual availability in a potential partner). The whole-organism adjustment to environmental challenge is often termed allostasis (McEwen, 1998; McEwen and Wingfield, 2003; Sterling and Eyer, 1988). Allostasis is defined as the process of achieving stability through physiological or behavioral change – in contrast with homeostasis, which maintains physiological stability by keeping the organism’s state at a fixed set point. The SRS mediates allostasis by coordinating brain/body changes in response to environmental challenges, both in the short and in the long term. Because allostasis is a broader concept than “stress response”, and because many of the challenges that activate the SNS and HPA are not “stressors” in the classical sense, the label “stress response system” is not entirely adequate to describe the function of the SRS. In the present paper we employ it for lack of a widely accepted alternative; however, we want to make it clear from the outset that the SRS is a general interface with the environment, mediating the organism’s adjustment to both positive and negative events (Boyce and Ellis, 2005; Ellis et al., 2006)4.
The second function of the SRS, closely connected to the first, is that of encoding and filtering information coming from the social and physical environment (section 4.2). Activation of the SRS components carries information about the likelihood of threats and opportunities in the environment, their type, and their severity. This information can be encoded by the SRS and, in the long run, provides the organism with a statistical “summary” of key dimensions of the environment, including the LH-relevant dimensions of extrinsic morbidity-mortality and unpredictability. An important corollary is that the system’s level of responsivity acts as an amplifier (when highly responsive) or filter (when unresponsive) of various types of environmental information. A highly responsive system makes an individual more informationally open and – as explained by the BSC theory – enhances his/her sensitivity to contextual influences, both “positive” and “negative”.
Finally, the role of the SRS extends far beyond the response to immediate challenges. Profiles of SRS baseline activity and responsivity are associated with individual differences in a range of LH-relevant domains including competitive risk-taking, learning, self-regulation, attachment, affiliation and reproductive functioning (section 4.3). In a life history framework, this is no coincidence: we argue that – together with sex hormones and relevant neurotransmitter systems – the SRS is a critical mediator of LH development, gathering information from the environment and translating it into broad-band individual differences in behavior and physiology (Figure 1; see also Korte et al., 2005; Worthman, 2009). In the remainder of this section we will discuss the functions of the SRS in more detail, from the coordination of the allostatic response to challenges (focusing specifically on psychosocial challenges) to the regulation of LH-relevant traits.
4.1. Response to psychosocial challenges
The SRS is organized in a hierarchical fashion, and its components differ in their response timing and in the physiological “depth” of their effects. The quickest and most immediate response is that of the parasympathetic system (PNS), followed by the sympathetic system (SNS) and by the HPA axis. The allostatic response of the SRS to a given challenge may involve activation of one or more of its components, depending on the nature, duration, and intensity of the challenge itself.
4.1.1. Anatomy and physiology
The parasympathetic branch of the autonomic system directly innervates internal organs via cholinergic fibres (with the exception of fibres releasing adenosine triphosphate in the digestive tract; see Lovallo and Sollers, 2007). The general function of the PNS is to promote vegetative functions and reduce physiological arousal. The PNS and SNS are in dynamic equilibrium, and tonic activation of the PNS counteracts sympathetic-induced arousal, thus promoting sustained attention, self-regulation, relaxation and social engagement. In mammals, a specific role in this respect may be played by myelinated vagal fibres originating in the nucleus ambiguus, which apply a parasympathetic “brake” on cardiac activity (Porges, 1995, 2001, 2007). If the PNS becomes deactivated, it stops counterbalancing sympathetic activation and releases the physiological effects of the SNS. Parasympathetic disengagement provides an extremely rapid way to increase arousal and re-orient attention in the face of unexpected events.
If parasympathetic withdrawal is not sufficient to cope with the present challenge, activation of the SNS provides a second layer of response. Sympathetic activation mediates fight/flight responses and is co-ordinated by the locus coeruleus (LC). From the LC, SNS activation follows two routes: a fast, direct pathway via the noradrenergic innervation of visceral organs, and a slower, hormonal pathway through innervation of the adrenal medulla (the sympathetic-adrenal-medullary pathway; See Goldstein and Kopin, 2008; Gunnar and Vazquez, 2006). Following sympathetic activation, the adrenal medulla secretes epinephrine (E) and smaller amounts of norepinephrine (NE). The resulting effects on physiology include heart rate increase, faster respiration, increased blood supply to skeletal muscles, glucose release in the bloodstream and suppression of vegetative functions. The norepinephrine/epinephrine balance is associated with specific types of behavioral reaction to the challenge, with high NE/E ratios associated with proactivity and dominance and low ratios associated with fear and anxiety (Korte et al., 2005; Netter, 1983, 1987, 1991).
More extreme defense reactions associated with “freeze/hide” behaviors may again involve activation of the PNS, although via different efferent fibres (Porges, 1995, 2007). In general, sympathetic and parasympathetic activity in response to challenge tend to be negatively associated with one another (e.g., PNS deactivation plus SNS activation), a pattern labeled “reciprocal activation” (Berntson et al., 1991). Only a minority of people show low activation of both systems (“coinhibition”), and even fewer show simultaneous high activation in both (“coactivation”; e.g., Alkon et al., 2003; El-Sheikh et al., 2009). Coactivation may also result from the specific demands of highly exciting situations where one needs to maintain tight self-control; for example, a recent study found a consistent pattern of autonomic coactivation during skydiving (Allison et al., in press). Thus, observed differences in autonomic profiles may partly reflect individual differences in the appraisal of a given stressful event.
The third component of the SRS is the HPA axis, which mounts a delayed, long-term response to environmental challenges through the release of cortisol. Neurons in the paraventricular nucleus of the hypothalamus (PVN) secrete corticotropin releasing hormone (CRH) and vasopressin (AVP) into the portal circulation system of the pituitary. In the anterior pituitary, CRH and AVP trigger secretion of pro-opiomelanocortin (POMC) polypeptide, which is then cleaved into various hormones, including adrenocotricotropic hormone (ACTH) and β–endorphin. Through systemic circulation, ACTH reaches the adrenal cortex where it stimulates cortisol release. Besides stimulating cortisol production, centrally released CRH also contributes on its own to the physiological and psychological aspects of response to challenge.
Cortisol elevation starts about 5 minutes after the triggering event, with a peak between 10 and 30 minutes. Some of the effects of cortisol begin after an hour and may be observed for several hours or more. Cortisol binds to nuclear receptors and regulates gene transcription; in addition, membrane-bound cortisol receptors have been found to mediate faster, nongenomic effects of cortisol (Oitzl et al., 2010). The main effects of cortisol are (1) to mobilize physiological and psychological resources (e.g., energy release, alertness and vigilance, memory sensitization; see Barsegyan et al., 2010; Flinn, 2006; Roozendaal, 2000; Sapolsky et al., 2000; Tops et al., 2006b; van Marle et al., 2009) and (2) in part, to counter-regulate the physiological effects of sympathetic activation, thereby facilitating recovery (Munck et al., 1984; discussed in Boyce and Ellis, 2005). The joint effects of the SNS and HPA axis are complex, and can be synergistic (especially in the short-term) as well as antagonistic (especially at later phases of the challenge response; Adam et al., 2007; Bauer et al., 2002; Sapolsky et al., 2000). In addition to the delayed cortisol response, HPA activation involves the secretion of AVP and β-endorphin, which can mediate rapid behavioral effects before cortisol levels begin to rise.
HPA activity is regulated by a hierarchy of feedback loops at different levels in the axis (see Gunnar and Vazquez, 2006), and the sensitivity of these feedback loops is a major factor in determining HPA responsivity. The HPA axis responds to chronic stressors with sustained cortisol elevation, resulting in a flattened diurnal rhythm of secretion (Miller et al., 2007)5. Chronic elevation is often followed by a rebound of the system below the previous baseline level after the stressor terminates (Koob and Le Moal, 2008). This hypocortisolism phase can last months; its function is probably to facilitate recovery and offset the physiological and immune costs of high circulating cortisol (Fries et al., 2005; Miller et al., 2007).
As required by tight functional integration, there is extensive cross-regulation among the various components of the SRS. For example, LC noradrenergic neurons project to the PVN, where they stimulate CRH release (Habib et al., 2001); in turn, cortisol exerts an inhibitory action on CRH-mediated activation of LC noradrenergic neurons (Valentino et al., 1998). At a higher hierarchical level, both the ANS and the HPA are centrally controlled by limbic structures, with the amygdala playing a crucial role (Ganzel et al., 2010; Gold and Chrousos, 2002; Herman et al., 2003). The organization of the SRS explains why correlations between the responses of its components are often found to be weak (e.g., response to a challenge may involve high sympathetic arousal but low cortisol elevation). Whereas weak correlations are usually interpreted as evidence of “dissociation” between components (e.g., Bauer et al., 2002; Schommer et al., 2003), they follow directly from the on-demand, quasi-sequential nature of the SRS response. An SRS component is activated only if the external situation calls for it, and usually after previous response steps have failed. For example, sympathetic activation can be avoided in favor of a rapid parasympathetic response; likewise, in most instances strong activation of the HPA axis is not required because the challenge is managed effectively by other fast-responding (and less costly) components of the SRS.
4.1.2. SRS responsivity and basal activity
Although the main focus of this paper is the development of individual differences in responsivity, the SRS shows background activity even when the individual is not engaging in any specific task. Indeed, basal activation levels have important implications for an individual’s psychological state. High basal activation of the PNS (especially the myelinated vagus) promotes calm, concentration, self-regulation and positive emotionality (e.g., Fabes and Eisenberg, 1997; Oveis et al., 2009; Porges, 2007), whereas high SNS baseline relates to anxiety (e.g., El-Sheikh et al., 2008); baseline cortisol secretion regulates energy mobilization and engagement with the physical and social environment (e.g., Booth et al., 2008). Patterns of basal SRS activity have been empirically linked to individual differences in a range of LH-relevant traits (see below). Interactions between baseline and responsivity of different systems are also possible: for example, when cortisol is low (e.g., after chronic stress), the consequent reduction of inhibitory feedback on CRH stimulation of the LC may amplify sympathetic activity and responsivity (Fries et al., 2005). Because of the important behavioral implications of basal SRS activity, the responsivity patterns predicted by our model (section 6) will also include information on basal activity profiles.
4.1.3. What activates the HPA axis?
Cortisol is a powerful mediator of the organism’s adaptation to the environment, but high levels of circulating cortisol also have physiological costs and a number of side effects (see Evans, 2003; Flinn and England, 2003; Gunnar and Vazquez, 2006; Korte et al., 2005; McEwen and Wingfield, 2003). Accordingly, the HPA axis does not respond indiscriminately to each and every challenge faced by the organism; rather, it is engaged most intensely by those challenges requiring extensive, sustained mobilization of metabolic and psychological resources.
In general, social-evaluative threats and uncontrollable outcomes elicit strong HPA responses in laboratory tasks (Dickerson and Kemeny, 2004; Mason, 1968). The largest increases in cortisol are observed when both elements are present. Public speaking in front of a panel of “judges” is an especially reliable elicitor of HPA activation; the likelihood of cortisol response can be augmented experimentally by increasing the social evaluation and/or uncontrollability components of the task, for example by increasing the number of judges, providing negative feedback, directing attention to the self, or modifying the length of time available to prepare the speech (Bosch et al., 2009; Denson et al., 2009; Dickerson et al., 2008; Westenberg et al., 2009). Social evaluation (e. g., public speaking, peer provocation) effectively activates the HPA axis in older children (juveniles) and adolescents as well (Gunnar et al., 2009b; Yim et al., 2010). Importantly, positive events involving a component of unpredictability/uncontrollability also activate the HPA axis; for example, anticipatory cortisol elevation in children has been observed on the day before Christmas, and the intensity of the response was proportional to the degree of children’s positive expectations (Flinn, 2006). Engagement in team sports (e.g., Bateup et al., 2002; Donzella et al., 2000; Gonzalez-Bono et al., 1999) and even video game contests (e.g., Mazur et al., 1997) also elicit HPA responses. The HPA axis responds strongly when “important events are going to happen, the child [or adult] does not know how to react so as to achieve desired outcomes, but is highly motivated to figure out what should be done. Cortisol release is associated with unpredictable, uncontrollable events that require full alert readiness and mental anticipation.” (Flinn, 2006, p.147; text in brackets added).
Separation from parents is a specific challenge activating the HPA response in infants and children. Infants 6–9 months old routinely respond with cortisol increase to mild separations from the mother. By 1 year of age, only infants with insecure-ambivalent attachment patterns (who must deal with unpredictable, inconsistently available caregivers) show HPA responses to brief separation, especially if they are also behaviorally inhibited (Spangler and Schieche, 1998). In contrast, insecure-avoidant infants (who experience consistent rejection by unavailable or intrusive caregivers) show little cortisol secretion to separation; their response profile is dominated by strong vagal withdrawal and sympathetic arousal, as though they were preparing to cope with possible dangers on their own (Hill-Soderlund et al., 2008). Whereas most 1-year-olds do not respond with HPA activation to brief separations from their caregivers, longer separations (e.g., entry in child care) can reliably elevate cortisol in 2-year-olds. It is noteworthy that the intensity of protest behavior during separation is often uncorrelated, or even inversely correlated, to cortisol secretion (Gunnar, 2005; see also Quas et al., 2000), suggesting that HPA response to separation takes place when more immediate coping mechanisms are ineffective. In one study, cortisol elevation and protest behavior were positively correlated in secure infants (who showed a weak cortisol response to separation) but not in insecure infants reacting with strong HPA activation (Ahnert et al., 2004).
Field studies clearly show that conflict, rejection and instability experienced in the family are powerful elicitors of children’s HPA response – much more so than physical challenges. In the Dominica studies by Flinn (2006; Flinn et al., 1996), the largest and most durable cortisol elevations in children followed family events such as punishment, quarreling, marital conflict and residence change. Socially anxious children also show HPA activation in tasks involving conflict or discussions with parents, perhaps because they are especially sensitive to cues of rejection (Gunnar et al., 2009b). When children respond with disengagement strategies, however, cortisol can decline acutely during family conflict (Kiecolt-Glaser et al., 1997, 2003; Klimes-Dougan et al., 2001; Malarkey et al., 1994). Cortisol decline may be observed more readily in response to laboratory-induced conflict, which takes place in a safe and controlled setting (with little risk of escalation or violence); actual conflict experienced at home is likely to be perceived as much more unpredictable/uncontrollable, thus eliciting a stronger HPA response in children.
In summary, the HPA is engaged (1) when the individual faces unpredictable and/or uncontrollable challenges requiring alertness and anticipation, and (2) when challenges involve social evaluation or a threat to one’s social/affective relationships. Relationships with parents (and between parents) have a vital importance for infants and children, whose HPA axis is exceptionally responsive to separation, rejection, and negative events in family life; in older children and adults, peer evaluation becomes a powerful elicitor of HPA response (Booth et al., 2008). From an evolutionary perspective, social evaluation is critical in two intertwined but distinct ways: being negatively judged can cause a loss in social status (thus being perceived as an agonistic threat), but can also lead to ostracism, rejection and loss of social bonds (thus resulting in a threat to affiliation and group membership). On both counts, HPA activation is crucially linked to sensitivity to social feedback; even in 4-year-olds, cortisol responsivity is associated with the experience of shame and embarrassment in self-evaluative tasks (Lewis and Ramsay, 2002; see also Tops et al., 2006a).
4.1.4. Sex differences in SRS functioning
Although the basic architecture of the SRS is the same in males and females, there are consistent sex differences in the type of events that elicit a SRS response and in the physiological and behavioral correlates of the response. Men often show larger HPA activation than women in achievement-related tasks (which may elicit status-related motives), whereas women show larger HPA activation in situations involving social rejection (Stroud et al., 2002; see also Ennis et al., 2001). During university entrance exams male students secrete more adrenaline, indicative of a stronger sympathetic response (Rauste von Wright et al., 1981). Cortisol elevation has also been found in male (but not female) undergraduates during periods of high examination stress (Weekes et al., 2006). A neuroimaging study employing mental arithmetic tasks showed clear sex differences in brain activation patterns; moreover, brain activity was more strongly correlated with cortisol elevation in males than in females (Wang et al., 2007).
In public speaking tasks, sex differences in HPA responsivity show an inconsistent pattern; when effects are found, they indicate stronger responses in men, although women show increased cortisol response in the luteal phase of the menstrual cycle (Kajantie and Phillips, 2006; Kudielka and Kirshbaum, 2005; Kudielka et al., 2009). As noted above, social evaluation tasks such as public speaking can elicit both status-related and affiliation-related motives, so they may activate the HPA axis in males and females for somewhat different reasons. Interestingly, two studies detected no elevation in cortisol after public speaking in 11–13 year old boys, but found the expected cortisol responses in younger and older boys and in girls (Gunnar et al., 2009c; Klimes-Dougan et al., 2001); the significance of this finding is not clear, but indicates that the pubertal transition may have sex-specific effects on HPA response.
Sex hormones are powerful determinants of sex differences in SRS functioning. Androgens and estrogen modulate cortisol synthesis in the adrenal cortex and CRH and AVP synthesis in the hypothalamus; the latter effect is mediated by sex hormone receptors in the amygdala (Viau, 2002; Viau et al., 2005). Estradiol typically stimulates HPA activity, whereas androgens tend to have inhibitory effects (Kudielka et al., 2005). Cross-talk between endocrine systems can even take place at the genomic level, since androgen and glucocorticoid receptors can interact in the regulation of gene transcription (Viau, 2002).
Taylor and colleagues (2000; Taylor, 2006) proposed an important and broad-ranging theory of sex differences in physiological and behavioral responses to stressors. They argued that, in humans as well as in other animals, the classical sympathetic fight-or-flight response is more typical of males, whereas females tend to react to threats with a “tend-and-befriend” behavioral pattern: (1) caring for and protecting offspring, if present (tending); and (2) affiliate with a group and seek social support, preferably from other females (befriending). They also proposed oxytocin secretion under stress as one of the key physiological mediators of the tend-and-befriend response. Oxytocin has anxiolytic and sedative effects (e.g., Heinrichs et al., 2003). In a series of experimental studies carried out in the last decade, oxytocin has been demonstrated to increase trust and altruism, stimulate affiliative social interactions and approach behavior, and enhance emotional empathy (e.g., Alvares et al., 2010; Ditzen et al., 2009; Domes et al., 2010; Kemp and Guastella, 2010; Hurlemann et al., 2010; Kosfeld et al., 2005; Theodoridou et al., 2009; Zak et al., 2005). Oxytocin production under stress is inhibited by androgens and stimulated by estrogen (Jezova et al., 1996; McCarthy, 1995; McCarthy et al., 1996); thus, sex hormones could drive sex differences in stress-related mechanisms through their effects on oxytocin in addition to direct modulation of SRS pathways.
Certainly, the model by Taylor and colleagues contains some simplifications. Long-term pair-bonding and paternal investment in humans mean that men are probably capable of expressing a “tending” response as well; it also means that male partners, and not just other females, can sometimes serve as effective providers of social support (but see Kirschbaum et al., 1995). Nevertheless, the model is consistent with women’s higher sensitivity to social rejection (see above) and stronger propensity to seek for social support in stressful situations (Belle, 1987; Tamres et al., 2002).
4.2. Information encoding and filtering
By modulating the organism’s response to environmental challenges, the SRS fulfills a major role in the transduction of information about the local environment. The view of neuroendocrine systems as involved in the transmission and integration of information is well established in neurobiology (e.g., Dufty et al., 2002; Ellison, 2009; Heyland et al., 2005). The SRS receives complex information about the external environment through limbic structures, and complex information about the organism from interaction with other neuroendocrine systems (e.g., the HPG axis6 and the immune system; see Herman et al., 2003). In the SRS, this information is integrated and translated into a physiological and behavioral response involving the whole organism. By binding to mineralocorticoid and glucocorticoid receptors, cortisol directly regulates gene expression in a wide range of tissues and organs (including the SRS itself), thus permitting the long-term encoding and storage of information about the frequency, type and severity of the challenges encountered by the organism. In other words, the SRS continuously “samples” the environment, and its pattern of activation over the years provides a statistical representation of key dimensions of the environment, which can then be used to orient the individual’s developing LH strategy. Crucially, different strategies may require different calibrations of the SRS itself; for example, a slow strategy in a safe environment could be optimally served by a responsive HPA axis and parasympathetic system, coupled with moderate sympathetic reactivity (section 6). SRS calibration can be expected to depend on the system’s previous history of activity (Adam et al., 2007), in interaction with factors such as the individual’s sex and developmental stage (Miller et al., 2007).
4.2.1. Environmental information
The amount of information encoded by each component of the SRS depends on the specificity of its response. Parasympathetic withdrawal occurs frequently and is a relatively non-specific response, so it comparatively conveys relatively little information about the local environment. Sympathetic activation, in contrast, is more specifically tied to challenges requiring fight-or-flight responses; patterns of SNS activation may thus provide reliable information about the dangerousness (or safety) of one’s environment. The most information-rich response (and the one with the longest lasting effects) is that of the HPA axis, which is strongly activated in unpredictable and/or uncontrollable situations, in those involving social evaluation, and in response to family conflict, rejection or separation. Secure attachment to parents buffers HPA activation (Ahnert et al., 2004; Nachmias et al., 1996), so that frequent, high-intensity cortisol elevation in the first years of life is a likely correlate of conflictual family dynamics, insecure attachment relations and insensitive parenting (Gunnar, 2005). Even if avoidant infants do not react with HPA activation to brief separations (section 4.1), they can still mount intense HPA responses to family conflict, punishment, or even longer separation episodes (as found by Ahnert et al., 2004).
In a life history perspective, extrinsic morbidity-mortality and unpredictability are two key dimensions of environmental variation (section 2). Extrinsic morbidity-mortality is conveyed both by frequent SNS activation (signaling a potentially dangerous ecology) and by repeated HPA activation. Extrinsic morbidity-mortality and environmental unpredictability have negative effects on family relationships and reduce both the quality and quantity of parental investment (Belsky et al., 1991; Chisholm, 1993, 1999; Ellis et al., 2009; Quinlan, 2007), thus indirectly leading to elevated cortisol in children. Because it responds strongly to uncontrollable challenges and novel situations, the HPA also encodes direct information about environmental unpredictability/uncontrollability, thus giving cortisol a central role in the regulation of LH strategies.
4.2.2. Responsivity and information filtering
If the SRS encodes environmental information as a statistical aggregation of repeated responses to challenge, it follows that SRS responsivity functions as an information filter. An unresponsive system has a higher threshold for letting environmental signals in: many potential challenges will not be encoded as such, and many potentially relevant events will fail to affect the organism’s physiology to a significant degree. Imagine an individual with an unresponsive HPA axis facing a socially evaluative event (e.g., public speaking). Note that it does not matter which specific mechanism is the cause of unresponsivity; it may be a high threshold for activation of the amygdala (e.g., Taylor et al., 2008), increased inhibitory activity in the prefrontal cortex (e.g., Kern et al., 2008; Pruessner et al., 2010), or an especially strong negative feedback loop at some level in the regulatory hierarchy. For this individual, public speaking will not entrain the chain of physiological and psychological effects that follow CRH and cortisol secretion. This will result in a number of potential costs (e.g., reduced alertness, reduced sensitivity to social feedback) as well as potential benefits (e.g., resource economization, avoidance of immune suppression). In fact, many of the possible consequences of a low-intensity HPA response can be read as either costs or benefits depending on context. Reduced sensitivity to social feedback, for example, can be optimal in highly competitive contexts, when taking deliberate risks, or when group non-conformity is the desired outcome. More generally, sometimes organisms do well to partially or totally shield themselves from the effects of environmental information.
A highly responsive SRS, by contrast, amplifies the signal coming from the environment and maximizes the chances that the organism will be modified by current experience. This, too, can have both costs and benefits. Potential costs of a highly responsive system include high physiological costs, hypersensitivity to social feedback, and exposure to psychological manipulation; in addition, the organism’s action plans can get easily interrupted by minor challenging events, and the ability to deal with future events may be reduced if physiological resources are already overwhelmed. On the other hand, a highly responsive system facilitates social learning, enhances mental activities in localized domains, focuses attention, and primes memory storage, thus improving cognitive processes for dealing with environmental opportunities and threats (Barsegyan et al., 2010; Flinn, 2006; Roozendaal, 2000; van Marle et al., 2009). Heightened sensitivity to social feedback can be advantageous in social contexts in which sharing in the emotions of another conveys social benefits and promotes bonding (Shirtcliff et al., 2009). In total, greater SRS responsivity generally increases “informational openness”; this is the basic insight of the theory of BSC (Boyce and Ellis, 2005; Ellis and Boyce, 2008) and a central building block of the model we develop here.
4.3. Regulation of life history-relevant traits
The biological function of the SRS extends beyond the immediate response to challenges and the encoding/filtering of environmental information. The SRS has a pervasive role in the regulation – and, most importantly, the integration – of physiology and behavior across the whole spectrum of human LH-relevant traits (Figure 1). Hundreds of studies document how individual differences in autonomic and HPA functioning are associated with individual differences in growth and maturation, learning, aggression and competitive risk-taking, attachment, and so on. Here we briefly summarize the most important findings on SRS functioning and individual differences in life-history related traits. Following LH theory we discuss traits in relation to two basic LH trade-offs: somatic versus reproductive effort and mating versus parenting (section 2.1).
4.3.1. Somatic effort: Growth and learning
The HPA is crucially involved in the regulation of metabolism, and chronic stress has been linked to individual differences in physical growth patterns (e.g., Hofer, 1984; Schanberg et al., 1984). Growth is largely regulated by hormones, including sex hormones like DHEA, testosterone and estrogen; moreover, release of growth hormones is integrally connected with the SRS, and growth hormones themselves are stress-responsive, especially in extremely stressful contexts (e.g., psychosocial dwarfism).
Physical growth is an important component of somatic effort but, from the biological point of view, learning can also be conceptualized as a form of investment in somatic capital (section 2.1). A learning organism spends time and energy accumulating knowledge and developing skills that may – or may not – become useful in the future. In formal models of human life history evolution, learning can be modeled as the accumulation of “embodied capital” with delayed returns (Kaplan et al., 2000). The SRS modulates learning in a number of different ways: HPA and autonomic profiles have been associated with individual differences in cognitive functioning (e.g., Staton et al., 2009), memory (e.g., Stark et al., 2006), and self-regulation/executive function (e.g., Blair et al., 2005; Shoal et al., 2003; Williams et al., 2009).
4.3.2. Reproductive effort: Maturation and fertility
The autonomic systems, HPA, and HPG axes are connected by extensive functional cross-talk (Ellis, 2004), and cortisol is a major regulator of fertility and sexual development. Given adequate bioenergetic resources to support growth and reproduction, exposures to psychosocial stressors—indexed by SRS activation and, over time, calibration during development—generally provoke early or accelerated development of the HPG axis but suppressed ovarian functioning in mature individuals (reviewed in Ellis, 2004). Thus, cues to extrinsic morbidity-mortality guide development toward LH trade-offs favoring an earlier pubertal transition from somatic to reproductive effort (e.g., Belsky et al., 1991; Ellis and Essex, 2007; Tither and Ellis, 2008) but future over current reproduction in adulthood, especially in females (e.g., Nakamura et al., 2008; Nepomnaschy et al., 2006). The effects of acute response to challenge, however, are much more variable; males and females do not respond in the same way, and whether acute stress suppresses or enhances fertility depends on individual characteristics such as dominance status (e.g., Chichinadze and Chichinadze, 2008; Korte et al., 2005; Tilbrook et al., 2000). Finally, cortisol responsivity has been linked to the age of first intercourse in women (Brody, 2002).
4.3.3. Mating effort: Intrasexual competition
Competition among members of the same sex is the inevitable outcome of sexual reproduction. Dominance-seeking, aggression, and risk-taking are all functionally connected to mating competition (Trivers, 1972), and all are associated with SRS functioning. There is a huge literature linking HPA and autonomic functioning to aggression, antisociality, and externalizing behavior (e.g., Alink et al., 2008; Boyce et al., 2001; Gunnar and Vazquez, 2006; Lorber, 2004; Shirtcliff et al., 2009; Shirtcliff and Essex, 2008; van Goozen et al., 2007). The widely adopted category of “externalizing behavior” in children and adolescents includes attention-seeking, defiance, bragging and boasting, disruption of (adult-imposed) discipline, teasing and threatening, in addition to physical aggression. In an evolutionary perspective, externalizing behaviors can be seen as high-risk tactics of social competition, and “disruptive behavior disorders” (conduct disorder and oppositional-defiant disorder) represent – at least in part – extreme manifestations of risky dominance-seeking (see also McIntyre and Hooven, 2009). Given the centrality of risk-taking and impulsivity in LH models of behavior (see section 2), it is noteworthy that HPA functioning has also been linked to risk-taking behavior in standardized laboratory tasks (e.g., Lighthall et al., 2009; van den Bos et al., 2009; van Honk, 2003). Moreover, executive function and self-regulation have a key role as (negative) mediators of risky and impulsive behavior (Figueredo and Jacobs, 2009). Stress exposure can also regulate mating behavior more directly by, for example, altering mate preferences and affecting the perceived attractiveness of potential sexual partners (e.g., Lass-Hennemann et al., 2010).
In the modulation of risky competition, the SRS interacts with sex hormones, serotonin (5-HT), and dopamine (DA). Studies of aggression and antisocial behavior often report interactions between cortisol, testosterone (T) and adrenal androgens such as DHEA and DHEAS (e.g., Popma et al., 2007; van Goozen et al., 2007). The general function of 5-HT is to regulate avoidance of threat, withdrawal from dangerous or aversive cues, and behavioral inhibition/restraint. Serotonergic activity is thus crucially involved in risk aversion and self-regulation (Cools et al., 2008; Deakin, 2003; Fairbanks, 2009; Tops et al., 2009). Serotonin is an upstream modulator of SRS activity through its action on the amygdala and hypothalamus; serotonergic neurotransmission, in turn, is reciprocally affected by cortisol (Porter et al., 2004; van Goozen et al., 2007). The effects of 5-HT on stress responsivity are complex: on the one hand, 5-HT up-regulates the HPA axis by promoting CRH secretion (Bagdy, 1992; Fuller, 1996); on the other hand, carrying low-transcription variants of the serotonin transoporter has been related to increased HPA responsivity (Gotlib et al., 2008; Mueller et al., 2010). In addition, 5-HT inhibits sympathetic activation via central 1A receptors (Nalivaiko and Sgoifo, 2009). Both 5-HT and sex hormones regulate LH-relevant traits in humans and other animals (e.g., Adkins-Regan, 2005; af Klinteberg et al., 2004; Fairbanks, 2009; Gray and Campbell, 2009; Hau, 2007; Knapp, 2004; Manuck et al., 2006; McGlothlin et al., 2007; Miczek and Fish, 2006; Worthman and Brown, 2005). Dopaminergic activity is also tightly linked to SRS functioning (Lexander et al., 2011); Gatze-Kopp (2010) has recently argued that reduced dopaminergic activity can be adaptive in highly dangerous and unstable environments (and especially so for males) by promoting sensation-seeking, risk-taking, and preference for immediate rewards.
4.3.4. Parenting effort: Pair bonding and caregiving
In humans, parenting effort is promoted by long-term bonding between romantic partners, which in turn is mediated by many of the same neurobiological mechanisms underlying parent-child attachment (Carter, 1998; Lancaster and Kaplan, 2009; Pedersen et al., 2005). Individual differences in SRS functioning have been associated with differences in romantic attachment styles (e.g., Diamond and Hicks, 2005; Quirin et al., 2008; Laurent and Powers, 2007; Oskis et al., 2010; Powers et al., 2006); in turn, romantic attachment predicts relationship stability, commitment and investment (reviewed in Del Giudice, 2009a). The key molecules that can be expected to interact with the SRS in the regulation of pair-bonding and parental investment are sex hormones, vasopressin, oxytocin, serotonin, and endogenous opioids (Fairbanks, 2009; Gray and Campbell, 2009). Oxytocin secretion, in particular, has been related to individual differences in romantic attachment styles (Chen et al., in preparation; Marazziti et al., 2006).
The SRS is also directly involved in the regulation of caregiving behavior. Stress responsivity in a parent can profoundly affect the way he/she responds to his/her child, especially in challenging situations and when the child’s behavior becomes especially demanding (for example following severe distress, illness, or prolonged separation). These are also the occasions in which parental behavior can have the strongest effects on the child’s emotional development and attachment security. Differences in SRS functioning (as well as in oxytocin- and serotonin-related genes) have been linked to individual differences in maternal sensitivity and parenting behavior (e.g., Bakermans-Kranenburg and van IJzendoorn, 2009; Martorell and Bugental, 2006).
In summary, the SRS not only collects and encodes crucial LH-relevant information but is also involved in the regulation of all the major trade-offs in human LH strategies. This is why we can employ LH theory as a guide to model the development of stress responsivity across the life course.
5. The Adaptive Calibration Model of stress responsivity
In this and the next section, we integrate the material presented so far into an evolutionary-developmental model of individual differences in stress responsivity. Here we summarize the key points of the model, review the underlying evolutionary logic, and describe four prototypical patterns of stress responsivity. In section 6 we present a detailed analysis of the four patterns, their behavioral correlates and their developmental trajectories.
Patterns I (sensitive), II (buffered) and III (vigilant) correspond to the extremes and midpoint of the distribution hypothesized in the original theory of BSC (Boyce and Ellis, 2005). We argue that pattern III (high responsivity in dangerous/unpredictable environments) actually comprises a distribution of subtypes, each with specific neurobiological and behavioral correlates; furthermore, pattern III subtypes are expected to develop in a sexually differentiated way. Finally, the male-typical pattern IV (unemotional)—a profile associated with antisocial and disruptive behavior, especially in males—was not described in the BSC theory and corresponds to low responsivity following severe/traumatic stress. These patterns are meant to describe relatively stable individual differences in SRS functioning, emerging over the course of years or even decades of development. That is, these patterns attempt to capture central tendencies in how different people characteristically respond to threats and opportunities in their environment. Although responsivity patterns are not fixed, and are sometimes expected to undergo substantial developmental change (see sections 5.4 and 6), major developmental transitions in responsivity should be distinguished as clearly as possible from temporary, reversible alterations in SRS functioning tied to specific life events. For example, highly responsive individuals (patterns I and III) may show an unresponsive phase after the termination of a prolonged stressor (section 4.1), but this alone does not amount to a shift to an unresponsive pattern (II or IV).
5.1. Summary of the model
The ACM can be summarized in seven points:
The SRS has three main biological functions: to coordinate the organism’s allostatic response to physical and psychosocial challenges; to encode and filter information from the environment, thus mediating the organism’s openness to environmental inputs; and to regulate a broad range of LH-relevant traits and behaviors.
The SRS works as a mechanism of conditional adaptation, regulating the development of alternative LH strategies. Different patterns of activation and responsivity in early development modulate differential susceptibility to environmental influence and shift susceptible children on alternative pathways, leading to individual differences in LH strategies and in the adaptive calibration of stress responsivity (Figure 1).
Activation of the SRS during the first years of life provides crucial information about LH-relevant dimensions of the child’s environment. Frequent, intense SNS/HPA activation carries information about extrinsic morbidity-mortality and environmental unpredictability; consequently, it tends to shift LH strategies toward the fast end of the LH continuum. In contrast, a safe environment and the buffer provided by investing, secure caregivers result in infrequent and low-intensity activation of the SNS and HPA axis, and shift development toward slow strategies oriented to high somatic effort and parental investment.
At a very general level, a nonlinear relationship exists between environmental stress during ontogenetic development and the optimal level of stress responsivity (Figure 2), as stipulated in the BSC theory. Note that the environment-responsivity relationship need not be the same for all the components of the SRS (see table 1 for detailed predictions for each of the main SRS components). Furthermore, stress responsivity is expected to show domain-specific effects; for example, a generally unresponsive component of the SRS may respond strongly to some particular type of challenge.
Because of sex differences in LH trade-offs and optimal strategies, sex differences are expected in the distribution of responsivity patterns and in their specific behavioral correlates. Sex differences should become more pronounced toward the fast end of the LH continuum; in environments characterized by severe/traumatic stress, we predict the emergence of a male-biased pattern of low responsivity.
Pre- and early postnatal development, the juvenile transition, and puberty are likely switch points for the calibration of stress responsivity. Individual and sex differences in SRS functioning are predicted to emerge according to the evolutionary function of each developmental stage.
Responsivity profiles develop under the joint effects of environmental and genetic factors. Genotypic variation may have directional effects on stress responsivity and associated LH strategies, thus predisposing some individuals to follow a certain developmental trajectory. Genotypic variation, in part through effects on the SRS, may also affect their sensitivity to environmental inputs, resulting in gene-environment interactions whereby some individuals display a broader range of possible developmental outcomes (i.e., broader “reaction norms”) than others.
Figure 2.
The Adaptive Calibration Model of individual differences in development of stress responsivity. At a very general level, a nonlinear relation exists between exposures to environmental stress and support during development and optimal levels of stress responsivity. Although this nonlinear relation is specified for the stress response system (SRS; see Table 1), it may apply to other neurobiological systems as well. The figure does not imply that all components of the SRS will show identical responsivity profiles, nor that they will activate at the same time or over the same time course. Male/female symbols indicate sex-typical patterns of responsivity, but substantial within-sex differences in responsivity are expected as well.
Table 1.
Predicted physiological profiles of the four responsivity patterns.
| Physiological profile | Responsivity patterns |
||||
|---|---|---|---|---|---|
| I Sensitive | II Buffered | III Vigilant | IV Unemotional | ||
| PNS | responsivity | High | Moderate | Low/moderate | Low* |
| basal | High | Moderate | Low | Low | |
| SNS | responsivity | High/moderate | Low/moderate | High | Low* |
| basal | Moderate | Low/moderate | High | Low | |
| HPA | responsivity | High | Moderate | High | Low |
| basal | Moderate | Moderate | High/moderate | Low | |
Unemotional individuals may display autonomic activation when faced with immediate physical threats and during agonistic confrontations, in contrast with their general pattern of unresponsivity to non-agonistic stressors.
5.2. Environmental stress and responsivity
The main tenet of the BSC theory is that optimal calibration of the stress response strongly depends on the characteristics of a child’s environment while growing up. In safe, protected, low-stress environments, a highly responsive SRS enhances social learning and engagement with the external world, allowing the child to benefit more fully from social resources and opportunities, thus favoring development of a sensitive phenotype (pattern I). A sensitive phenotype in this context may make children better at detecting positive opportunities and learning to capitalize on them (e.g., seeing a teacher as a prospective mentor, taking advice from a parent). Social learning and sensitivity to context are especially adaptive in the context of slow LH strategies, as a form of protracted somatic investment. It is important to note that in very safe and protected settings, sensitive individuals will rarely experience strong, sustained activation of the SNS and HPA systems; precisely because of the high quality of the environment, they will most likely experience a pattern of low-key, short-lived activations followed by quick recovery. Thus, the individual enjoys the benefits of responsivity without paying significant fitness costs (e.g., immune, energetic, and so on). At moderate levels of environmental stress, however, the cost/benefit balance begins to shift; the optimal level of HPA and SNS responsivity falls downward, leading to buffered phenotypes (pattern II).
The benefits of increased responsivity rise again when the environment is perceived as dangerous and/or unpredictable. A responsive SRS enhances the individual’s ability to react appropriately to dangers and threats while maintaining a high level of engagement with the social and physical environment. Moreover, engaging in fast LH strategies should lead the individual to allocate resources in a manner that discounts the long-term physiological costs of the stress response in favor of more immediate advantages. In this context, the benefits of successful defensive strategies outweigh the costs of frequent, sustained HPA and SNS activation, leading to vigilant phenotypes (pattern III). High HPA and SNS responsivity, however, can be associated with rather different behavioral patterns, leaning toward the “fight” (vigilant-agonistic, III-A) or “flight” (vigilant-withdrawn, III-W) side of the sympathetic response. Furthermore, evolutionary theory provides reasons to expect males and females to differ in the distribution of agonistic versus withdrawn patterns (see below). Increased SRS responsivity in dangerous environments can be expected to go together with increased responsivity in other neurobiological systems; for example, hyper-dopaminergic function may contribute to the vigilant phenotype by boosting attention to threat-related cues and fast associative learning (Gatze-Kopp, 2010).
What happens in extremely dangerous environments characterized by severe or traumatic stress? We argue that the balance shifts again toward low responsivity, especially for males who adopt a fast, mating-oriented LH strategy characterized by antagonistic competition and extreme risk-taking. Such a strategy requires outright insensitivity to threats, dangers, social feedback and the social context. For an extreme risk-taker, informational insulation from environmental signals of threat is an asset, not a weakness. In particular, adopting an exploitative/antisocial interpersonal style requires one to be shielded from social rejection, disapproval, and feelings of shame (all amplified by heightened HPA responsivity). In summary, an unemotional pattern of generalized low responsivity (pattern IV) can be evolutionarily adaptive (i.e., fitness-maximizing) at the high-risk end of the environmental spectrum, despite its possible negative consequences for the social group and for the individual’s subjective well-being. The same principle applies to other neurobiological systems involved in the regulation of risk-taking; for example, hypodopaminergic function is likely adaptive in severely stressful environments (Gatze-Kopp, 2010).
Figure 2 depicts the overall predicted relations between developmental context and stress responsivity, extending the original BSC curve to the right and showing the male-biased pattern of low responsivity in high-risk environments. In section 6 we further supplement this broad-band analysis with a more fine-grained description of the profiles of basal activity and responsivity of the various SRS components (see table 1 for details). It is important to stress again that, although in this paper we mainly restrict our attention to the SRS, similar environment-dependent curves may potentially be described for other neurobiological systems as well (e.g., the DA system [Gatze-Kopp, 2010], the OT and AVP systems, and so on).
Responsivity profiles respond adaptively to environmental conditions, but are also co-determined by genetic factors and genotype-by-environment (GxE) interactions (section 2.2). For reasons of space, in the present paper we focus our attention on the role of the environment, and only cursorily describe the most likely forms of GxE interactions that we expect to emerge in the development of responsivity patterns (section 6). Future treatments of the ACM will need to integrate the role of specific genes in more detail (including individual differences in reaction norms), as well as consider the possibility of epigenetic effects mediating intergenerational transmission of stress responsivity patterns (Champagne, 2010; Meaney, 2010).
5.2.1. Adaptation versus pathology
Whereas the ACM emphasizes adaptive calibration to a wide range of developmental conditions, not all developmental outcomes reflect evolutionary adaptation. Some rearing contexts may go beyond the range to which humans adapted during their evolutionary history, and the developmental response to such contexts may be better described as truly pathological; i.e., as a failure of an otherwise adaptive system. Maltreatment, neglect, abandonment and infanticide have been consistently evident across human evolution and across cultures, and parental violence and neglect are found in many other species as well (e.g., Daly and Wilson, 1984; Hrdy, 1999; McCormack et al., 2006; Mock, 2004; Sanchez et al., 2001). For this reason, and in contrast to a common view among developmentalists (e.g., Cicchetti and Lynch, 1995; Scarr, 1992), our model assumes that children have the evolved capacity to respond in biologically adaptive ways to harsh and unsupportive family environments, not only to loving and protective ones (see also Meaney, 2010).
Of course, a biologically adaptive response does not necessarily result in psychological well-being or socially valued outcomes. Indeed, the evolutionary meaning of “adaptive” is fundamentally different from the meaning often given to the term in the developmental and psychopathology literatures. Further, optimal adaptation (in the evolutionary sense) to challenging environments is not without real consequences and costs, as adaptive processes in this context often involve “making the best of a bad job.” Indeed, harsh environments can harm or kill children, and the fact that children can developmentally adapt to such rearing conditions does not imply that such conditions either promote child well being or should be accepted as inevitable facts of life. Relatedly, it is likely that some extreme and evolutionarily novel rearing conditions, such as orphanages and peer-reared “street children,” can push evolved mechanisms beyond their adaptive limits and lead to pathological developmental outcomes. Likewise, at least some genotypic variants that contribute to individual differences in responsivity may just be harmful mutations with no previous history of natural selection.
Another possible source of biologically maladaptive outcomes is phenotype-environment mismatch, occurring as a side effect of conditional adaptation (e.g., Gluckman et al., 2007). For example, a child exposed to stressful and dangerous conditions in the first years of life could develop a physiological and behavioral profile designed to deal with a competitive, dangerous world; however, the same phenotype would become maladaptive if the environment changed suddenly to a safe, low-stress one (e.g., because of adoption or other dramatic improvements in life conditions; see Rutter et al., [2004] for a discussion of developmental programming in post-institutionalized children). Even if the extended plasticity of LH development in humans (section 3.3) can be expected to reduce the likelihood of prolonged phenotype-environment mismatches, any predictive-adaptive mechanism is vulnerable to the effects of a rapidly changing environment.
5.2.2. Domain generality versus specificity
The SRS responds to a wide range of external and internal events (Figure 1), and in that sense can be described as a domain-general mechanism of response to the environment (Bugental, 2000; Nesse et al., 2007). In fact, a certain degree of domain generality is required by the broad integrative function served by the SRS in the regulation of life history trade-offs (section 4.3). At the same time, the individual components of the SRS respond in specific ways to different types of challenges (section 4), and the physiological profile of the stress response has been shown to change subtly with different types of stressors (e.g., Nair et al., 2007; Pacák and Palkovits, 2001; Thrivikraman et al., 2000). We therefore expect a mixture of domain-general and domain-specific effects in the development of responsivity patterns. Two instances of domain specificity in the present version of the model are (1) the prediction of increased sensitivity to social support in adolescent and adult females (section 5.4) and (2) the prediction of high autonomic responsivity to agonistic encounters in unemotional individuals (section 6.4). As future research will progress in mapping domain-specific effects in the SRS, the resulting knowledge could be usefully incorporated in the ACM to increase its descriptive and predictive power.
5.3. Sex differences
Because the costs and benefits associated with LH trade-offs are not the same for males and females, LH strategies show consistent differences between the sexes (section 2.1). On average, men engage in faster LH strategies and invest more in mating effort (and less in parenting effort) than women. The extent of sex differences in LH-related behavior, however, is not fixed but depends in part on the local environment.
At the slow end of the LH continuum, both sexes engage in high parental investment, and male and female interests largely converge on long-term, committed pair bonds; sex differences in behavior are thus expected to be relatively small. As environmental danger and unpredictability increase, males benefit by shifting to low-investment, high-mating strategies; females, however, do not have the same flexibility since they benefit much less from mating with multiple partners and incur higher fixed costs through childbearing. Thus, male and female strategies should increasingly diverge at moderate to high levels of environmental danger/unpredictability. Only in high-risk environments are females predicted to reduce their parental investment in a male-like way (Del Giudice, 2009a, 2009b); nevertheless, males can successfully engage in higher mating and lower parenting effort than females, even at the fast end of the LH continuum. In addition, sexual competition takes different forms in males and females, with males engaging in more physical aggression and substantially higher levels of risk-taking behavior (e.g., Archer, 2009; Byrnes et al., 1999; Kruger and Nesse, 2006; Wilson et al., 2002). As LH strategies become faster, sexual competition becomes stronger, and sex differences in competitive strategies become more apparent. For these reasons, sex differences in responsivity patterns and in the associated behavioral phenotypes should be relatively small at low to moderate levels of environmental stress (patterns I and II) and increase in stressful environments (pattern III). Finally, males should be over-represented as high-risk, low-investment LH strategists (pattern IV) because of the larger potential benefits they enjoy from extreme mating-oriented behavior.
5.4. Developmental stages and switch points
The human life history is organized as a sequence of stages and transitions (section 3). LH strategies unfold progressively, according to the evolutionary function of each life stage. The juvenile transition can be expected to be a critical turning point in the development of stress responsivity. Juvenility witnesses a dramatic increase in the sexual differentiation of behavior and physiology, and early forms of sexual selection are already at work in this life stage; thus, we predict that sex differences in the developmental trajectories of stress responsivity will become apparent starting from the beginning of middle childhood, with a further increase at puberty.
We also expect that individual changes in responsivity will be especially frequent in the transition from early to middle childhood. Early childhood affords an “evaluation” period in which the child can sample the environment – both directly and through the mediation of parents. With juvenility, however, stress responsivity becomes an integral component of the child’s emerging LH strategy; indeed, the SRS contributes to regulation of what are probably the main evolutionary functions of juvenility: learning, self-regulation, and peer competition (section 4.3). For this reason, it may be adaptive for some children to adjust their levels of responsivity when transitioning from early to middle childhood, possibly under the effect of adrenal androgens. For example, a highly responsive boy living in a severely traumatic environment may show a marked decrease in responsivity when transitioning to middle childhood. Such a decrease in SRS responsivity would reduce the boy’s sensitivity to social feedback, increase risk-taking, and promote disruptive, exploitative patterns of social behavior. Despite the obvious personal and social costs, this outcome can be seen as an evolutionarily adaptive “emergency” strategy, one that promotes individual fitness (or has done so in past environments) in the face of threatening environmental conditions.
With the onset of puberty, sexual behavior and romantic attachment come to the forefront, and social competition further intensifies. Puberty affords another opportunity to revise one’s LH strategy, depending for example on the success enjoyed – or the level of competition experienced – during juvenility. The activation of sex hormone pathways also provides a possible source of novel genetic effects on LH-related behavior. Thus, individual and sex-related changes in stress responsivity are expected to take place in adolescence as well.
5.4.1. Social support and fertility in females
In females, stress has a powerful regulatory effect on fertility, a pivotal component of female LH strategies (section 4.3). We predict that adolescent females should exhibit increased stress responsivity to cues of reduced social support, a key determinant of successful reproduction (Hrdy, 2009; Wasser, 1994; Wasser and Barash, 1983; see also Troisi, 2001). The function of increased responsivity would be to allow females to track the fluctuations of the social and physical environment and to suppress fertility when conditions are unsupportive (e.g., because of a sudden decrease in social support or partner loss). Basal cortisol often increases in adolescent girls (reviewed in Adam et al., 2007), and cortisol in adolescent girls is more state-dependent (i.e., context-sensitive) than in same-age boys (Booth et al., 2008; Shirtcliff et al., 2005). If our prediction was supported, we would expect the oxytocin system to be involved in the context-specific modulation of the stress response (section 4.1 and 4.3).
Following the logic of LH theory, we also predict that responsivity to loss of social support should be weaker in women engaging in fast LH strategies; when social resources become scarce, it only makes sense to delay reproduction if (1) the social context can be reasonably expected to improve (Wasser and Barash, 1983), (2) the delay in reproduction can be repaid by an increase in offspring quality, and (3) extrinsic morbidity-mortality is not too high. By contrast, fertility can be expected to become relatively decoupled from social support in women engaging in fast, low-investment LH strategies. This may be achieved by reduced sensitivity to social support or by functional decoupling between SRS activation and fertility. This kind of strategy-specific effect has been recently documented in side-blotched lizards: administration of corticosterone (the analogue of cortisol) suppresses reproduction in females with a slow LH phenotype, but increases the reproductive rate of females with a fast LH phenotype (Lancaster et al., 2007).
6. Patterns of responsivity
In this section we provide a detailed description of the four patterns of responsivity arising in the ACM. Each pattern is characterized by a physiological profile of SRS responsivity and basal activity, the behavioral correlates of that profile, and one or more hypothetical developmental trajectories. Organizing the description of individual differences in responsivity around a limited number of patterns is an effective expository device and helps derive clear-cut, falsifiable empirical predictions. It should be stressed, however, that the ACM is a dimensional model rather than a typological one; accordingly, many individuals are expected to show responsivity patterns intermediate between the “pure” prototypes (Figure 2). Furthermore, the present classification is not intended to be exhaustive. Knowledge about individual differences in stress responsivity is still limited, and it is likely that additional pathways and patterns will be described in the future.
6.1. Sensitive pattern (I)
6.1.1. Physiological profile
The logic of sensitive phenotypes is that responsivity increases the organism’s openness to the social and physical environment. In our model, sensitive phenotypes are characterized by high PNS responsivity and basal activation (e.g., vagal tone), favoring sustained but flexible attention and engagement with the external environment. In contrast, SNS responsivity and (especially) basal activation are predicted to be in the moderate range: high trait anxiety (associated with high SNS baseline) would interfere with learning and exploration rather than promoting them, and an oversensitive fight/flight response is not needed in a safe, protected environment. Thus, the autonomic profile of pattern I is expected to be relatively dominated by the activity of the parasympathetic system. HPA responsivity is expected to be high, with moderate basal activity; a highly responsive HPA promotes sensitivity to social feedback and the mobilization of metabolic and psychological resources (Table 1). High HPA responsivity also allows the organism to adjust rapidly to temporary perturbations in the environment, for example by suppressing fertility following reductions in social support.
6.1.2. Behavioral and life history correlates
The sensitive phenotype has been described extensively in the BSC literature (see Boyce and Ellis, 2005; Ellis et al., 2006; Ellis and Boyce, 2008). Sensitive children and adults are reflective, self- and other-conscious, and engaged with the environment; they are also high in inhibitory control, executive function, and delay of gratification. Collectively, these traits promote sustained learning (a form of somatic investment) and social cooperation, hallmarks of a slow LH strategy; accordingly, we predict that sensitive phenotypes will be associated with slow physical and sexual maturation (Ellis, 2004). Because of the overall tendency of males to engage in faster LH strategies than females, small sex differences can be expected in the distribution of patterns I and II, with females somewhat over-represented in pattern I and under-represented in pattern II.
High parasympathetic baseline and responsivity have been linked to socio-emotional competence, engagement, and self-regulation (e.g., Beauchaine, 2001; Calkins, 1997; Fabes and Eisenberg, 1997; Stifter and Corey, 2001). High PNS baseline also predicts measures of cognitive efficiency and working memory (Staton et al., 2009). Cortisol responsivity has been related to executive function and self-regulation in preschoolers (Blair et al., 2005) and task engagement and sensitivity to feedback in adults (Tops et al., 2006a). Associations between HPA responsivity and social competence are found in some (but not all) studies of children (see Doussard-Roosvelt et al., 2003). From the vantage point of our model, contradictory associations between HPA responsivity and social competence may be explained by the existence of functionally differentiated patterns with similar levels of responsivity (e.g., cortisol responsivity alone does not discriminate well between pattern I and III). Intriguing evidence in this respect comes from a study by Legendre and Trudel (1996), who found strong cortisol responses to peer group entry both in shy/withdrawn children and in the most outgoing, extraverted ones (see also Gunnar et al., 1997). A relevant study in another species is the experiment by Macrì and colleagues (2007): as adults, mouse pups raised in protected, low-stress conditions showed both high HPA responsivity and the highest levels of novelty-seeking behavior, a clear indicator of openness to environmental stimulation.
Sensitive phenotypes may hold the solution to a long-standing paradox in psychological research: that of the “repressive coping style”. It has been observed that some individuals display high responsivity to social stressors while at the same time reporting low levels of trait anxiety. They also tend to score high on scales purportedly assessing socially desirable response styles (specifically “impression management”; Weinberger et al., 1979). This response profile has been labeled repressive, based on the assumption that these individuals are repressing the experience of anxiety out of defensive self-presentation motives. However, subsequent research has shown that “repressive” individuals do in fact experience lower levels of negative emotion, and – contrary to predictions – do not incur increased health risks (reviewed in Uziel, 2010). Instead, the evidence indicates that “repressors” actively respond to social evaluation with SRS activation and an increased sense of challenge (Derakshan and Eysenck, 1997; Newton and Contrada, 1992), which in our model can reflect heightened sensitivity to social feedback. Moreover, there is overwhelming evidence that impression management scales do not tap defensiveness but a very different construct, which has been recently labeled interpersonally oriented self-control (IOSC). IOSC predicts increased prosocial orientation, optimism, and emotional stability, higher social skills and frustration tolerance, and lower risk-taking (Uziel, 2010). Most intriguingly in a life history perspective, IOSC also predicts increased marital stability and lower levels of intimate violence (Bradburn et al., 1979; Sugarman and Hotaling, 1997). It is likely that many “repressors” identified in this research tradition are in fact type I individuals, who display high stress responsivity and low anxiety and whose behavioral profiles reflect slow, cooperative, low-risk LH strategies.
6.1.3. Development
What are the most likely developmental pathways leading to sensitive responsivity patterns? First, infrequent SNS and/or HPA activation in the first years of life could up-regulate the SRS, leading to increasing responsivity in those children with sufficiently wide reaction norms. Second, at least some children are already born with a highly responsive profile (because of genetic predisposition and/or prenatal effects of maternal hormones); a protected environment would then maintain responsivity, whereas moderate stress would down-regulate the SRS during the first years of life. Consistent with this view, a large study by Blair et al. (2006) found that high maternal sensitivity is associated with higher cortisol responsivity in 6-months-old infants. A relation between positive early family relations and cortisol responsivity in adults was also found by Luecken et al. (2009). Further, in a longitudinal study of bereaved children by Hagan et al. (2010), elevated basal cortisol was found in adolescents who had experienced unsupportive parenting and high levels of concurrent stress, but also in those who had enjoyed highly supportive parenting and low stress levels. Finally, some children may have a narrow reaction norm, so that they almost always develop highly responsive phenotypes (sensitive or vigilant, depending on the level of stress experienced).
Highly responsive infants and young children may be emotionally labile and characterized by difficult or inhibited temperament; as they grow up in a protected environment, however, they are expected to become more emotionally stable, low-anxious, and socially confident, possibly even more so than their temperamentally stable peers (Belsky, 2005; Belsky and Pluess, 2009). At the physiological level, this shift is likely to be marked by increased parasympathetic tone and responsivity (e.g., Propper et al., 2008). It is important not to confuse a protected, safe environment (characterized by secure attachment relationships) with overprotective, intrusive, and anxious parenting. The latter is associated with insecure attachment patterns, and has been shown to promote persistent inhibition/withdrawal and low vagal tone/responsivity in children (Rubin, 2002), a physiological profile consistent with vigilant patterns (III). The developmental trajectory from difficult temperament to openness and stability is expected to consolidate in middle childhood and adolescence if the social context remains protective, and if new genetic influences do not emerge during the juvenile transition or puberty.
6.2. Buffered pattern (II)
6.2.1. Physiological profile
Buffered phenotypes are predicted to develop preferentially in conditions of moderate environmental stress, where they strike a balance between the costs and benefits of responsivity. The responsivity profile of pattern II is in the low-to-moderate range, and should not be confused with the extreme, generalized unresponsivity of pattern IV (unemotional). We predict that the autonomic balance should be tilted toward PNS activity, reflecting the lower costs associated with parasympathetic response and the benefits of an active engagement with the social environment (Table 1).
6.2.2. Behavioral and life history correlates
In our model, buffered phenotypes (like sensitive ones) lie in the slow range of the LH spectrum. We do not expect large sex differences in the overall frequency of this pattern; still, buffered females are expected to show domain-specific responsivity to cues of reduced social support starting from adolescence. Compared with fast LH strategists (patterns III and IV), buffered individuals are predicted to be lower in anxiety and aggression, less risk-prone, more sensitive to social feedback and more oriented to long-term couple relationships. Buffered responsivity can thus look like a “protective factor”, especially when the study sample does not include many sensitive individuals. This is the essence of the stress inoculation hypothesis (Garmezy, 1991; Rutter, 1993). Indeed, most current approaches to stress development imply an “optimal” responsivity profile very close to the buffered pattern, with higher or lower responsivity usually considered as “dysfunctional” outcomes.
6.2.3. Development
Buffered responsivity is predicted to arise primarily through moderate, repeated SRS activation during the first years of life. Moderate stress would then down-regulate (or fail to up-regulate) responsivity in children with a potentially responsive phenotype. On the other hand, genetic and/or epigenetic factors may narrow the reaction norm of some infants, leading to the development of buffered phenotypes over most of the range of possible environments. Indeed, an infant born with a moderately unresponsive SRS would partially filter out the cues of danger/unpredictability even in a dangerous and unpredictable environment. “Buffering” genetic variants would therefore tend to shift development toward slow LH strategies, irrespective of the local environment. Conversely, buffering genetic variants should impede development of a potentially costly slow-LH strategy, instantiated through pattern I (sensitive), in response to highly supportive environments. As a result, we predict that buffered phenotypes – though more prevalent at moderate levels of stress – will be distributed widely across environmental conditions; in contrast, sensitive phenotypes are expected to cluster tightly in protective, low-stress developmental contexts.
6.3. Vigilant pattern (III)
6.3.1. Physiological profile
Broadly stated, vigilant patterns develop in stressful contexts, where they enable people to cope effectively with dangers and threats in the physical and social environment. They are characterized by high responsivity and basal activity in both the HPA and the sympathetic system, and by low parasympathetic tone and responsivity, especially as concerns the myelinated vagus (Table 1). This SNS-dominated physiological profile mediates heightened attention to threats and high trait anxiety (which is adaptive in dangerous contexts; see also Porges, 2005). In response to severe challenges, however, vigilant individuals may also display “freezing” PNS responses mediated by the dorsal vagal complex (Porges, 1995, 2007). Compared with highly responsive individuals with a sensitive phenotype (pattern I), vigilant individuals may show slower HPA recovery (i.e., they may take longer to return to baseline after HPA activation) and slower habituation (see Gunnar and Vazquez, 2006; Netter, 2004; Pruessner et al., 1997). For this reason, they are also likely to show stronger hypocortisolism following prolonged stress (section 4.1).
Within this general responsivity profile, we predict the existence of a distribution of behavioral patterns, whose extremes are represented by two sex-typical subtypes: a vigilant-agonistic pattern (III-A), typical of males, in which “fight” responses predominate; and a vigilant-withdrawn pattern (III-W), typical of females, characterized by protective “flight” behavior. The III-A and III-W subtypes are similar in their overall profile of SRS responsivity; both should involve lower serotonergic activity than patterns I and II (Carver et al., 2008; Tops et al., 2010); however, they can be expected to differ in a number of functionally related physiological parameters. Agonistic patterns should be associated with a high NE/E ratio and higher levels of androgens such as testosterone and DHEA/DHEAS (van Goozen et al., 2007). Withdrawn patterns, in contrast, should be characterized by a lower NE/E ratio, lower androgen levels, and – at least in females – higher levels of oxytocin, as predicted by the tend-and-befriend hypothesis.
6.3.2. Behavioral and life history correlates
In our model, vigilant responsivity patterns reflect fast LH strategies characterized by early maturation, low investment in somatic effort, low levels of cooperation, and – especially in males –increased mating effort and risk-taking. Psychological resources are employed to monitor (and cope with) possible sources of threat and/or social competition, rather than to maximize learning and relaxed exploration as in the sensitive pattern. In males, we expect vigilant phenotypes to be associated more often with increased risk-taking, impulsivity, agonistic social competition and aggression (the agonistic subtype; III-A). In females, the typical pattern should involve social anxiety, lower risk-taking and impulsivity, and fearful/withdrawn behavior (the withdrawn subtype; III-W). Consistent with the tend-and-befriend theory, vigilant females should be highly sensitive to social support and especially distressed by cues of rejection; these effects may be partly mediated by oxytocinergic activity. The vigilant-agonistic pattern overlaps with the hostility/anger dimension of the “type A” personality profile, which is characterized by high SNS responsivity, low vagal responsivity, and increased cardiovascular risk (e.g., Muranaka et al., 1988; Williams et al., 1982;). In contrast, the vigilant-withdrawn pattern may overlap with the anxious, socially inhibited “type D” personality profile (Denollet et al., 1996). Both profiles are related to increased risk of cardiovascular disease (see Denollet, 2000; Pedersen and Denollet, 2003; Williams, 1987; Yan et al., 2003).
A number of studies have found positive relationships between aggressive/externalizing behavior and HPA and/or SNS responsivity, especially in non-clinical groups, in preschoolers, and when aggressive/disruptive behaviors coexists with high levels of anxiety (see Bauer et al., 2002; Gunnar and Vazquez, 2006; Lorber, 2004; Netter, 2004; Shirtcliff et al., 2009; van Goozen et al., 2000, 2007). At the same time, many studies report associations between high HPA and/or SNS responsivity and fearful, anxious, or internalizing behavior (see Adam et al., 2007; Boyce et al., 2001; Flinn et al., 1996; Gunnar et al., 2009c; Gunnar and Vazquez, 2006; Li et al., 2007). There are large and robust sex differences in the prevalence of externalizing versus internalizing behaviors, with males higher in externalizing and lower in internalizing compared to females (e.g., Boyce et al., 2001; Crijnen et al., 1997; Leadbeater et al., 1999; Shirtcliff and Essex, 2008). Both externalizing and internalizing behaviors correlate with precocious sexuality in juveniles (Lévesque et al., 2010; van Goozen et al., 2002), which is to be expected if they are manifestations of fast, mating-oriented LH strategies.
Consistent with our hypothesis, impulsivity is high in externalizers but low in internalizers, whereas both types of behavior are associated with lower self-regulation and increased irritability (Baldwin and Dadds, 2008; Eisenberg et al., 2003; Oldehinkel et al., 2004). Serotonin is a key modulator of impulsivity and risk-taking and is implicated in the regulation of stress responsivity; moreover, serotonergic activity interacts with sex hormones at various levels (see Amin et al., 2005; Fink et al., 1999; Rubinow et al., 1998). Through their interaction with 5-HT, sex hormones can contribute to the sexually differentiated distribution of agonistic and withdrawn patterns; for example, a sex-specific association between the 5-HT transporter polymorphism and aggression has been reported by Verona and colleagues (2006).
The kind of aggression displayed by pattern III individuals is expected to be mostly reactive, that is, enacted in response to threats or social confrontation (e.g., aggression may follow from a challenge to the individual’s status or dominance). Reactive, emotionally charged aggression is down-regulated by 5-HT and potentiated by cortisol; by contrast, proactive (instrumental, cold-blooded) aggression appears to be unrelated to serotonergic function and more typical of low-cortisol phenotypes such as the unemotional pattern IV (Haller and Kruk, 2006; Haller et al., 2004, 2005; Hawes et al., 2009; van Bokhoven et al., 2005; van Goozen et al., 2007; see below).
In the ACM, high HPA/SNS responsivity in pattern III is not associated with a single behavioral pattern, but rather with a distribution of patterns involving different mixtures of aggressive/externalizing and withdrawn/internalizing behaviors. Moreover, there is a high-responsivity subgroup (pattern I) characterized by low fearfulness and low aggression. We believe that such a complex, nonlinear relation between responsivity and behavior may explain the many inconsistent findings in the literature and the very small overall correlations found in meta-analysis (Alink et al., 2008). The fact that internalizing and externalizing behaviors are both associated with high responsivity in pattern III may contribute to the observed positive correlation between the two (e.g., Keiley et al., 2003; Oldehinkel et al., 2004; Shirtcliff and Essex, 2008); the progressive sexual differentiation of agonistic versus withdrawn patterns may explain why this correlation seems to decrease over the course of adolescence (Scaramella et al., 1999). Vigilant children who display high levels of both agonistic and withdrawn behaviors (Rogosch and Cicchetti, 1994) may be best described as belonging to a third subtype, the vigilant-agonistic/withdrawn pattern (III-A/W).
6.3.3. Development
We expect vigilant phenotypes to develop primarily in temperamentally difficult/inhibited infants who grow up in stressful rearing contexts. Frequent stress experienced in early life may up-regulate (or fail to down-regulate) SRS responsivity; however, some highly responsive children with narrow reaction norms may develop a pattern III profile even in conditions that would prompt the development of a buffered pattern II in most of their peers. Intrusive, anxious and over-protective parenting (which signals to the child a dangerous, unpredictable environment) seems to be especially conducive to the development of vigilant phenotypes in temperamentally labile infants (Rubin, 2002; see also Calkins et al., 1998).
There are two main reasons to predict that the juvenile transition will be a crucial turning point in the development of vigilant patterns. First, sex differences in the distribution of agonistic versus withdrawn patterns are expected to emerge consistently only in middle childhood (under the influence of adrenal androgens) and further intensify in adolescence. Second, some children (especially males) who grow up under conditions of severe stress may display a highly responsive profile in early childhood, then shift to low responsivity as social competition becomes a central developmental task. Thus, we expect a number of individuals – likely the most aggressive ones – to display a transition from pattern III-A to pattern IV during juvenility or adolescence. This may help to explain a puzzling finding in the literature: that the overall association between basal cortisol and aggressive/externalizing behavior tends to be positive in preschoolers but negative in middle childhood and adulthood (Alink et al., 2008; Shirtcliff et al., 2005, 2009).
6.4. Unemotional pattern (IV)
6.4.1. Physiological profile
The last pattern we discuss is the unemotional one, characterized by low responsivity and basal activity in all the components of the SRS (Table 1). Generalized unresponsivity inhibits social learning and sensitivity to social feedback; it can also increase risk-taking by blocking information about dangers and threats in the environments. Based on LH theory, the distribution of pattern IV is expected to be male-biased; in addition, the behavioral correlates of low stress responsivity seem to depend on the action of sex hormones and may differ between the sexes (see below). Low serotonergic and dopaminergic activity are also expected to be associated with unresponsive phenotypes, especially when associated with aggression and risk-taking (Gatze-Kopp, 2010; Moore et al., 2002; Susman, 2006).
Whereas low responsivity can be adaptive in severely stressful contexts, there is no reason to expect that unemotional individuals will always show a blunted response to challenge. More specifically, we anticipate that pattern IV individuals (especially males) will be unresponsive to performance-related stressors and social evaluation, but will respond with autonomic activation when facing immediate physical threats and during agonistic encounters (e.g., physical confrontations or dominance conflicts). The SNS response is partly an adaptation to fight, and it would make little biological sense to suppress it precisely when it is most needed. At the same time, the aggression exhibited by unemotional individuals is often of the instrumental/proactive, cold-blooded type (Haller and Kruk, 2006; Haller et al., 2004, 2005; van Goozen et al., 2007). Maintaining calm and vigilance during aggressive confrontation is likely to involve parasympathetic upregulation, preceding or complementing SNS activation. Putting a parasympathetic “brake” on sympathetic activation when threatened can also work as a signaling handicap (see Zahavi and Zahavi, 1997); by showing that he/she is not preparing for immediate physical response, an individual sends opponents a credible signal that he/she is not scared, and is ready to withstand an attack. In summary, we predict that the autonomic pattern of unemotional individuals facing agonistic threats will be complex, possibly involving SNS/PNS coactivation and a delayed “fight-or-flight” response. So far, the results of human studies employing agonistic stressors are consistent with our prediction7. Using a peer provocation paradigm, Katz (2007) found that conduct-disordered children coming from violent families showed vagal augmentation (rather than suppression) during anticipation of conflict with another child. In a study by Waschbusch et al. (2002), the initial autonomic response to provocation was similar in controls and in boys with conduct disorders; however, conduct-disordered boys showed reduced heart rate recovery, which could result from increased vagal activation. Finally, inspection of the data collected by van Goozen and colleagues (2000) reveals that, during a peer provocation task, youth with disruptive disorders and low autonomic baseline displayed an increase in sympathetic activity at least as large as that of controls (p. 1443).
6.4.2. Behavioral and life history correlates
Unemotional patterns lie at the fast end of the LH spectrum and can be expected to show a male-biased distribution and an association with early sexual maturation and behavior. In males, extreme fast strategies are characterized by low empathy and cooperation, impulsivity, competitive risk-taking, and a higher likelihood of antisocial behavior. Low basal activity and responsivity in both the autonomic and HPA systems are consistently associated with disruptive, antisocial, and externalizing behavior (e.g., Beauchaine et al., 2007; Boyce et al., 2001; Lorber, 2004; O’Neal et al., 2010; Shirtcliff et al., 2005, 2009). In particular, low stress responsivity seems to be specifically related to callous-unemotional traits (e.g., lack of empathy and guilt, manipulativeness, emotional constrictedness), which are a core feature of psychopathic and antisocial personalities (Enebrink et al., 2005; Frick et al., 2000, 2003; Hawes et al., 2009; Shirtcliff et al., 2009). Despite the clear overlap between the unemotional pattern and psychopathic traits, it should be stressed that the two constructs are not equivalent. Different dimensions of psychopathy have different physiological correlates, and some individuals high in psychopathic traits do not display generalized unresponsivity to stress, nor high levels of impulsivity or antisocial behavior (see Hall and Benning, 2006; Fowles and Dindo, 2006).
The specific behavioral correlates of low stress responsivity seem to depend at least in part on the action of sex hormones. In several studies of adults and juveniles, severe antisocial/disruptive behavior is associated with a profile of low cortisol coupled with high levels of androgens (testosterone, DHEA or DHEAS; see Popma et al., 2007; van Goozen et al., 2007). Androgens may also mediate the male-specific association found between low HPA activity/responsivity, psychopathic traits, and externalizing behavior (O’Leary et al., 2007; Shirtcliff et al., 2005; but see Pajer et al., 2001). At the fast extreme of the LH continuum, male and female strategies are expected to diverge; indeed, the typical behavioral profile of unemotional females may primarily involve reduced cooperation and parental investment rather than high-risk competition of the externalizing kind. Booth and colleagues (2008) found that, during adolescence, low basal cortisol in females (but not in males) was associated with a generalized pattern of aloof social relationships with parents, siblings and peers. Given that social resources are a key determinant of women’s ability to invest in children (Hrdy, 2009), detached social/kin relationships may indicate reduced parental investment. Low basal cortisol and psychopathic traits in females also predict high levels of relational aggression (Marsee et al., 2005; Murray-Close et al., 2008); indeed, this may be the preferred type of aggression in unemotional females. In turn, both low HPA responsivity and relational aggression toward peers in adolescence are associated with earlier age at first intercourse, a key indicator of fast LH strategy (Brody, 2002; White et al., 2010). In view of these data, we expect unemotional females to display low stress responsivity even to cues of reduced social support. Insensitivity to social support would decouple fertility from the perceived availability of social resources, as required by a low-investment LH strategy adapted to high-risk environments.
6.4.3. Development
We hypothesize two main developmental pathways leading to unemotional responsivity patterns. In the first pathway, an initially responsive phenotype shifts toward unresponsivity following chronic severe stress (Gunnar and Vazquez, 2006; Gustafsson et al., 2010; Tarullo and Gunnar, 2006). The shift to pattern IV should often take place during juvenility or adolescence (see section 6.3); indeed, the typical age of onset of disruptive behavior disorders is between 8 and 15 years (Kessler et al., 2005). In the second pathway, unresponsivity may develop even in low-stress environments because of strong genetic predispositions, and may be apparent already in early childhood (e.g., Hawes et al., 2009; Raine et al., 1997). The difference between these two pathways may have crucial implications for the long-term development of stress responsivity: unemotional individuals coming from a history of high responsivity may revert to higher responsivity levels if environmental conditions improve for a sufficiently long time, and/or if their high-risk behavioral strategies turns out to be successful (e.g., if they are able to acquire social status and mating success).
The importance of wide versus narrow reaction norms in the development of unemotional patterns and their behavioral correlates is well illustrated by a recent experimental study of children at risk for antisocial behavior (O’Neal et al., 2010). The effectiveness of an aggression-reducing intervention was largely mediated by changes in cortisol responsivity: the children who became more responsive to social stress following intervention also became less aggressive, whereas children whose HPA axis remained unresponsive (possibly because of their narrow reaction norms) continued to show high levels of aggression.
7. Conclusions
In this paper we presented an evolutionary-developmental theory of individual differences in stress responsivity. The ACM provides a framework for research on stress and development, one that is explicitly built on the foundation of modern evolutionary biology. The ACM reorganizes a substantial amount of empirical findings from different research fields, weaving them together in a theoretically coherent fashion; even more importantly, it makes many novel and testable predictions about behavior, development, and neurobiology. The stress literature has contributed a wealth of insight into the mechanics of how SRS responsivity is elicited, yet the same research seldom asks why or for what purpose; evolutionary theory allows researchers to formulate these questions. As a result, research can move beyond purely inductive theory-building, dramatically increasing the researchers’ ability to make targeted hypotheses about individual differences and their development.
Perhaps the single most important implication of the ACM for empirical research is that the relationships between developmental factors, stress responsivity, and behavior are expected to be complex and intrinsically non-linear. This aspect was already present in the original BSC theory, and the ACM further reinforces it. If one considers the environment-responsivity curves shown in Figure 2, it is apparent that the results of any single study looking at linear statistical relationships can range from positive to null to negative, depending on the portion of the curve sampled in each case (Boyce and Ellis, 2005; Ellis et al., 2005). The many inconsistent results in the stress literature may depend, at least in part, on the failure to consider nonlinear relationships between environmental factors and SRS parameters, or the failure to assess the full range of environmental variance necessary to capture all four patterns of responsivity specified by the ACM. Moreover, the behavioral correlates of a single physiological parameter (for example high HPA responsivity) are predicted to vary widely depending on an individual’s developmental history, age, and sex, as explored in detail in section 6. The good news is that the complexity we envision in the ACM is lawful and predictable: although the development of stress responsivity is a highly multifactorial process, evolutionary theory makes it possible to “carve nature at its joints” and advance principled hypotheses about the most critical factors, their effects, and their interaction.
7.1. The ACM in context
How does our model relate to other theories of stress responsivity? First, the ACM incorporates the key elements of Porges’ polyvagal theory of autonomic functioning (Porges, 1995, 2007). Like the ACM, the polyvagal theory recognizes that different autonomic profiles can be adaptive in different environmental contexts, thus implying the concept of conditional adaptation (e.g., Porges, 2005, 2007). Two other models that we see as fully consistent with the ACM – and whose insights we drew on in this paper – are the neurobiological model of antisocial behavior by van Goozen and colleagues (2007) and the tend-and-befriend theory by Taylor and colleagues (2000). Similarly, the ACM employs the concept of allostasis (McEwen and Wingfield, 2003); across development, allostatic processes help canalize the individual along their developmental trajectory based on information encoding and filtering.
Finally, the ACM shares some features with the evolutionary hawk-dove model by Korte and colleagues (2005). These authors argue that, in many animal species, it is possible to describe two distinct behavioral phenotypes: aggressive, bold, proactive “hawks” and non-aggressive, cautious, reactive “doves”. These phenotypes have different costs and benefits and are biologically adaptive in different environmental contexts. The physiological profile of hawks includes high testosterone, low HPA and PNS responsivity coupled with high SNS responsivity, and low serotonergic activity, whereas doves show the opposite pattern. We believe that the ACM provides a better fit to humans by describing four responsivity patterns instead of two; however, it is possible to draw some parallels between the two models. The “dove” phenotype is similar to patterns I and III (especially III-W), whereas the “hawk” phenotype shares features with both pattern IV and pattern III-A. There is no equivalent of pattern II in the hawk-dove model. Another difference is that the hawk-dove model conceptualizes the alternative phenotypes as maintained by frequency-dependent selection (i.e., adaptive genetic variation) and does not consider conditional adaptation.
In summary, building on the key features of extant models the ACM aspires to become a truly integrative framework for the study of stress responsivity. In our opinion, what the field really needs is more fundamental theory, not a multitude of alternative micro-models without a common frame of reference.
7.2. Current limitations and future directions
In spite of its broad scope, the ACM is far from comprehensive or complete. The model still lacks a detailed account of genetic and epigenetic effects and GxE interactions (see Champagne, 2010; Meaney, 2010), and further work remains to be done before achieving full integration with the theory of differential susceptibility (for discussion, see Ellis et al., 2011a). Another current limitation is the relative neglect of domain-specificity (section 5.2); although the ACM is a refinement over the BSC in this respect, it is likely that, as research progresses, the model will need to accommodate many more domain-specific effects than those described so far. Third, the ACM adopts a simplified definition of responsivity as the magnitude of the response to challenge. Responsivity, however, can be measured on several possible dimensions; for example, Boyce and colleagues (1995a) proposed peak intensity, between-task variability, and attenuation over time in addition to classical difference measures. Such a fine-grained analysis may help formulate even more specific functional hypotheses and possibly reveal important differences between apparently similar patterns of responsivity. Finally, the present version of the ACM focuses on developmental trajectories and does not explicitly deal with the occurrence of traumatic stress in adults or the etiology of post-traumatic stress disorder (PTSD).
Thanks to the common framework of LH theory, the ACM has the potential to be easily connected with other evolutionary models of individual differences. For example, Figueredo and colleagues (2006) formulated some intriguing hypotheses about the relation between individual differences in LH strategies and the development of specific brain regions including the amygdala, the prefrontal cortex, and the hippocampus. This is especially interesting in view of the role played in the regulation of the SRS by these structures and other functionally related regions, such as the insula and the anterior cingulate cortex (Shirtcliff et al., 2009).
Other potentially fruitful connections can be made with the LH-based theory of attachment by Del Giudice (2009a), which extends the model proposed by Belsky and colleagues (1991; see Del Giudice and Belsky, 2010a). Patterns of parent-child attachment in early life and romantic attachment in adulthood can be linked to individual differences in LH strategies, as they influence crucial LH dimensions such as parental investment, mating style, social bonding/cooperation, and so on (see section 4.3). Thus, it should be possible to link individual and sex differences in attachment styles to the patterns of stress responsivity described in this paper. It should also be possible to formulate principled hypotheses about the reciprocal relations between attachment and stress responsivity across the life course, and to relate both to individual patterns of brain development (Figueredo et al, 2006).
In conclusion, the ACM can contribute to the progress of research in two distinct but related ways. On the one hand, it reorganizes present knowledge and helps formulate new predictions within the domain of stress research. On the other hand, it promotes integration with other research areas by anchoring stress research to the common framework of evolutionary biology. We believe that – in the not so distant future – large-scale integration based on evolutionary theory will be the key to breaking obsolete boundaries between disciplines to finally build a unified science of human development.
Acknowledgments
Marco Del Giudice was supported by the Regione Piemonte, bando Scienze Umane e Sociali 2008, L.R. n. 4/2006. Elizabeth Shirtcliff was supported by a grant from the National Institute of Mental Health for the duration of this project (K01 MH077687). International collaboration on this project was supported by the John & Doris Norton Endowment for Fathers, Parenting, and Families at the University of Arizona.
Footnotes
In evolutionary biology the term “strategy” denotes an organism’s realized phenotype among a set of possible phenotypes; adoption of a given strategy can depend on both environmental and genetic factors (see section 3). The term does not imply conscious planning, deliberation, or even awareness – a “choice” between phenotypic strategies (even behavioral ones) can be implemented by low-level physiological means such as hormonal switches or modifications of genetic expression.
A pleiotropic gene is involved in the expression of more than one phenotypic trait, so that allelic variation can affect many traits simultaneously. With respect to stages, pleiotropy refers to genes that affect physiology and behavior at different time points, thus creating genetic correlations across life history stages.
Note that we use “competition” in a broad sense as the pursuit of social acceptance, centrality, and influence; in this sense, social competition often involves cooperative and prosocial behaviors in addition to agonistic and aggressive ones (e.g., Hawley, 1999; Pellegrini and Bartini, 2001).
Note that in this paper we focus on the mechanisms mediating the allostatic response (allostatic mediators) rather than on the long-term cost of allostasis (allostatic load). Allostatic load has received much attention in the health-related literature (McEwen and Wingfield, 2003); however, the evolutionary approach suggests a broader perspective, emphasizing the trade-offs involved in allostasis and the overall cost-benefit balance of the organism’s strategy.
Circadian rhythm refers to the 24 hour cycle of the biological processes shifting their basal levels of activity. When limited within the waking hours, the term is more accurately described as diurnal (i.e., daily, or daytime) rhythm. Cortisol levels, for example, are at their zenith within an hour of awakening, decline sharply across the morning hours and then reach their lowest diurnal levels around bedtime.
In keeping with our definition of the SRS as a functional (rather than anatomical) system, we treat the HPG axis as a related but functionally distinct system, despite its anatomical overlap with the HPA axis. Of course, such distinctions are always arbitrary to some degree, since – at a higher organization level – the whole organism can be considered as a single integrated system.
Haller and colleagues (2004) showed that, in mice, chronically induced hypocortisolism promotes predatory aggression but also causes a smaller increase in heart rate during fights. However, heart rate reflects the action of both the SNS and the PNS, and a blunted increase in heart rate is compatible with a pattern of autonomic coactivation such as the one we hypothesize here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marco Del Giudice, Email: marco.delgiudice@unito.it.
Bruce J. Ellis, Email: bjellis@email.arizona.edu.
Elizabeth A. Shirtcliff, Email: birdie.shirtcliff@uno.edu.
References
- Adam EK, Klimes-Dougan B, Gunnar MR. Social regulation of the adrenocortical response to stress in infants, children and adolescents: Implications for psychopathology and education. In: Coch D, Dawson G, Fischer KW, editors. Human behavior, learning, and the developing brain: Atypical development. Guilford; New York: 2007. pp. 264–304. [Google Scholar]
- Adkins-Regan E. Hormones and animal social behavior. Princeton University Press; Princeton, NJ: 2005. [Google Scholar]
- af Klinteberg B, von Knorring L, Oreland L. On the psychobiology of impulsivity. In: Stelmack RM, editor. On the psychobiology of personality. Elsevier; Oxford, UK: 2004. pp. 429–451. [Google Scholar]
- Ahnert L, Gunnar M, Lamb M, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion and cortisol elevations. Child Dev. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene—environment interactions predict cortisol responses after acute stress: Implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Alexander N, Osinsky R, Mueller E, Schmitz A, Guenthert S, Kuepper Y, Hennig J. Genetic variants within the dopaminergic system interact to modulate endocrine stress reactivity and recovery. Behav Brain Res. 2011;216:53–58. doi: 10.1016/j.bbr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Alink LRA, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in cbildren and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol. 2008;50:427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce T the MacArthur Assessement Battery Working Group. Developmental and contextual influences on autonomic reactivity in young children. Dev Psychobiol. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Allison AL, Peres JC, Boettger C, Leonbacher U, Hastings PD, Shirtcliff EA. Fight, flight or fall: Coactivation of the autonomic nervous system during skydiving in press. [Google Scholar]
- Alvares GA, Hickie IB, Guastella AJ. Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Exp Clin Psychopharmacol. 2010;18:316–321. doi: 10.1037/a0019719. [DOI] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN. Effect of Estrogen-Serotonin Interactions on Mood and Cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Archer J. Does sexual selection explain human sex differences in aggression? Behav Brain Sci. 2009;32:249–266. doi: 10.1017/S0140525X09990951. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Rainey WE. Adrenarche – Physiology, biochemistry and human disease. Clin Endocrinol. 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learning Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. SCAN. 2009;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JS, Dadds MR. Examining alternative explanations of the covariation of ADHD and anxiety symptoms in children: a community study. J Abnorm Child Psychol. 2008;36:67–79. doi: 10.1007/s10802-007-9160-1. [DOI] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Booth A, Shirtcliff EA, Granger DA. Testosterone, cortisol, and women’s competition. Evol Hum Behav. 2002;23:181–192. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Dev Behav Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle D. Gender differences in the social moderators of stress. In: Barnett RC, Biener L, Baruch GK, editors. Gender and stress. The Free Press; New York: 1987. pp. 257–277. [Google Scholar]
- Belsky J. Variation in susceptibility to rearing influences: An evolutionary argument. Psychol Inquiry. 1997;8:182–186. [Google Scholar]
- Belsky J. Differential susceptibility to rearing influences: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary Psychology and Child Development. Guilford; New York: 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential Susceptibility to environmental influences. Curr Dir Psychol Sci. 2007;16:300–304. [Google Scholar]
- Belsky J, Houts RM, Pasco, Fearon RM. Infant attachment security and the timing of puberty: Testing an evolutionary hypothesis. Psychol Sci. 2010;21:1195–2001. doi: 10.1177/0956797610379867. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis-stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Psychol Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, Jones LL. Executive functions after age 5: Changes and correlates. Dev Rev. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, Razza RP. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Dev. 2005;76:554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Kivlighan K, et al. Maternal sensitivity is related to hypothalamic-pituitary-adrenal axis stress reactivity and regulation in response to emotion challenge in 6-month-old infants. Ann NY Acad Sci. 2006;1094:263–267. doi: 10.1196/annals.1376.031. [DOI] [PubMed] [Google Scholar]
- Bogin B. Evolutionary hypotheses for human childhood. Yearbook Phys Anthropol. 1997;40:63–89. [Google Scholar]
- Bogin B. Evolutionary perspective on human growth. Ann Rev Anthropol. 1999;28:109–153. doi: 10.1146/annurev.anthro.28.1.109. [DOI] [PubMed] [Google Scholar]
- Booth A, Granger DA, Shirtcliff EA. Gender- and age-related differences in the association between social relationship quality and trait levels of salivary cortisol. J Res Adol. 2008;18:239–260. [Google Scholar]
- Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJ, et al. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med. 2009;71:877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT. Biobehavioral reactivity and injuries in children and adolescents. In: Bornstein MH, Genevro J, editors. Child development and behavioral pediatrics: Toward understanding children and health. Erlbaum; Mahwah, NJ: 1996. [Google Scholar]
- Boyce WT, Alkon A, Tschann JM, Chesney MA, Alpert BS. Dimensions of psychobiologic reactivity: cardiovascular responses to laboratory stressors in preschool children. Ann Behav Med. 1995a;17:315–323. doi: 10.1007/BF02888596. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, Cohen F, Kaiser P, Folkman S, Wara D. Psychobiologic reactivity to stress and childhood respiratory illnesses: results of two prospective studies. Psychosom Med. 1995b;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. Br J Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bradburn NM, Sudman S, Blair E, Locander W, Miles C, et al. Reinterpreting the Marlowe-Crowne Scale. In: Bradburn NM, Sudman S, editors. Improving interview method and questionnaire design. Jossey-bass; San Francisco, CA: 1979. pp. 85–106. [Google Scholar]
- Brody S. Age at first intercourse is inversely related to female cortisol stress reactivity. Psychoneuroendocrinology. 2002;27:933–943. doi: 10.1016/s0306-4530(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Brown GR, Laland KN, Borgerhoff Mulder M. Bateman’s principles and human sex roles. Trends Ecol Evol. 2009;24:297–304. doi: 10.1016/j.tree.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubier JB, Drabick DA, Breiner T. Autonomic functioning moderates the relations between contextual factors and externalizing behaviors among inner-city children. J Fam Psychol. 2009;23:500–510. doi: 10.1037/a0015555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugental DB. Acquisition of the algorithms of social life: A domain-based approach. Psychol Bull. 2000;126:187–219. doi: 10.1037/0033-2909.126.2.187. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: A meta-analysis. Psychol Bull. 1999;125:367–383. [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Dev Psychobiol. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Smith CL, Gill K, Johnson MC. Maternal interactive style across contexts: Relations to emotional, behavioral and physiological regulation during toddlerhood. Soc Dev. 1998;7:350–369. [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PloS ONE. 2008a;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J Neuroendocrinol. 2008b;20:795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Campbell B. Adrenarche and the evolution of human life history. Am J Hum Biol. 2006;18:569–589. doi: 10.1002/ajhb.20528. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychol Bull. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Early adversity and developmental outcomes: interaction between genetics, epigenetics, and social experiences across the life span. Perspect Psychol Sci. 2010;5:564–574. doi: 10.1177/1745691610383494. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joëls M, Krugers H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Simpson JA, Rholes WS. Oxytocin and social support in romantic relationships: An attachment perspective in preparation. [Google Scholar]
- Chichinadze K, Chichinadze N. Stress-induced increase of testosterone: Contributions of social status and sympathetic reactivity. Physiol Behav. 2008;94:595–603. doi: 10.1016/j.physbeh.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Chisholm JS. Death, hope, and sex: life-history theory and the development of reproductive strategies. Curr Anthropol. 1993;34:1–24. [Google Scholar]
- Chisholm JS. The evolutionary ecology of attachment organization. Hum Nat. 1996;7:1–38. doi: 10.1007/BF02733488. [DOI] [PubMed] [Google Scholar]
- Chisholm JS. Attachment and time preference: Relations between early stress and sexual behavior in a sample of American university women. Hum Nat. 1999;10:51–83. doi: 10.1007/s12110-999-1001-1. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology Vol. 2. Risk, Disorder and Adaptation. Wiley; New York: 1995. pp. 32–71. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crawford CB, Anderson JL. Sociobiology: An environmentalist discipline? Amer Psychol. 1989;44:1449–1459. [Google Scholar]
- Crijnen AAM, Achenbach TM, Verhulst FC. Comparisons of problems reported by parents of children in 12 cultures: Total problems, externalizing, and internalizing. J Am Acad Child Adolesc Psychiatry. 1997;36:1269–1277. doi: 10.1097/00004583-199709000-00020. [DOI] [PubMed] [Google Scholar]
- Curry OS, Price ME, Price JG. Patience is a virtue: cooperative people have lower discount rates. Pers Indiv Diff. 2008;44:780–785. [Google Scholar]
- Daly M, Wilson M. A sociobiological analysis of human infanticide. In: Hausfater G, Hrdy SB, editors. Infanticide: Comparative and evolutionary perspectives. Aldine de Gruyter; New York, NY: 1984. pp. 487–502. [Google Scholar]
- Daly M, Wilson M. Carpe diem: Adaptation and devaluing the future. Q Rev Biol. 2005;80:55–60. doi: 10.1086/431025. [DOI] [PubMed] [Google Scholar]
- Davis J, Werre D. Agonistic stress in early adolescence and its effects on reproductive effort in young adulthood. Evol Hum Behav. 2007;28:228–233. [Google Scholar]
- Deakin JFW. Depression and antisocial personality disorder: two contrasting effects of 5- HT function. J Neural Transm. 2003;64:79–93. doi: 10.1007/978-3-7091-6020-6_5. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Sex, attachment, and the development of reproductive strategies. Behav Brain Sci. 2009a;32:1–67. doi: 10.1017/S0140525X09000016. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Human reproductive strategies: An emerging synthesis? Behav Brain Sci. 2009b;32:45–67. doi: 10.1017/S0140525X09000016. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Angeleri R, Manera V. The juvenile transition: a developmental switch point in human life history. Dev Rev. 2009;29:1–31. [Google Scholar]
- Del Giudice M, Belsky J. Sex differences in attachment emerge in middle childhood: An evolutionary hypothesis. Child Dev Perspect. 2010a;4:97–105. [Google Scholar]
- Del Giudice M, Belsky J. The development of life history strategies: Toward a multistage theory. In: Buss DM, Hawley PH, editors. The evolution of personality and individual differences. Oxford University Press; New York: 2010b. pp. 154–176. [Google Scholar]
- Denollet J. Type D personality: A potential risk factor refined. J Psychosom Res. 2000;49:255–266. doi: 10.1016/s0022-3999(00)00177-x. [DOI] [PubMed] [Google Scholar]
- Denollet J, Rombouts H, Gillebert TC, Brutsaert DL, Sys SU, Brutsaert DL, Stroobant N. Personality as an independent predictor of long-term mortality in patients with coronary heart disease. Lancet. 1996;347:417–421. doi: 10.1016/s0140-6736(96)90007-0. [DOI] [PubMed] [Google Scholar]
- Denson TF, Fabiansson EC, Creswell JD, Pedersen WC. Experimental effects of rumination styles on salivary cortisol responses. Motivation Emotion. 2009;33:42–48. [Google Scholar]
- Derakshan N, Eysenck MW. Interpretive biases for one’s own behaviour and physiology in high trait-anxious individuals and repressors. J Pers Soc Psychol. 1997;73:816–825. [Google Scholar]
- Diamond LM, Hicks AM. Attachment style, current relationship security, and negative emotions: The mediating role of physiological regulation. J Soc Pers Relat. 2005;22:499–518. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychol. 2008;27:116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Réale D. Natural selection and animal personality. Behaviour. 2005;142:1165–1190. [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bdenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donzella B, Gunnar M, Krueger WK, Alwin J. Cortisol and vagal tone responses to competitive challenge in preschoolers: associations with temperament. Dev Psychobiol. 2000;37:209–220. doi: 10.1002/1098-2302(2000)37:4<209::aid-dev1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosvelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Dev Psychobiol. 2003;43:230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Draper P, Harpending H. A sociobiological perspective on the development of human reproductive strategies. In: MacDonald KB, editor. Sociobiological perspectives on human development. Springer-Verlag; NY: 1988. pp. 340–372. [Google Scholar]
- Dufty AM, Clobert J, Møller AP. Hormones, developmental plasticity and adaptation. Trends Ecol Evol. 2002;17:190–196. [Google Scholar]
- Ebling FJP. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad T, Fabes RA, Shepard SA, Reiser M, Murphy BC, Losoya SH, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Dev. 2003;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychol Bull. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Toward an evolutionary-developmental explanation of alternative reproductive strategies: The central role of switch-controlled modular systems. In: Buss DM, Hawley PH, editors. The evolution of personality and individual differences. Oxford University Press; New York: 2010. [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Curr Dir Psychol Sci. 2008;17:183–187. [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: A neurodevelopmental theory. Development and Psychopathology. 2011a;23(1) doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche and sexual maturation: A longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum Nat. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response system: universality and adaptive individual differences. Dev Rev. 2006;26:175–212. [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011b;23(1) doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PT. On fertile ground: a natural history of human reproduction. Harvard University Press; Cambridge, MA: 2001. [Google Scholar]
- Ellison PT. Social relationships and reproductive ecology. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge, MA: 2009. pp. 54–73. [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. J Abnorm Child Psychol. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children’s externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monogr Soc Res Child Dev. 2009;74:1–78. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enebrink P, Andershed H, Langstrom N. Callous–unemotional traits are associated with clinical severity in referred boys with conduct problems. Nordic J Psychiatry. 2005;59:431–440. doi: 10.1080/08039480500360690. [DOI] [PubMed] [Google Scholar]
- Ennis M, Kelly KS, Lambert PL. Sex differences in cortisol excretion during anticipation of a psychological stressor: Possible support for the tend–and–befriend hypothesis. Stress Health. 2001;17:253–261. [Google Scholar]
- Essex MJ, Armstrong JM, Burk LR, Goldsmith HH, Boyce WT. Biological sensitivity to context moderates the effects of the early teacher-child relationship on the development of mental health by adolescence. Development and Psychopathology. 2011;23(1) doi: 10.1017/S0954579410000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults’ stress-related responses to daily life events. J Pers Soc Psychol. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Hormonal and neurochemical influences on aggression in group-living monkeys. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge, MA: 2009. pp. 159–195. [Google Scholar]
- Figueredo AJ, Jacobs WJ. Aggression, risk-taking, and alternative life history strategies: The behavioral ecology of social deviance. In: Frias-Armenta M, Corral-Verdugo V, editors. Biopsychosocial Perspectives on Aggression. Nova Science Publishers; Hauppauge, NY: 2009. [Google Scholar]
- Figueredo AJ, Vásquez G, Brumbach BH, Schneider SMR. The heritability of life history strategy: the K-factor, covitality, and personality. Soc Biol. 2004;51:121–143. doi: 10.1080/19485565.2004.9989090. [DOI] [PubMed] [Google Scholar]
- Figueredo AJ, Vásquez G, Brumbach B, Sefcek JA, Krisner BR, Jacobs WJ. The K-Factor: Individual differences in life-history strategy. Pers Indiv Diff. 2005;39:1349–1360. [Google Scholar]
- Figueredo AJ, Vásquez G, Brumbach B, Schneider SMR, Sefcek JA, Tal IR, et al. Consilience and life history theory: From genes to brain to reproductive strategy. Dev Rev. 2006;26:243–275. [Google Scholar]
- Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Flinn MV. Evolution and ontogeny of the stress response to social challenges in the human child. Dev Rev. 2006;26:138–174. [Google Scholar]
- Flinn MV, England BG. Childhood stress: Endocrine and immune responses to psychosocial events. In: Wilce JM, editor. Social and cultural lives of immune systems. Routledge press; London: 2003. pp. 107–147. [Google Scholar]
- Flinn MV, Quinlan RJ, Decker SA, Turner MT, England BG. Male-female differences in effects of parental absence on glucocorticoid stress response. Hum Nat. 1996;7:125–162. doi: 10.1007/BF02692108. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain Cognit. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC, Dindo L. A dual-deficit model of psychopathy. In: Patrick CJ, editor. Handbook of psychopathy. Guilford; New York: 2006. pp. 14–34. [Google Scholar]
- Frick PJ, Bodin SD, Barry CT. Psychopathic traits and conduct problems in community and clinic-referred samples of children: Further development of the psychopathy screening device. Psychol Assessment. 2000;12:382–393. [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous–unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. J Abnorm Child Psychol. 2003;31:457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Fuller RW. The involvement of serotonin in regulation of pituitary-adrenocortical function. Front Neuroendocrinol. 1992;13:250–270. [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: trade-offs and strategic pluralism. Behav Brain Sci. 2000;23:573–587. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Pychol Rev. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmezy N. Resilience in children’s adaptation to negative life events and stressed environments. Pediatr Ann. 1991;20:463–466. doi: 10.3928/0090-4481-19910901-05. [DOI] [PubMed] [Google Scholar]
- Gatze-Kopp LM. The canary in the coalmine: The sensitivity of mesolimbic dopamine to environmental adversity during development. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Geary DC. Evolution and proximate expression of human paternal investment. Psychol Bull. 2000;126:55–77. doi: 10.1037/0033-2909.126.1.55. [DOI] [PubMed] [Google Scholar]
- Geary DC. Sexual selection and human life history. Adv Child Dev Behav. 2002;30:41–101. doi: 10.1016/s0065-2407(02)80039-8. [DOI] [PubMed] [Google Scholar]
- Geary DC. Evolution of paternal investment. In: Buss DM, editor. The evolutionary psychology handbook. John Wiley & Sons; Hoboken, NJ: 2005. pp. 483–505. [Google Scholar]
- Geary DC. Male, female: the evolution of human sex differences. 2. APA Press; Washington, DC: 2010. [Google Scholar]
- Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, et al. Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83:3695–3701. doi: 10.1210/jcem.83.10.5070. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs. low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bono E, Salvador A, Serrano MA, Ricarte J. Testosterone, cortisol, and mood in a sports team competition. Horm Behav. 1999;35:55–62. doi: 10.1006/hbeh.1998.1496. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PB, Campbell BC. Human male testosterone, pair-bonding, and fatherhood. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge, MA: 2009. pp. 270–293. [Google Scholar]
- Grumbach MM, Styne DM. Puberty: Ontogeny, neuroendocrinology, physiology and disorders. In: Larsen PR, Kronenberg HM, Melmed SM, Polonsky KS, editors. Williams textbook of endocrinology. 10. Philadelphia, PA: Saunders; 2003. [Google Scholar]
- Gunnar MR. Attachment and stress in early development: Does attachment add to the potency of social regulators of infant stress? In: Carter CS, Ahnert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, Sascher N, editors. Attachment and bonding: A new synthesis. MIT Press; Cambridge, MA: 2005. pp. 245–256. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Vol. 2. Wiley; Hoboken, NJ: 2006. [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009a;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009b;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, deHaan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Dev Psychobiol. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in HPA activity over the transition to adolescence: Normative changes and associations with puberty. Dev Psychopathol. 2009c;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsäter H, Lichtenstein P, Nelson N, Gustafsson PA. Does quantity have a quality all its own? Cumulative adversity and up- and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology. 2010;35:1410–1415. doi: 10.1016/j.psyneuen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Maternal prenatal stress and 4–6 year old children’s salivary cortisol concentrations pre- and post-vaccination. Stress. 2004;7:257–260. doi: 10.1080/10253890500044521. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Neuroendocrinology. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Hagan MJ, Roubinov DS, Gress-Smith J, Luecken LJ, Sandler IN, Wolchik S. Positive parenting during childhood moderates the impact of recent negative events on cortisol activity in parentally bereaved youth. Psychopharmacology. 2010 doi: 10.1007/s00213–010–1889–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Benning SD. The “successful” psychopath. In: Patrick CJ, editor. Handbook of psychopathy. Guilford; New York: 2006. pp. 459–475. [Google Scholar]
- Haller J, Halász J, Mikics E, Kruk MR. Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. J Neuroendocrinol. 2004;16:550–557. doi: 10.1111/j.1365-2826.2004.01201.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Haller J, Mikics E, Halász J, Toth M. Mechanisms differentiating normal from abnormal aggression: Glucocorticoids and serotonin. Eur J Pharmacol. 2005;526:89–100. doi: 10.1016/j.ejphar.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr Opin Psychiatry. 2009;22:357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Hawley PH. The ontogenesis of social dominance: A strategy-based evolutionary perspective. Dev Rev. 1999;19:97–132. [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Genetics of puberty. Horm Res. 2007;68:75–79. doi: 10.1159/000110583. [DOI] [PubMed] [Google Scholar]
- Herdt G, McClintock M. The magical age of 10. Arch Sex Behav. 2000;29:587–606. doi: 10.1023/a:1002006521067. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Heyland A, Hodin J, Reitzel AM. Hormone signaling in evolution and development: A non-model system approach. BioEssays. 2005;27:64–75. doi: 10.1002/bies.20136. [DOI] [PubMed] [Google Scholar]
- Hill K. Life history theory and evolutionary anthropology. Evol Antropol. 1993;2:78–88. [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins SD, Granger DA, Moore GA, Gariepy J, Cox MJ. Parasympathetic and sympathetic responses to the strange situation in infants and mothers from avoidant and securely attached dyads. Dev Psychobiol. 2008;50:361–376. doi: 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- Hofer M. Relationships as regulators. Psychosom Med. 1984;46:183. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. Mothers and others: The evolutionary origins of mutual understanding. Harvard University Press; Cambridge, MA: 2009. [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche – normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671–696. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- Jackson JJ, Ellis BJ. Synthesizing life history theory with sexual selection: Toward a comprehensive model of alternative reproductive strategies. Behav Brain Sci. 2009;32:31–32. [Google Scholar]
- Janson CH, van Schaik CP. Ecological risk aversion in juvenile primates: slow and steady wins the race. In: Pereira ME, Fairbanks LA, editors. Juvenile primates. Life history, development, and behavior. Oxford University Press; NY: 1993. pp. 57–74. [Google Scholar]
- Jezova D, Jurankova E, Mosnarova A, Kriska M, Skultetyova I. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol Exp. 1996;56:779–785. doi: 10.55782/ane-1996-1183. [DOI] [PubMed] [Google Scholar]
- Joffe TH. Social pressures have selected for an extended juvenile period in primates. J Hum Evol. 1997;32:593–605. doi: 10.1006/jhev.1997.0140. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kaplan HS, Gangestad SW. Life history theory and evolutionary psychology. In: Buss DM, editor. Handbook of evolutionary psychology. Wiley; New Jersey: 2005. pp. 68–95. [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- Kaplan HS, Lancaster JB. An evolutionary and ecological analysis of human fertility, mating patterns, and parental investment. In: Wachter KW, Bulatao RA, editors. Offspring: Human fertility behavior in biodemographic perspective. National Academies Press; Washington, DC: 2003. pp. 170–223. [PubMed] [Google Scholar]
- Katz LF. Domestic violence and vagal reactivity to peer provocation. Biol Psychol. 2007;74:154–164. doi: 10.1016/j.biopsycho.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiley MK, Lofthouse N, Bates JE, Dodge KA, Pettit GS. Differential risks of covarying and pure components in mother and teacher reports of externalizing and internalizing behavior across ages 5 to 14. J Abnorm Chid Psychol. 2003;31:267–283. doi: 10.1023/a:1023277413027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. Oxytocin: Prosocial behavior, social salience, or approach-related behavior? Biol Psychiatry. 2010;67:e33–e34. doi: 10.1016/j.biopsych.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, et al. Marital conflict in older adults: endocrinological and immunological correlates. Psychosom Med. 1997;59:339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: newlyweds’ stress hormones foreshadow relationship changes. J Consult Clin Psychol. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Blomberg SP, Duffy DL, Heath AC, Owens IPF, Martin NG. Natural selection and quantitative genetics of life-history traits in Western women: A twin study. Evolution. 2001;55:423–435. doi: 10.1111/j.0014-3820.2001.tb01304.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Knapp R. Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integr Comp Biol. 2004;43:658–668. doi: 10.1093/icb/43.5.658. [DOI] [PubMed] [Google Scholar]
- Kokko H, Jennions M. Parental investment, sexual selection and sex ratios. J Evol Biol. 2008;21:919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc Lond B. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kramer KL. Variation in juvenile dependence: Helping behavior among Maya children. Hum Nat. 2002;13:299–325. doi: 10.1007/s12110-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Kramer KL. Children’s help and the pace of reproduction: Cooperative breeding in humans. Evol Anthropol. 2005;14:224–237. [Google Scholar]
- Kruger DJ, Nesse RM. An evolutionary life-history framework for understanding sex differences in human mortality rates. Hum Nat. 2006;17:74–97. doi: 10.1007/s12110-006-1021-z. [DOI] [PubMed] [Google Scholar]
- Kruger DJ, Reischl T, Zimmerman MA. Time perspective as a mechanism for functional developmental adaptation. J Soc Evol Cult Psychol. 2008;2:1–22. [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychopneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Bélanger A, Lin SG, Simard J, Pelletier G, et al. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- Lancaster JB, Kaplan HS. The endocrinology of the human adaptive complex. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge, MA: 2009. pp. 95–120. [Google Scholar]
- Lancaster LT, Hazard LC, Clobert J, Sinervo BR. Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J Evol Biol. 2007;21:556–565. doi: 10.1111/j.1420-9101.2007.01478.x. [DOI] [PubMed] [Google Scholar]
- Lancy DF, Grove MA. Getting noticed: Middle childhood in cross-cultural perspective. Hum Nat. doi: 10.1007/s12110-011-9117-5. in press. [DOI] [PubMed] [Google Scholar]
- Lass-Hennemann J, Deuter CE, Kuehl LK, Schulz A, Blumenthal TD, Schachinger H. Effects of stress on human mating preferences: stressed individuals prefer dissimilar mates. Proc R Soc B. 2010 doi: 10.1098/rspb.2010.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H, Powers S. Emotion regulation in emerging adult couples: Temperament, attachment, and HPA response to conflict. Biol Psychol. 2007;76:61–71. doi: 10.1016/j.biopsycho.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbeater BJ, Kuperminc GP, Blatt SJ, Hertzog C. A multivariate model of gender differences in adolescents’ internalizing and externalizing problems. Dev Psychol. 1999;35:1268–1282. doi: 10.1037//0012-1649.35.5.1268. [DOI] [PubMed] [Google Scholar]
- Legendre A, Trudel M. Cortisol and behavioral responses of young children coping with a group of unfamiliar peers. Merrill–Palmer Quart. 1996;42:554–577. [Google Scholar]
- Leimar O, Hammerstein P, Van Dooren TJM. A new perspective on developmental plasticity and the principles of adaptive morph determination. Amer Nat. 2006;167:367–376. doi: 10.1086/499566. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bigras M, Pauzé R. Externalizing problems and problematic sexual behaviors: same etiology? Aggress Behav. 2010;36:358–370. doi: 10.1002/ab.20362. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuoendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Cortisol response to embarrassment and shame. Child Dev. 2002;73:1034–1045. doi: 10.1111/1467-8624.00455. [DOI] [PubMed] [Google Scholar]
- Li I, Chiou H, Shen P. Correlations between cortisol level and internalizing disposition of young children are increased by selecting optimal sampling times and aggregating data. Dev Psychobiol. 2007;49:633–639. doi: 10.1002/dev.20239. [DOI] [PubMed] [Google Scholar]
- Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the balloon analogue task. PloS ONE. 2009;4:e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JL, Bogin B. Language and life history: A new perspective on the evolution and development of human language. Beha Brain Sci. 2006;29:259–325. doi: 10.1017/s0140525x0600906x. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta- analysis. Psychol Bull. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Sollers JJ., III . Autonomic nervous system. In: Fink J, editor. Encyclopedia of stress. 2. Academic Press; 2007. pp. 282–289. [Google Scholar]
- Luecken LJ, Kraft A, Hagan MJ. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Horm Behav. 2009;55:412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KB. An evolutionary perspective on human fertility. Popul Environ. 1999;21:223–246. [Google Scholar]
- Macrì S, Granstrem O, Shumilina M, dos Santos FJAG, Berry A, Saso L, Laviola G. Resilience and vulnerability are dose-dependently related to neonatal stressors in mice. Horm Behav. 2009;56:391–398. doi: 10.1016/j.yhbeh.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Macrì S, Pasquali S, Bonsignore LT, Pieretti S, Cirulli F, Chiarotti F, Laviola G. Moderate neonatal stress decreases within-group variation in behavioral, immune and HPA responses in adult mice. PLoS One. 2007;10:e1015. doi: 10.1371/journal.pone.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey WB, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosom Med. 1994;56:41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Lotrich FE. Brain serotonin and aggressive disposition in humans and nonhuman primates. In: Nelson RJ, editor. Biology of aggression. Oxford University Press; New York: 2006. pp. 65–113. [Google Scholar]
- Marazziti D, Dell’Osso B, Baroni S, Mungai F, Catena M, Rucci P, et al. A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemiol Ment Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe F. Paternal investment and the human mating system. Behav Process. 2000;51:45–61. doi: 10.1016/s0376-6357(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Marlowe F. The mating system of foragers in the Standard Cross-Cultural Sample. Cross-Cult Res. 2003;37:282–306. [Google Scholar]
- Marsee MA, Silverthorn P, Frick PJ. The association of psychopathic traits with aggression and delinquency in non-referred boys and girls. Behav Sci Law. 2005;23:803–817. doi: 10.1002/bsl.662. [DOI] [PubMed] [Google Scholar]
- Martorell GA, Bugental DB. Maternal variations in stress reactivity: Implications for harsh parenting practices with very young children. J Fam Psychol. 2006;20:641–647. doi: 10.1037/0893-3200.20.4.641. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom Med. 1968;30:576–608. [PubMed] [Google Scholar]
- Mazur A, Susman EJ, Edelbrock S. Sex differences in testosterone response to a video game contest. Evol Hum Behav. 1997;18:317–326. [Google Scholar]
- Maynard Smith J. Evolutionary genetics. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- McCarthy MM. Estrogen modulation of oxytocin and its relation to behavior. In: Ivell R, Russell J, editors. Oxytocin: Cellular and molecular approaches in medicine and research. Plenum Press; NEW YORK: 1995. pp. 235–242. [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McClintock MK, Herdt G. Rethinking puberty: The development of sexual attraction. Curr Dir Psychol Sci. 1996;5:178–183. [Google Scholar]
- McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Dev Psychobiol. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Amer Nat. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- McIntyre MH, Hooven CK. Human sex differences in social relationships: Organizational and activational effects of androgens. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge, MA: 2009. pp. 225–245. [Google Scholar]
- McNamara JM, Houston AI. State-dependent life histories. Nature. 1996;380:215–221. doi: 10.1038/380215a0. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual diVerences in stress reactivity across generations. Ann Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Turkheimer E, Van Hulle CA, D’Onofrio BM, Brooks-Gunn J, Rodgers JL, Emery RE, Lahey BB. Associations between father absence and age of first sexual intercourse. Child Dev. 2009;80:1463–1480. doi: 10.1111/j.1467-8624.2009.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Fish EW. Monoamines, GABA, glutamate, and aggression. In: Nelson RJ, editor. Biology of aggression. Oxford University Press; New York: 2006. pp. 114–149. [Google Scholar]
- Miller G. The mating mind. How sexual choice shaped the evolution of human nature. Heinemann; London: 2000. [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psycholl Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mock DW. More than kin and less than kind: The evolution of family conflict. Belknap Press; Harvard, MA: 2004. [Google Scholar]
- Möhler E, Parzer P, Brunner R, Wiebel A, Resch F. Emotional stress in pregnancy predicts human infant reactivity. Early Hum Dev. 2006;82:731–737. doi: 10.1016/j.earlhumdev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Moore T, Scarpa A, Raine A. A meta-analysis of serotonin metabolite 5-HIAA and antisocial behavior. Aggressive Behav. 2002;28:299–316. [Google Scholar]
- Mueller A, Brocke B, Fries E, Lesch K, Kirschbaum C. The role of the serotonin transporter polymorphism for the endocrine stress response in newborns. Psychoneuroendocrinology. 2010;35:289–296. doi: 10.1016/j.psyneuen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–43. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Muranaka M, Lane JD, Suarez EC, Anderson NB, Suzuki J, Williams RB. Stimulus-specific patterns of cardiovascular reactivity in Type A and B subjects: Evidence for enhanced vagal reactivity in Type B. Psychophysiology. 1988;25:330–338. doi: 10.1111/j.1469-8986.1988.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Han G, Cicchetti D, Crick NR, Rogosch FA. Neuroendocrine regulation and physical and relational aggression: The moderating roles of child maltreatment and gender. Dev Psychol. 2008;44:1160–1176. doi: 10.1037/a0012564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: Moderating role of attachment security. Child Dev. 1996;67:508–522. [PubMed] [Google Scholar]
- Nakamura K, Sheps S, Arck PC. Stress and reproductive failure: past notions, present insights and future directions. J Assist Rep Gen. 2008;25:47–62. doi: 10.1007/s10815-008-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaiko E, Sgoifo A. Central 5-HT receptors in cardiovascular control during stress. Neurosci Biobehav Rev. 2009;33:95–106. doi: 10.1016/j.neubiorev.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. PNAS. 2006;103:3938–3942. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Bhatnagar S, Young EA. Evolutionary origins and functions of the stress response. In: Fink J, editor. Encyclopedia of stress. 2. Academic Press; 2007. pp. 965–970. [Google Scholar]
- Netter P. Activation and anxiety as represented by patterns of catecholamine levels in hyper-and normotensives. Neuropsychobiology. 1983;10:148–55. doi: 10.1159/000118002. [DOI] [PubMed] [Google Scholar]
- Netter P. Psychological aspects of catecholamine response patterns to pain and mental stress in essential hypertensive patients and controls. J Clin Hypertens. 1987;3:727–742. [PubMed] [Google Scholar]
- Netter P. Do biochemical response patterns tell us anything about trait anxiety? In: Spielberger CD, Sarason IG, Kulcsar S, van Heck G, editors. Stress and anxiety. Vol. 14. Hemisphere; Washington, DC: 1991. pp. 187–214. [Google Scholar]
- Netter P. Personality and hormones. In: Stelmack RM, editor. On the psychobiology of personality. Elsevier; Ottawa, Canada: 2004. pp. 353–377. [Google Scholar]
- Newton TL, Contrada RJ. Repressive coping and verbal-autonomic response dissociation: The influence of social context. J Pers Soc Psychol. 1992;32:790–804. doi: 10.1037//0022-3514.62.1.159. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. Development and the evolution of adaptive polyphenisms. Evol Dev. 2003;5:9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush N, Boyce WT. The effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Dev Psychopathol. 2011;23 doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce TW. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veeen R, de Kloet R. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, De Winter AF, Veenstra R, Ormel J. Temperament profiles associated with internalizing and externalizing problems in preadolescence. Dev Psychopathol. 2004;16:421–440. doi: 10.1017/s0954579404044591. [DOI] [PubMed] [Google Scholar]
- O’Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32:183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- O’Neal CR, Brotman LM, Huang K, Gouley KK, Kamboukos D, Calzada EJ, Pine DS. Understanding relations among early family environment, cortisol response, and child aggression via a prevention experiment. Child Dev. 2010;81:290–305. doi: 10.1111/j.1467-8624.2009.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Anxious attachment style and salivary cortisol dysregulation in healthy female children and adolescents. J Child Psychol Psychiatry. 2010 doi: 10.1111/j.1469–7610.2010.02296.x. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arsenault L, Barr RG, Pérusse D, Tremblay RE. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch Gen Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9:265–270. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Arch Gen Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Jr, Crowley WF, Jr, Chandler, et al. The longitudinal study of adrenal maturation during gonadal suppression: Evidence that adrenarche is a gradual process. J Clin Endocrinol Metab. 2001;86:4536–4542. doi: 10.1210/jcem.86.9.7863. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ahnert L, Anzenberger G, Belsky J, Draper P, Fleming AS, Grossmann K, Sachser N, Sommer S, Tietze DP, Young LJ. Beyond infant attachment: the origins of bonding in later life. In: Carter CS, Ahnert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, Sachser N, editors. Attachment and bonding: a new synthesis. MIT Press; Cambridge, MA: 2005. pp. 385–428. [Google Scholar]
- Pedersen S, Denollet J. Type D personality, cardiac events, and impaired quality of life: a review. Eur J Cardiovasc Prev Rehab. 2003;10:241–248. doi: 10.1097/00149831-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Pellegrini AD, Archer J. Sex differences in competitive and aggressive behavior: a view from sexual selection theory. In: Ellis BJ, Bjorklund DF, editors. Origins of the social mind: evolutionary psychology and child development. Guilford; New York: 2005. pp. 219–244. [Google Scholar]
- Pellegrini AD, Bartini M. Dominance in early adolescent boys: Affiliative and aggressive dimensions and possible functions. Merrill Palmer Quart. 2001;47:142–163. [Google Scholar]
- Penke L. Bridging the gap between modern evolutionary psychology and the study of individual differences. In: Buss DM, Hawley PH, editors. The evolution of personality and individual differences. Oxford University Press; New York: 2010. [Google Scholar]
- Penke L, Denissen JJA, Miller GF. The evolutionary genetics of personality. Eur J Pers. 2007;21:549–587. [Google Scholar]
- Pennington R, Harpending H. Fitness and fertility among Kalahari !Kung. American J Phys Anthropol. 1988;77:303–319. doi: 10.1002/ajpa.1330770304. [DOI] [PubMed] [Google Scholar]
- Pereira ME, Fairbanks LA. What are juvenile primates all about? In: Pereira ME, Fairbanks LA, editors. Juvenile primates. Life history, development, and behavior. Oxford University Press; NY: 1993. pp. 3–12. [Google Scholar]
- Pettay JE, Kruuk LEB, Jokela J, Lummaa V. Heritability and genetic constraints of life-history trait evolution in pre-industrial humans. Proc Natl Acad Sci USA. 2005;102:2838–2843. doi: 10.1073/pnas.0406709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi T, Seger J. Hedging one’s evolutionary bets, revisited. Trends Ecol Evolut. 1989;4:41–44. doi: 10.1016/0169-5347(89)90138-9. [DOI] [PubMed] [Google Scholar]
- Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- Poggi Davis E, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2010 doi: 10.1111/j.1469–7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popma A, Vermeiren R, Geluk CAML, Rinne T, van den Brink W, Knol DL, Jansen LMC, van Engeland H, Doreleijers TAH. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biol Psychiatry. 2007;61:405–411. doi: 10.1016/j.biopsych.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid-serotonin interactions in depression: A review of the human evidence. Psychopharmacology. 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- Powers SI, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples’ attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. J Pers Soc Psychol. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Tucker Halpern C, Hill-Soderlund AL, Calkins SD, Carbone MA, Cox M. Gene–environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Dev. 2008;79:1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlation between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22:615–625. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Quas JA, Bauer A, Boyce WT. Physiological reactivity, social support, and memory in early childhood. Child Dev. 2004;75:797–814. doi: 10.1111/j.1467-8624.2004.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas JA, Hong M, Alkon A, Boyce WT. Dissociations between psychobiological reactivity and emotional expression in children. Dev Psychobiol. 2000;37:153–175. doi: 10.1002/1098-2302(200011)37:3<153::aid-dev4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Quinlan RJ. Human parental effort and environmental risk. Proc R Soc Lond B. 2007;274:121–125. doi: 10.1098/rspb.2006.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RJ. Human pair-bonds: Evolutionary functions, ecological variation, and adaptive development. Evol Anthropol. 2008;17:227–238. [Google Scholar]
- Quirin M, Pruessner JC, Kuhl J. HPA system regulation and adult attachment anxiety: Individual differences in reactive and awakening cortisol. Psychoneuroendocrinology. 2008;33:581–590. doi: 10.1016/j.psyneuen.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius child health project. J Am Acad Child Adolesc Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Dev. 2003;74:456–464. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Rauste von Wright M, von Wright J, Frankenhauser M. Relationship between sex related psychological characteristics during adolescence and catecholamine excretion during achievement stress. Psychophysiology. 1981;18:362–370. doi: 10.1111/j.1469-8986.1981.tb02467.x. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol Evolut. 2002;17:462–468. [Google Scholar]
- Rodgers JL, Hughes K, Kohler H, Christensen K, Doughty D, Rowe DC, Miller WB. Genetic influence helps explain variation in human fertility: Evidence from recent behavioral and molecular genetic studies. Curr Dir Psychol Sci. 2001;10:184–188. [Google Scholar]
- Roff DA. Life history evolution. Sinauer; Sunderland, MA: 2002. [Google Scholar]
- Rogosch FA, Chicchetti D. Illustrating the interface of family and peer relations through the study of child maltreatment. Soc Dev. 1994;3:291–308. [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Rowe DC. Environmental and genetic influences on pubertal development: Evolutionary life history traits? In: Rodgers JL, Rowe DC, Miller WB, editors. Genetic influences on human fertility and sexuality: recent empirical and theoretical findings. Kluwer; Boston: 2000. pp. 147–168. [Google Scholar]
- Rubin KH. “Brokering” emotion dysregulation: the moderating role of parenting in the relation between child temperament and children’s peer interactions. In: Zuckerman BS, Lieberman AF, Fox NA, editors. Emotional Regulation and Developmental Health: Infancy and Early Childhood. Johnson & Johnson; New Brunswick, NJ: 2002. pp. 81–99. [Google Scholar]
- Rubinow DR, Scmidt PJ, Roca CA. Estrogen-serotonin interaction: implications for affective regulation. Biol Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience: Some conceptual considerations. J Adol Health. 1993;14:690–696. doi: 10.1016/1054-139x(93)90196-v. [DOI] [PubMed] [Google Scholar]
- Rutter M, O’Connor T the English and Romanian Adoptees Research Team. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Haith MM, editors. The five to seven year shift: the age of reason and responsibility. University of Chicago Press; Chicago, IL: 1996. [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scaramella LV, Conger RD, Simons RL. Parental protective influences and gender-specific increases in adolescent internalizing and expternalizing problems. J Res Adol. 1999;9:111–141. [Google Scholar]
- Scarr S. Developmental theories for the 1990s: Development and individual differences. Child Dev. 1992;63:1–19. [PubMed] [Google Scholar]
- Schanberg S, Evoniuk G, Kuhn C. Tactile and nutritional aspects of maternal care: Specific regulators of neuroendocrine function and cellular development. Proc Soc Exp Biol Med. 1984;175:135. doi: 10.3181/00379727-175-41779. [DOI] [PubMed] [Google Scholar]
- Schlegel A. A cross-cultural approach to adolescence. Ethos. 1995;23:15–32. [Google Scholar]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wust S. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosom Med. 2008;70:787–796. doi: 10.1097/PSY.0b013e3181810658. [DOI] [PubMed] [Google Scholar]
- Schmitt DP. Sociosexuality from Argentina to Zimbabwe: A 48-nation study of sex, culture, and strategies of human mating. Behav Brain Sci. 2005;28:247–311. doi: 10.1017/s0140525x05000051. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Ruttle P. Immunological and neuroendocrine dysregulation following early deprivation and stress. In: Heinz Brisch K, editor. Attachment and early disorders of development. Klett-Cotta & Stuttgart; Munich: in press. [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behav Sci Law. 2009;27:137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillovac GP. Salivare cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. J Amer Acad Child Adol Psychiatry. 2003;42:1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Simon NG, Lu S. Androgens and aggression. In: Nelson RJ, editor. Biology of aggression. Oxford University Press; New York: 2006. pp. 211–230. [Google Scholar]
- Smith PK. Play: types and functions in human development. In: Ellis BJ, Bjorklund DF, editors. Origins of the social mind: evolutionary psychology and child development. Guilford; New York: 2005. pp. 271–291. [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the Strange Situation: The differential function of emotional expression. Int J Behav Dev. 1998;22:681–706. [Google Scholar]
- Sroufe LA, Bennett C, Englund M, Urban J, Shulman S. The significance of gender boundaries in preadolescence: contemporary correlates and antecedents of boundary violation and maintenance. Child Dev. 1993;64:455–466. [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann NY Acad Sci. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Staton L, El-Sheikh M, Buckhalt JA. Respiratory sinus arrhythmia and cognitive functioning in children. Dev Psychobiol. 2009;51:249–258. doi: 10.1002/dev.20361. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley & Sons; New York: 1988. pp. 629–649. [Google Scholar]
- St George IM, Williams S, Silva PA. Body size and menarche: The Dunedin study. J Adol Health. 1994;15:573–576. doi: 10.1016/1054-139x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Corey JM. Vagal regulation and observed social behavior in infancy. Soc Dev. 2001;10:189–201. [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Stroud L, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to corticotropin releasing hormone challenge. Ann NY Acad Sci. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Sugarman DB, Hotaling GT. Intimate violence and social desirability. J Interpers Violence. 12:275–290. [Google Scholar]
- Surbey MK. Parent and offspring strategies in the transition at adolescence. Hum Nat. 1998;9:67–94. doi: 10.1007/s12110-998-1012-3. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Tamres LK, Janicki D, Helgeson VS. Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Pers Soc Psychol Rev. 2002;6:2–30. [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend: Biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci. 2006;15:273–277. [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenenwald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56:128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Nemeroff CB, Plotsky PM. Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic–pituitary–adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000;870:87–101. doi: 10.1016/s0006-8993(00)02405-7. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev Reprod. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- Tither JM, Ellis BJ. Impact of fathers on daughters’ age at menarche: A genetically- and environmentally-controlled sibling study. Dev Psychol. 2008;44:1409–1420. doi: 10.1037/a0013065. [DOI] [PubMed] [Google Scholar]
- Tops M, Boksem MAS, Luu P, Tucker DM. Brain substrates of behavioral programs associated with self-regulation. Front Psychol. 2010;1:152. doi: 10.3389/fpsyg.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Boksem MAS, Wester AE, Lorist MM, Meijman TF. Task engagement and the relationships between the error-related negativity, agreeableness, behavioral shame proneness and cortisol. Psychoneuroendocrinology. 2006b;31:847–858. doi: 10.1016/j.psyneuen.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Tops M, van Peer JM, Wijers AA, Korf J. Acute cortisol administration reduces subjective fatigue in healthy women. Psychophysiology. 2006a;43:653–656. doi: 10.1111/j.1469-8986.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- Tops M, Russo S, Boksem MAS, Tucker DM. Serotonin: Modulator of a drive to withdraw. Brain Cognit. 2009;71:427–436. doi: 10.1016/j.bandc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man 1871–1971. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Troisi A. Gender differences in vulnerability to social stress: A Darwinian perspective. Physiol Behav. 2001;73:443–449. doi: 10.1016/s0031-9384(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Uziel L. Rethinking social desirability scales: From impression management to interpersonally oriented self-control. Perspect Psychol Sci. 2010;5:243–262. doi: 10.1177/1745691610369465. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: Circuitry, consequences, and regulation. Adv Pharmacol. 1998;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, Van Goozen SHM, van Engeland H, Schaal B, Arseneault L, Séguin JR, Nagin DS, Vitaro F, Tremblay RE. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. J Neural Transm. 2005;112:1083–1096. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- van den Bos R, Harteveld M, Stoop H. Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34:144–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Cohen-Kettenis PT, Matthys W, Van Engeland H. Preference for aggressive and sexual stimuli in children with disruptive behavior disorder and normal controls. Arch Sex Behav. 2002;31:247–253. doi: 10.1023/a:1015248803022. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Faichild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Thijssen JHH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biol Psychiatry. 1998;43:156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Buitelaar JK, Van Engeland H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. J Am Acad Child Adol Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14:1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- van Lenthe FJ, Kemper HCG, van Mechelen W, Post GB, Twisk JWR, Welten DC, Snel J. Biological maturation and the distribution of subcutaneous fat from adolescence into adulthood: The Amsterdam growth and health study. Int J Obesity. 1996;20:121–129. [PubMed] [Google Scholar]
- van Marle HJF, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Verona E, Joiner TE, Johnson F, Bender TW. Gender specific gene-environment interactions on laboratory-assessed aggression. Biol Psychol. 2006;71:33–41. doi: 10.1016/j.biopsycho.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic–pituitary–gonadal–adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Altenberg L. Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Mezey J, Calabretta R. Natural Selection and the origin of modules. In: Callabaut W, Rasskin-Gutman D, editors. Modularity. Understanding the development and evolution of complex natural systems. MIT Press; Cambridge, MA: 2005. pp. 33–49. [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. SCAN. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschbusch DA, Pelham WE, Jr, Jennings JR, Greiner AR, Tarter RE, Moss HB. Reactive aggression in boys with disruptive behavior disorders: Behavior, physiology, and affect. J Abnorm Child Psychol. 2002;30:641–656. doi: 10.1023/a:1020867831811. [DOI] [PubMed] [Google Scholar]
- Wasser SK. Psychosocial stress and infertility: Cause or effect? Hum Nat. 1994;5:293–306. doi: 10.1007/BF02692156. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Barash DP. Reproductive suppression among female mammals: Implications for biomedicine and sexual selection theory. Q Rev Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- Weekes N, Lewis R, Patel F, Garrison-Jakel J, Berger DE, Lupien SJ. Examination stress as an ecological inducer of cortisol and psychological responses to stress in undergraduate students. Stress. 2006;9:199–206. doi: 10.1080/10253890601029751. [DOI] [PubMed] [Google Scholar]
- Weinberger DA, Schwartz JE, Davidson RJ. Low-anxious, high-anxious, and repressive coping styles: Psychometric patterns and behavioural and physiological responses to stress. J Abnorm Psychol. 1979;88:369–380. doi: 10.1037//0021-843x.88.4.369. [DOI] [PubMed] [Google Scholar]
- Weisfeld GE. Evolutionary principles of human adolescence. Basic Books; New York: 1999. [Google Scholar]
- Weisfeld GE, Coleman DK. Further observations on adolescence. In: Burgess RL, MacDonald K, editors. Evolutionary perspectives on Human Development. 2. Sage; Thousand Oaks: 2005. pp. 331–356. [Google Scholar]
- Weisfeld GE, Janisse HC. Some functional aspects of human adolescence. In: Ellis BJ, Bjorklund DF, editors. Origins of the social mind: evolutionary psychology and child development. Guilford; New York: 2005. pp. 189–218. [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; New York: 2003. [Google Scholar]
- Westenberg PM, Bokhorst CL, Miers AC, Sumter SR, Kallen VL, van Pelt J, et al. A prepared speech in front of a pre-recorded audience: Subjective, physiological, and neuroendocrine responses to the Leiden Public Speaking Task. Biol Psychol. 2009;82:116–124. doi: 10.1016/j.biopsycho.2009.06.005. [DOI] [PubMed] [Google Scholar]
- White DD, Gallup AC, Gallup GG. Indirect peer aggression in adolescence and reproductive behavior. Evol Psychol. 2010;8:49–65. doi: 10.1177/147470491000800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: Implications for stress regulation. Ann Behav Med. 2009;37:126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Williams RB. Refining the Type A hypothesis: Emergence of the hostolity complex. Am J Cardiol. 1987;60:j27–j32. doi: 10.1016/0002-9149(87)90680-1. [DOI] [PubMed] [Google Scholar]
- Williams RB, Lane JD, Kuhn CM, Melosh W, White AD, Schanberg SM. Type A behavior and elevated physiological and neuroendocrine responses to cognitive tasks. Science. 1982;218:483–485. doi: 10.1126/science.7123248. [DOI] [PubMed] [Google Scholar]
- Wilson DS. Adaptive genetic variation and human evolutionary psychology. Ethol Sociobiol. 1994;15:219–235. [Google Scholar]
- Wilson DS, Yoshimura J. On the coexistence of specialists and generalists. Amer Nat. 1994;144:692–707. [Google Scholar]
- Wilson M, Daly M, Pound N. An evolutionary psychological perspective on the modulation of competitive confrontation and risk-taking. In: Pfaff D, et al., editors. Hormones, brain and behavior. Academic Press; San Diego: 2002. pp. 381–408. [Google Scholar]
- Winking J, Kaplan H, Gurven M, Rucas S. Why do men marry and why do they stray? Proc R Soc Lond B. 2007;274:1643–1649. doi: 10.1098/rspb.2006.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, van Doorn GS, Leimar O, Weissing FJ. Life-history trade-offs favour the evolution of animal personalities. Nature. 2007;447:581–585. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: Effects on cognition and emotion in animals and humans. Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Worthman CM. Habits of the heart: Life history and the developmental neuroendocrinology of emotion. Amer J Hum Biol. 2009;21:772–781. doi: 10.1002/ajhb.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM, Brown RA. A biocultural life history approach to the developmental psychobiology of male aggression. In: Stoff DM, Susman EJ, editors. Developmental psychobiology of aggression. Cambridge university Press; New York: 2005. [Google Scholar]
- Worthman CM, Kuzara J. Life history and the early origins of health differentials. Am J Hum Biol. 2005;17:95–112. doi: 10.1002/ajhb.20096. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko IS, van Rossum EFC, Koper JW, Kumsta R, Entriger S, Hellhammer DH. A psychobiological perspective on genetic determinants of hypothalamus-pituitary-adrenal axis activity. Ann NY Acad Sci. 2004;1032:52–62. doi: 10.1196/annals.1314.005. [DOI] [PubMed] [Google Scholar]
- Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension. JAMA. 2003;290:2138–2148. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, Hayawaka CM. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 2010;35:241–248. doi: 10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Zahavi A, Zahavi A. The handicap principle: A missing piece of Darwin’s puzzle. Oxford University Press; New York: 1997. [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]