Abstract
The pancreas has both endocrine and exocrine functions. As an endocrine organ, stimulation of the pancreatic β-cells results in insulin secretion to control systemic glucose levels. The exocrine function of the pancreas and the need for alkaline pancreatic secretion (pH 8.0–8.5) have been appreciated for more than 40 years. Yet, our knowledge of the cellular mechanisms (signaling, transporters and channels) which accomplish these critical functions has evolved greatly. In the mid-1990s, basolateral Na-bicarbonate (HCO3−) uptake by NBCe1 (Slc4a4) was shown to be critical for the generation of approximately 75% of stimulated HCO3− secretion. In the last 10 years, several new HCO3− transporters in the Slc26 family and their interaction with the cystic fibrosis transmembrane conductance regulator-chloride channel have elucidated the HCO3− exit step at the ductal lumen. Most recently, both IRBIT (inositol 1,4,5-trisphosphate receptor-binding protein) and WNK [with no lysine (K)] kinase have been implicated as additional HCO3− secretory controllers.
Key Words: Cystic fibrosis transmembrane conductance regulator, SLC26 family, Pancreatic duct
The pancreas has both endocrine and exocrine functions. As an endocrine organ, stimulation of the pancreatic β-cells results in insulin secretion to control systemic glucose levels. The exocrine function of the pancreas and the need for alkaline pancreatic secretion have been appreciated for more than 40 years. Yet, our knowledge of the cellular mechanisms (signaling, transporters and channels) which accomplish these critical functions has evolved greatly.
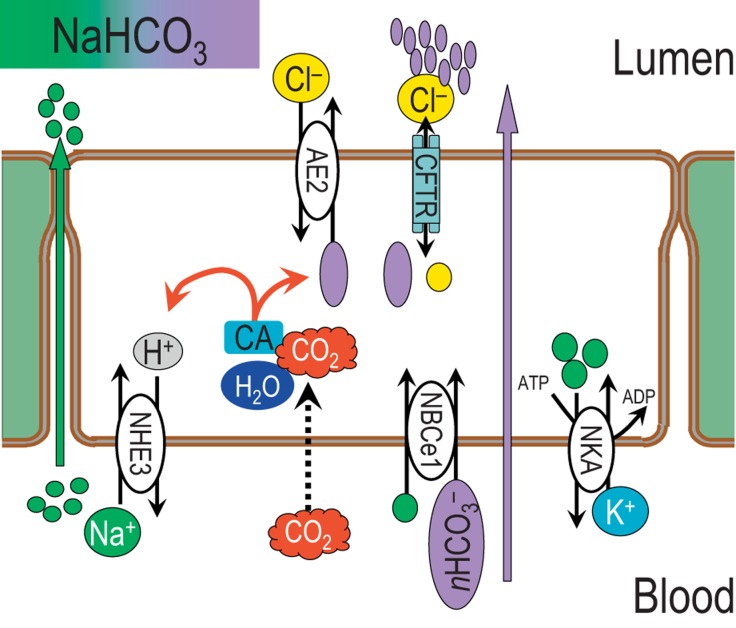
The major function of the exocrine pancreas is to produce and secrete digestive enzymes into the duodenum. To prevent the pancreas from digesting itself, these digestive enzymes are kept inactive by elevated pH (>8.0) of the pancreatic juice. The high pH is the result of co-secretion of bicarbonate (HCO3−). That said, the HCO3− concentration of secretions varies greatly between species: approximately 70–75 mM for rats and mice, and about 150 mM (isotonic NaHCO3) for cats, guinea pigs and humans. It is this difference in the 2-fold HCO3− concentration which has perplexed physiologists for 30 years. Reaching 75 mM NaHCO3 can be accomplished by the channels and transporters shown in figure 1. However, additional HCO3− secretion must occur in the context of a continually decreasing ductal Cl− concentration, which, based on the transport shown in figure 1, violates thermodynamics.
Fig. 1.
HCO3− transport by the pancreatic duct, prior to 2004. Transductal NaHCO3 secretion is accomplished by predominantly (75%) basolateral NBCe1-B and apical CFTR Cl− channel. Other transporters involved in sodium, potassium and chloride are anion exchanger 2 (AE2) and NHE3. CO2 from the blood is hydrated and then carbonic anhydrase (CA) makes HCO3− and H+. For each H+ excluded from the cell, 1 HCO3− is transported in the lumen of the pancreatic duct or exchanged for Cl− on the basolateral membrane. NKA = Na+/K+ ATPase.
Pancreatic acinar cells are responsible for enzyme secretion while pancreatic ductal cells are responsible for the secretion of ions (predominantly Na+ and HCO3−) and water. Failure in the transport of different ions including HCO3− in the pancreatic duct is most obvious in cystic fibrosis [mutations in the cystic fibrosis transmembrane conductance regulator (CFTR)], whereas in the pancreas the mucus secretion blocks the ducts [1].
In order to secrete HCO3−, ductal cells have HCO3− or H+ transporters on both basolateral and apical membranes. Until recently, the major player in HCO3− secretion in the apical membrane was consider to be CFTR (fig. 1) [2,3,4]. At that time, CFTR was consider to be a chloride channel capable of HCO3− transport activated by an increase in intracellular cAMP [5,6]. The model indicated that activity of carbonic anhydrase produces HCO3−, which is then secreted into the ductal lumen. At the same time, an H+ is transported into the cell by a Na+/H+ exchanger, NHE3 (fig. 1). This model later evolved to include an apical HCO3− exchanger, presumed to be anion exchanger 1, 2 or 3 (Slc4a1–3)1.
At the basolateral membrane, a HCO3−transporter was not initially proposed. A combination of CO2 ‘diffusion across the membrane’ and a Na+/H+ exchanger was sought and then shown to mediate net HCO3− entry [2,7,8]. The model indicated that activity of carbonic anhydrase produces HCO3−, which is then secreted into the ductal lumen. At the same time, an H+ is transported into the cell by a Na+/H+ exchanger (fig. 1). Discovery [9] and cloning of the electrogenic Na+/nHCO3− co-transporter (NBCe1, Slc4a4) [10] revealed another basolateral entry pathway from the blood [8]. NBCe1 transports HCO3− into the ductal cells using the electrochemical gradients (Na+, HCO3−, voltage) maintained by Na+/K+ ATPase. Thus, the Slc4 HCO3− transporters were believed to account for the major apical and basolateral transporters.
In 1999, Melvin and associates [11] discovered that Slc26a3 (downregulated in adenoma) functions as a Cl−-HCO3− exchanger. This discovery revealed that another protein family (Slc26) could have HCO3− transport activities, which has forever changed the dogma that only Slc4 proteins transport HCO3−.
With the discovery of Slc26 Cl−-HCO3− exchangers, the physiological role of CFTR-HCO3− transport by CFTR is becoming more disputed in the pancreatic ductal cells [12]. This dispute was further complicated by the discoveries that Cl−:HCO3− coupling in mice is 2:1 for Slc26a3 [13] and 1:2 for Slc26a6 [13,14]. A further feature is that human SLC26A6 coupling is 1:1 [15]. Three of 11 members of the Slc26 family seem involved in pancreatic HCO3− secretion (Slc26a3, Slc26a6 and Slc26a9) [13,16,17]. All are found on apical epithelial membranes [17,18].
Slc26 proteins transport anions as exchangers or channels [18,19,20,21]. Slc26a6 also transports oxalate, formate and sulfate [14,22,23], whereas Slc26a9 possesses several transport modes: nCl−-HCO3− exchanger, anion channel [20,24,25,26] and Na+ transporter [26].
The relationship of Slc26 proteins with CFTR is a 2-way street. When R-CFTR binds the sulfate transporter anti-σ domain of Slc26a3 or Slc26a6, exchange activity is increased [13] while the same interaction with Slc26a9 inhibits transport [27]. This interaction also increases CFTR-Cl− channel activity [16,21], although Slc26a9-sulfate transporter anti-σ interaction activation requires cAMP [25,28]. Not surprisingly, Slc26a9 does not stimulate ΔF508-CFTR activity [29], the most common mutation in cystic fibrosis, implying that Slc26a9 may be associated with the severity of cystic fibrosis phenotypes.
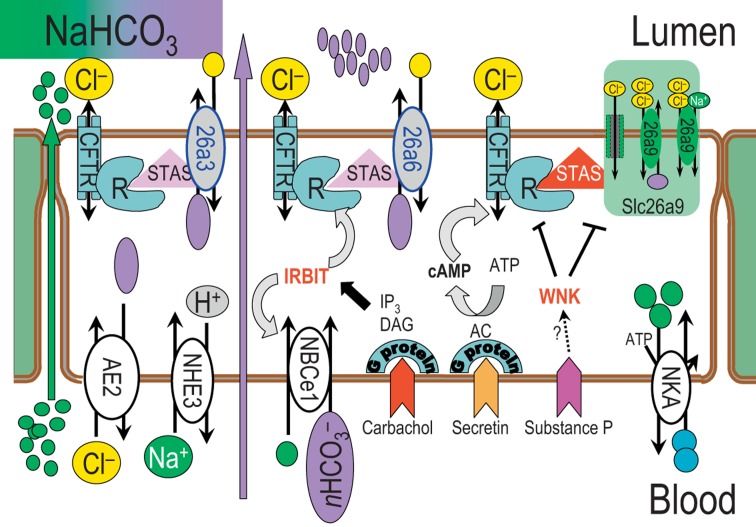
Several of these interactions and regulatory cascades are controversial. Cl− exit via CFTR and H+ recirculating by NHE3 [30] are necessary for Cl−-HCO3− exchange activity of Slc26a3/Slc26a6 and HCO3− secretion. Slc26a6-like Cl−-HCO3− exchange activity increases with CFTR inhibition in the apical membrane of guinea pig pancreatic ductal cells [31], opposed to the results in HEK-293 cells [13]. Similarly, Slc26a9 [24] as well as CFTR [32] can be inhibited by WNK1/4 [with no lysine (K) 1/4] kinases, which are mutated in familial hyperkalemic hypertension. At physiological inositol-3-phosphate, its receptor releases IRBIT (inositol 1,4,5-trisphosphate receptor-binding protein) to apparently activate downstream molecules. Recently, IRBIT was found to increase basolateral NBCe1 [33,34] and CFTR activity [34] (fig. 2). Thus, increasing IRBIT in pancreatic ductal cells would lead to stimulation of transductal HCO3− secretion [34].
Fig. 2.
An updated model of the HCO3− transport by the pancreatic duct. Apical players are Slc26a3, Slc26a6 and Slc26a9 members which can interact with CFTR. Slc26a9 and CFTR are inhibited by WNK kinases and possibly activated by substance P. NBCe1-B is the major basolateral HCO3− transporter (pNBCe1), which is activated by IRBIT, thereby increasing HCO3− secretion by pancreatic duct cells. R = R region; STAS = sulfate transporter anti-σ; AE2 = anion exchanger 2; IP3 = inositol-3-phosphate; DAG = diacyl-glycerol; AC = adenylate cyclase; NKA = Na+/K+ ATPase.
Future studies will almost certainly reveal additional details of the regulation as well as the pathophysiology of pancreatic HCO3− secretion. Fortunately, we can now at least account for the mechanism of isotonic NaHCO3 secretion found in humans.
Acknowledgements
We thank our collaborators and past members of the Romero lab who contributed to some of the basic physiology. Our apologies to colleagues whose work we could not cite due to space constraints. This work was supported by NIH EY017732, DK083007 (M.F.R.), the Cystic Fibrosis Foundation (Sindic-06F0, Romero-06G0), the American Heart Association (C.R.S.) and the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30-DK084567).
Footnotes
SLC is the Human Genome Organization nomenclature for solute carriers. There are presently 46 known Slc gene families. Human genes are represented with capitals, while genes from other organism are given in lowercase letters. See http://www.bioparadigms.org/slc/menu.asp for detailed explanation of these Slc gene families.
References
- 1.Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3– transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Case RM, Argent BE. Pancreatic secretion of electrolytes and water. In: Schultz SG, Forte JG, Fauner BB, editors. Handbook of Physiology. The Gastrointestinal System. New York: Oxford University; 1989. pp. 383–417. [Google Scholar]
- 3.Sohma Y, Gray MA, Imai Y, Argent BE. HCO3– transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol. 2000;176:77–100. doi: 10.1007/s00232001077. [DOI] [PubMed] [Google Scholar]
- 4.Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl–/HCO3– exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 5.Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol. 1993;264:C591–C602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- 6.Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl– conductance. Am J Physiol. 1994;266:L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- 7.Sohma Y, Gray MA, Imai Y, Argent BE. A mathematical model of the pancreatic ductal epithelium. J Membr Biol. 1996;154:53–67. doi: 10.1007/s002329900132. [DOI] [PubMed] [Google Scholar]
- 8.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3– by Na+-HCO3– cotransport in interlobular ducts from guinea-pig pancreas. J Physiol (Lond) 1996;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3– transport. J Gen Physiol. 1983;81:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3– cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- 11.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl–/HCO3– exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- 12.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 13.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3– transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular and functional characterization of the Slc26A6 anion exchanger, functional comparison to Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 15.Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem. 2005;280:8564–8580. doi: 10.1074/jbc.M411703200. [DOI] [PubMed] [Google Scholar]
- 16.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sindic A, Mount DB, Plata C, Sussman CR, Romero MF. The rat Slc26a9 anion transporter is located in many epithelial tissues. Pediatr Pulmonol. 2007;42:283. [Google Scholar]
- 18.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol. 2011;73:14.1–;14.21. doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 20.Romero MF, Chang M-H, Plata C, Zandi-Nejad K, Broumand V, Sussman CR, Mount DB. Physiology of electrogenic SLC26 paralogues. Novartis Found Symp. 2006;273:126–147. [PubMed] [Google Scholar]
- 21.Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- 22.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 24.Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J. Characterization of SLC26A9, facilitation of Cl– transport by bicarbonate. Cell Physiol Biochem. 2008;22:15–30. doi: 10.1159/000149780. [DOI] [PubMed] [Google Scholar]
- 26.Chang M-H, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26A9 – anion exchanger, channel and Na+ transporter. J Membr Biol. 2009;128:125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang M-H, Plata C, Sinđić A, Ranatunga WK, Chen AP, Zandi-Nejad K, Chan KW, Thompson J, Mount DB, Romero MF. Slc26a9 is inhibited by the R-region of CFTR via the STAS domain. J Biol Chem. 2009;284:28306–28318. doi: 10.1074/jbc.M109.001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133:421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avella M, Loriol C, Boulukos K, Borgese F, Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J Cell Physiol. 2011;226:212–223. doi: 10.1002/jcp.22328. [DOI] [PubMed] [Google Scholar]
- 30.Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCOFormula secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol. 2010;298:C1057–C1065. doi: 10.1152/ajpcell.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl–/HCO3– exchange with CFTR in stimulated HCO3– secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1307–G1317. doi: 10.1152/ajpgi.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH. WNK1 and WNK4 modulate CFTR activity. Biochem Biophys Res Commun. 2007;353:535–540. doi: 10.1016/j.bbrc.2006.11.151. [DOI] [PubMed] [Google Scholar]
- 33.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3– cotransporter 1 (pNBC1) Proc Natl Acad Sci USA. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3– secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]