Abstract
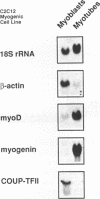
COUP-TF II is an 'orphan steroid receptor' that binds a wide variety of AGGTCA repeats and represses thyroid hormone (T3) and retinoid dependent trans-activation; however, very little is known of its functional and/or developmental role during mammalian cell differentiation. T3 and retinoids have been demonstrated to promote terminal muscle differentiation via activation of the muscle specific myoD gene family (myoD, myogenin, myf-5 and MRF-4). The myoD gene family can direct the fate of mesodermal cell lineages, repress proliferation, activate differentiation and the contractile phenotype. Hence, we investigated the expression and functional role of COUP-TF II during muscle differentiation. Proliferating C2C12 myoblasts expressed COUP-TF II mRNA which was repressed when cells were induced to differentiate into post-mitotic multinucleated myotubes by serum withdrawal. Concomitant with the decrease of COUP-TF II mRNA was the appearance of muscle specific mRNAs (e.g. myogenin, alpha-actin). We show that Escherichia coli expressed full length and truncated COUP-TF II bound in a sequence specific manner to the T3 response elements (TREs) in the myoD and myogenin regulatory HLH genes [Olson (1992) Dev. Biol. 154, 261-272]; and the TRE in the skeletal alpha-actin contractile protein gene. COUP-TF II diminished the homodimeric binding of the thyroid hormone receptor and the heterodimeric binding of thyroid hormone and retinoid X receptor complexes to these TREs. Constitutive over-expression of COUP-TF II cDNA in mouse C2C12 myogenic cells suppressed the levels of myoD mRNA and blocked the induction of myogenin mRNA, whereas constitutive expression of anti-sense COUP-TF II cDNA significantly increased the steady state levels of myoD mRNA and hyper-induced myogenin mRNA. These studies demonstrate for the first time (i) that COUP-TF II, functions as a physiologically relevant antagonistic regulator of myogenesis via direct effects on the myoD gene family and (ii) direct evidence for the developmental role of COUP-TF II during mammalian cell differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Berrodin T. J., Marks M. S., Ozato K., Linney E., Lazar M. A. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol Endocrinol. 1992 Sep;6(9):1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- Cooney A. J., Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993 Feb 25;268(6):4152–4160. [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Downes M., Griggs R., Atkins A., Olson E. N., Muscat G. E. Identification of a thyroid hormone response element in the mouse myogenin gene: characterization of the thyroid hormone and retinoid X receptor heterodimeric binding site. Cell Growth Differ. 1993 Nov;4(11):901–909. [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Green S. Nuclear hormone receptors. Promiscuous liaisons. Nature. 1993 Feb 18;361(6413):590–591. doi: 10.1038/361590a0. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Tsai S. Y., Tsai M. J., O'Malley B. W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J Biol Chem. 1992 Sep 5;267(25):17617–17623. [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang N., Teng C. T. COUP-TF acts as a competitive repressor for estrogen receptor-mediated activation of the mouse lactoferrin gene. Mol Cell Biol. 1993 Mar;13(3):1836–1846. doi: 10.1128/mcb.13.3.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990 Jan 26;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- Muscat G. E., Gobius K., Emery J. Proliferin, a prolactin/growth hormone-like peptide represses myogenic-specific transcription by the suppression of an essential serum response factor-like DNA-binding activity. Mol Endocrinol. 1991 Jun;5(6):802–814. doi: 10.1210/mend-5-6-802. [DOI] [PubMed] [Google Scholar]
- Muscat G. E., Griggs R., Downes M., Emery J. Characterization of the thyroid hormone response element in the skeletal alpha-actin gene: negative regulation of T3 receptor binding by the retinoid X receptor. Cell Growth Differ. 1993 Apr;4(4):269–279. [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Mynett-Johnson L., Dowhan D., Downes M., Griggs R. Activation of myoD gene transcription by 3,5,3'-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors. Nucleic Acids Res. 1994 Feb 25;22(4):583–591. doi: 10.1093/nar/22.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Conneely O. M. Orphan receptors: in search of a unifying hypothesis for activation. Mol Endocrinol. 1992 Sep;6(9):1359–1361. doi: 10.1210/mend.6.9.1331771. [DOI] [PubMed] [Google Scholar]
- Olson E. N. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992 Dec;154(2):261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson E. N. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993 Nov;7(11):1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- Power R. F., Conneely O. M., O'Malley B. W. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992 Aug;13(8):318–323. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Schnegelsberg P. N., Stead R. H., Braun T., Arnold H. H., Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993 Dec 31;75(7):1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P., Zhang X. K., Salbert G., Hermann T., Lehmann J. M., Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992 Oct;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Sagami I., Wang H., Tsai M. J., O'Malley B. W. Interactions between a DNA-binding transcription factor (COUP) and a non-DNA binding factor (S300-II). Cell. 1987 Aug 28;50(5):701–709. doi: 10.1016/0092-8674(87)90328-x. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- Widom R. L., Rhee M., Karathanasis S. K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992 Aug;12(8):3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]