Abstract
Described is a systematic study of the effects of varied backbone structure on the stabilities of pyr.pur.pyr triple helices. The effects were measured using six circular 34 base oligonucleotides containing DNA (D), RNA (R) and/or 2'-O-methyl-RNA (M) residues designed to bind a complementary single-stranded purine target strand by triple helix formation. Eighteen different backbone combinations were studied at pH 5.5 and 7.0 by optical melting experiments and the results compared with the stabilities of the corresponding Watson-Crick duplexes. When the target purine strand is DNA, all circles form pH-dependent triple helical complexes which are considerably stronger than the duplexes alone. When RNA is the target, five of the nine complexes studied are of the pH-dependent triplex type and the other four complexes are not significantly stronger than the corresponding duplexes. The results are useful in the design of the highest affinity ligands for single- and double-stranded DNAs and RNAs and also point out novel ways to engender DNA- or RNA-selective binding.
Full text
PDF
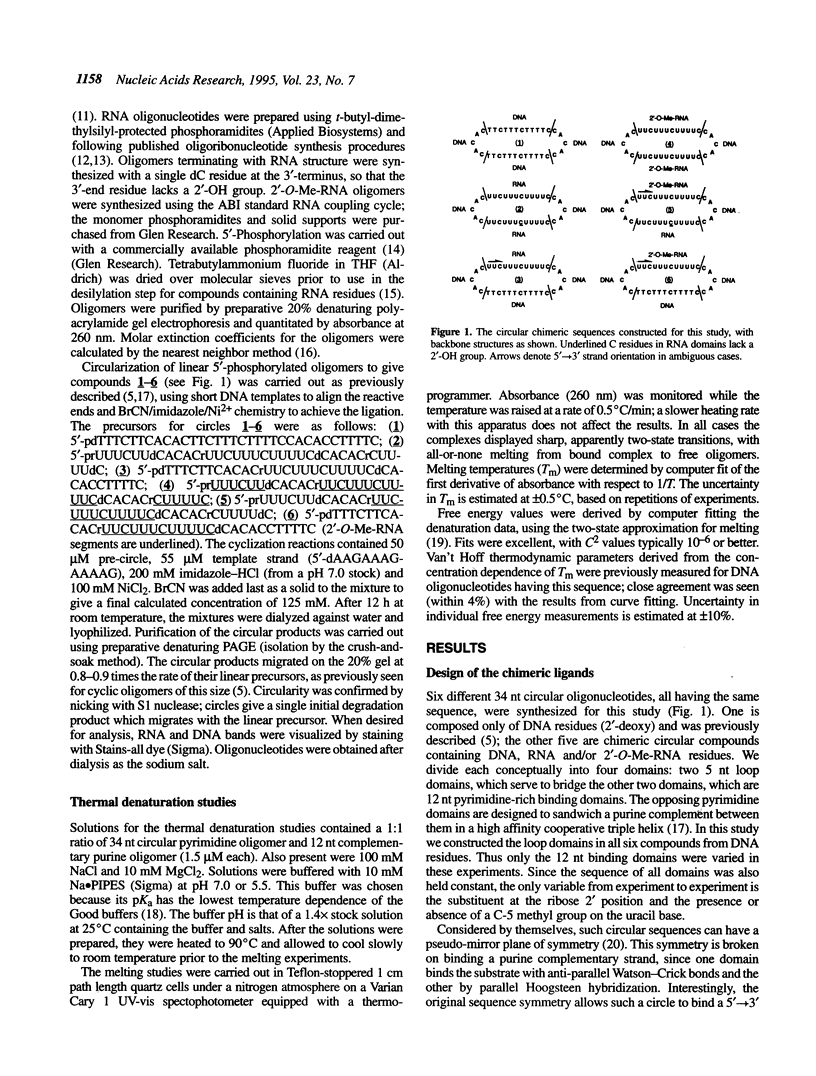
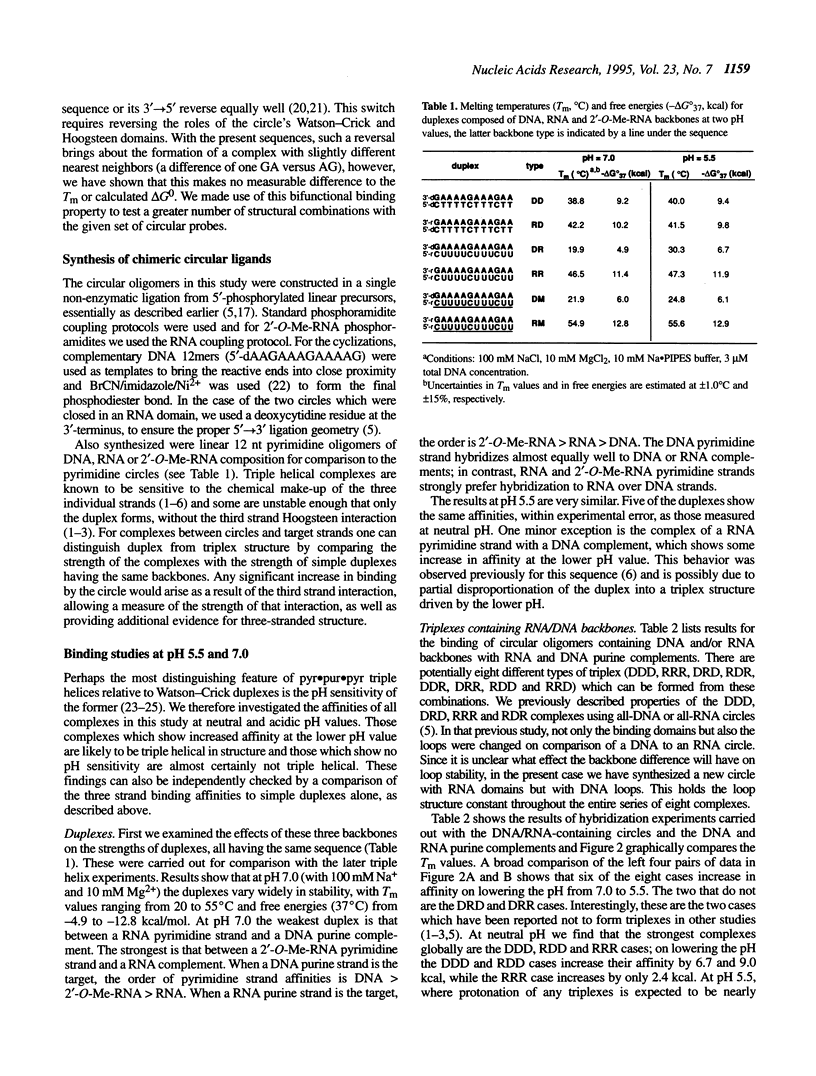
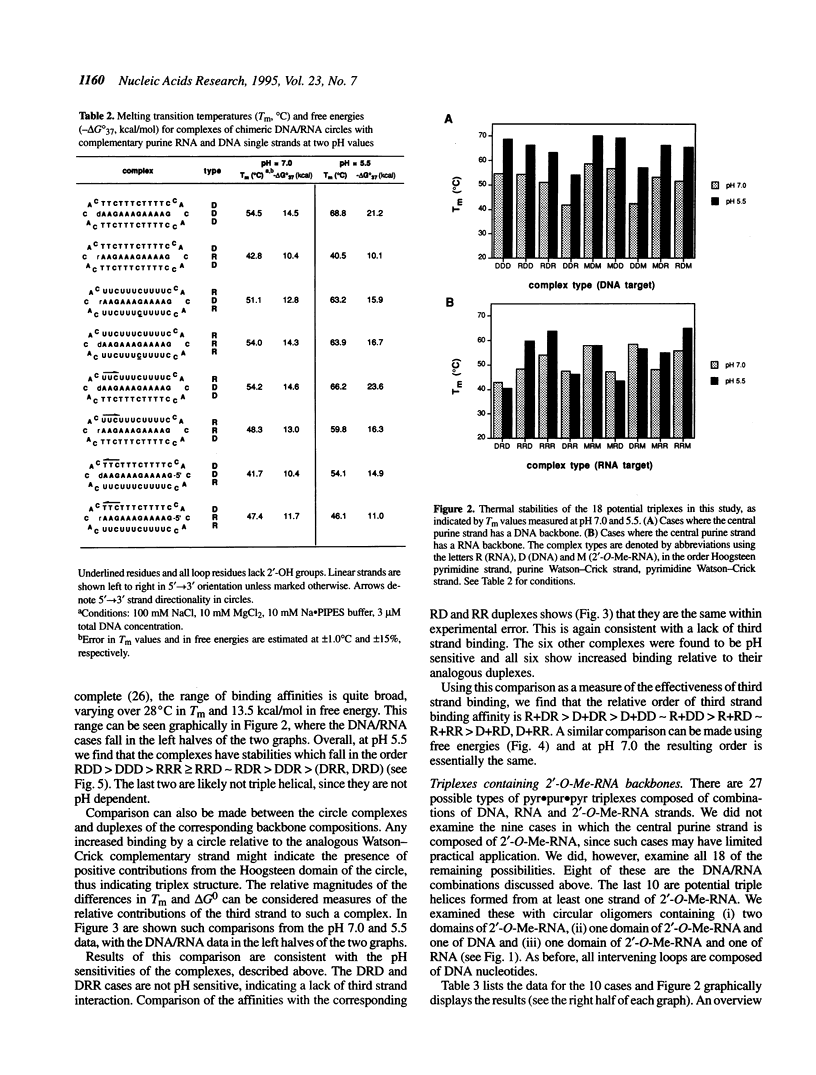
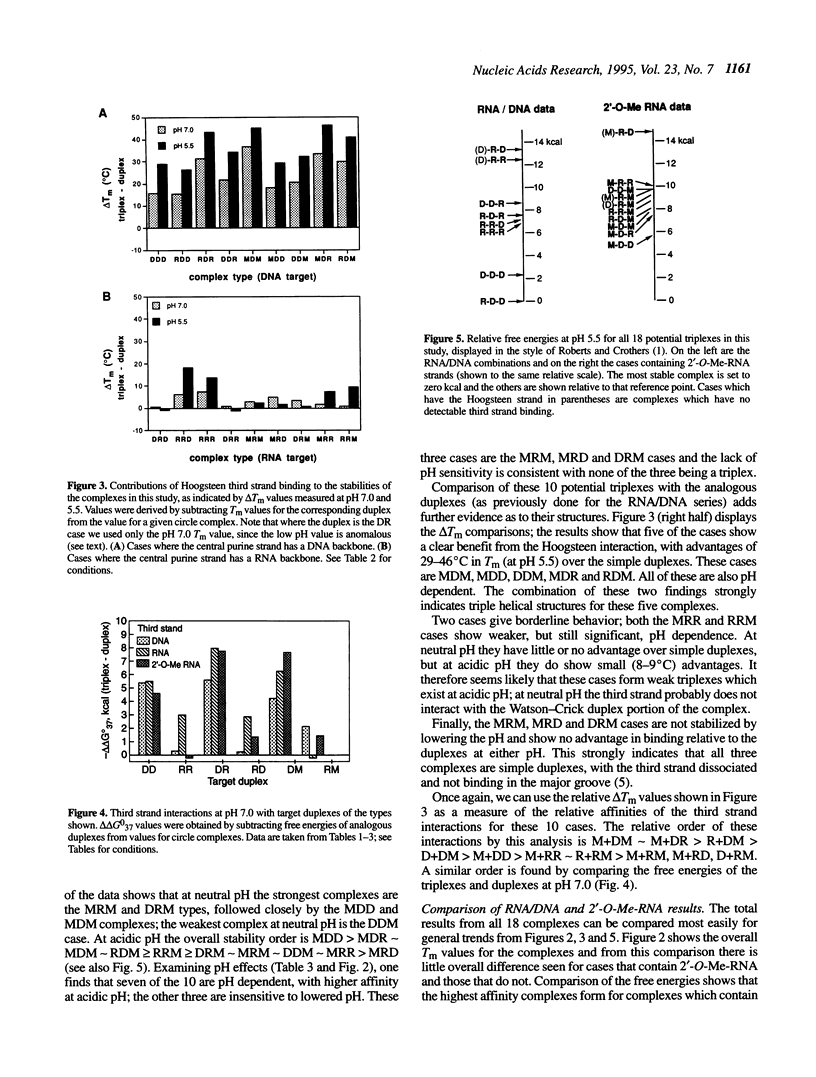



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan D. E., Trapane T. L., Miller P. S., Ts'o P. O., Kan L. S. Comparative circular dichroism and fluorescence studies of oligodeoxyribonucleotide and oligodeoxyribonucleoside methylphosphonate pyrimidine strands in duplex and triplex formation. Biochemistry. 1991 Feb 12;30(6):1650–1655. doi: 10.1021/bi00220a030. [DOI] [PubMed] [Google Scholar]
- D'Souza D. J., Kool E. T. Strong binding of single-stranded DNA by stem-loop oligonucleotides. J Biomol Struct Dyn. 1992 Aug;10(1):141–152. doi: 10.1080/07391102.1992.10508634. [DOI] [PubMed] [Google Scholar]
- Escudé C., François J. C., Sun J. S., Ott G., Sprinzl M., Garestier T., Hélène C. Stability of triple helices containing RNA and DNA strands: experimental and molecular modeling studies. Nucleic Acids Res. 1993 Dec 11;21(24):5547–5553. doi: 10.1093/nar/21.24.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudé C., Sun J. S., Rougée M., Garestier T., Hélène C. Stable triple helices are formed upon binding of RNA oligonucleotides and their 2'-O-methyl derivatives to double-helical DNA. C R Acad Sci III. 1992;315(13):521–525. [PubMed] [Google Scholar]
- Giannaris P. A., Damha M. J. Oligoribonucleotides containing 2',5'-phosphodiester linkages exhibit binding selectivity for 3',5'-RNA over 3',5'-ssDNA. Nucleic Acids Res. 1993 Oct 11;21(20):4742–4749. doi: 10.1093/nar/21.20.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannangeli C., Thuong N. T., Hélène C. Oligonucleotide clamps arrest DNA synthesis on a single-stranded DNA target. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10013–10017. doi: 10.1073/pnas.90.21.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gryaznov S. M., Lloyd D. H. Modulation of oligonucleotide duplex and triplex stability via hydrophobic interactions. Nucleic Acids Res. 1993 Dec 25;21(25):5909–5915. doi: 10.1093/nar/21.25.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Thermodynamic and structural properties of pentamer DNA.DNA, RNA.RNA, and DNA.RNA duplexes of identical sequence. Biochemistry. 1991 Nov 5;30(44):10606–10613. doi: 10.1021/bi00108a002. [DOI] [PubMed] [Google Scholar]
- Han H., Dervan P. B. Sequence-specific recognition of double helical RNA and RNA.DNA by triple helix formation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3806–3810. doi: 10.1073/pnas.90.9.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe R. I., McCaffrey A. P., Borozdina L. U., McCampbell E. S., Vaghefi M. M. Effect of excess water on the desilylation of oligoribonucleotides using tetrabutylammonium fluoride. Nucleic Acids Res. 1993 Oct 11;21(20):4739–4741. doi: 10.1093/nar/21.20.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R. H., Damha M. J. Association of branched nucleic acids. Nucleic Acids Symp Ser. 1993;(29):97–99. [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya E., Yanagawa H. Template-directed polymerization of oligoadenylates using cyanogen bromide. Biochemistry. 1986 Nov 18;25(23):7423–7430. doi: 10.1021/bi00371a026. [DOI] [PubMed] [Google Scholar]
- Kandimalla E. R., Agrawal S. Single-strand-targeted triplex formation: stability, specificity and RNase H activation properties. Gene. 1994 Nov 4;149(1):115–121. doi: 10.1016/0378-1119(94)90419-7. [DOI] [PubMed] [Google Scholar]
- LIPSETT M. N. COMPLEX FORMATION BETWEEN POLYCYTIDYLIC ACID AND GUANINE OLIGONUCLEOTIDES. J Biol Chem. 1964 Apr;239:1256–1260. [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Ratmeyer L., Vinayak R., Zhong Y. Y., Zon G., Wilson W. D. Sequence specific thermodynamic and structural properties for DNA.RNA duplexes. Biochemistry. 1994 May 3;33(17):5298–5304. doi: 10.1021/bi00183a037. [DOI] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semerad C. L., Maher L. J., 3rd Exclusion of RNA strands from a purine motif triple helix. Nucleic Acids Res. 1994 Dec 11;22(24):5321–5325. doi: 10.1093/nar/22.24.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Konishi A., Shimada Y., Inoue H., Ohtsuka E. Oligo(2'-O-methyl)ribonucleotides. Effective probes for duplex DNA. FEBS Lett. 1992 May 11;302(2):155–158. doi: 10.1016/0014-5793(92)80428-j. [DOI] [PubMed] [Google Scholar]
- Skoog J. U., Maher L. J., 3rd Repression of bacteriophage promoters by DNA and RNA oligonucleotides. Nucleic Acids Res. 1993 May 11;21(9):2131–2138. doi: 10.1093/nar/21.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Lamond A. I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989 May 11;17(9):3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Booher M. A., Kool E. T. Stabilities of nucleotide loops bridging the pyrimidine strands in DNA pyrimidine.purine.pyrimidine triplexes: special stability of the CTTTG loop. Biochemistry. 1994 Apr 19;33(15):4639–4644. doi: 10.1021/bi00181a026. [DOI] [PubMed] [Google Scholar]
- Wang S., Kool E. T. Circular RNA oligonucleotides. Synthesis, nucleic acid binding properties, and a comparison with circular DNAs. Nucleic Acids Res. 1994 Jun 25;22(12):2326–2333. doi: 10.1093/nar/22.12.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xodo L. E., Manzini G., Quadrifoglio F. Spectroscopic and calorimetric investigation on the DNA triplex formed by d(CTCTTCTTTCTTTTCTTTCTTCTC) and d(GAGAAGAAAGA) at acidic pH. Nucleic Acids Res. 1990 Jun 25;18(12):3557–3564. doi: 10.1093/nar/18.12.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]


