Abstract
Endothelial cells (ECs) are constantly exposed to blood flow-induced shear forces in the vessels and this is a major determinant of endothelial function. Ion channels have a major role in endothelial function and in the control of vascular tone. We hypothesized that shear force is a general regulator of ion channel expression, which will have profound effects on endothelial function. We examined this hypothesis using large-scale quantitative real-time RT-PCR. Human coronary artery ECs were exposed to two levels of flow-induced shear stress for 24 h, while control cells were grown under static conditions. The expression of ion channel subunits was compared between control and flow-adapted cells. We used primers against 55 ion channel and exchanger subunits and were able to detect 54 subunits. Five dyn/cm2 of shear induced downregulation of 1 (NCX1) and upregulation of 18 subunits, including KCa2.2, KCa2.3, CX37, Kv1.5 and HCN2. Fifteen dyn/cm2 of shear stress induced the expression of 30 ion channel subunits, including KCa2.3, KCa2.2, CX37, Kir2.3 and KCa3.1. Our data demonstrate that substantial remodeling of endothelial ion channel subunit expression occurs with flow adaptation and suggest that altered ion channel expression may significantly contribute to vascular pathology associated with flow-induced alterations.
Key Words: Coronary artery, Endothelium, Ion channel, Shear
Introduction
Endothelial cells (ECs) line in the luminal side of blood vessels and are constantly exposed to shear stress due to fluctuations in blood flow. Changes in these shear forces are sensed by the endothelium and are responsible for mechanochemical signal transduction [1]. It is well documented that flow-sensitive ion channels, integrins, receptor tyrosine kinases, G protein-coupled receptors, lipid domains of the membrane and the cytoskeleton are all involved in sensing of shear [2,3]. This results in activation of intracellular signaling pathways and subsequent changes in structural elements as well as alterations in gene expression patterns of the EC. Cumulatively, these responses lead to major changes in endothelial functions, including proliferation, apoptosis, alignment, migration and permeability [3,4]. The ability of ECs to sense flow allows acute flow-mediated arterial vasoconstriction and vasodilation as well as wall remodeling due to chronic changes in flow [5]. Moreover, abnormalities in flow-mediated responses of ECs, for example during endothelial dysfunction, are associated with vascular pathology. Accumulating data suggest that disturbed or oscillatory flow is proatherogenic, through the induction of proinflammatory, procoagulant, proliferative and proapoptotic molecules, and sustained laminar flow provides protection from atherosclerosis through the induction of antithrombotic and antiproliferative factors [3,5,6].
Among the first cellular components identified to mediate the endothelial response to shear were ion channels. In particular, it was shown that shear stress induces rapid activation of inward-rectifying K+currents that result in membrane hyperpolarization and Ca2+ entry. Subsequently, various types of Ca2+-permeable cation channels, K+ channels, and Cl– channels have also been implicated in endothelial mechanosensing [7,8,9,10,11]. The long-term effects that flow has on ion channel expression and function are poorly investigated. It has been reported that flow mediates changes in the expression of specific ion channel subunits in the endothelium. For example, the intermediate-conductance Ca2+ channel subunit KCa3.1 [12], ATP-sensitive K+ (KATP) channel subunit Kir6.2 [13] and the T-type voltage-gated Ca2+ channel subunit Cav3.1 [14] are reported to be upregulated by flow. Based on these findings, we hypothesized that shear force is a general regulator of ion channel expression, which will have profound effects on endothelial function. In order to test our hypothesis, we used a large-scale quantitative RT-PCR assay (qRT-PCR) to examine the transcriptional levels of about 60 ion channel and exchanger subunits in ECs that have been cultured under static or flow-adapted conditions. We found that a remarkable transcriptional upregulation of ion channels occurs with flow adaptation that is also dependent on the levels of flow.
Materials and Methods
Flow Adaptation of ECs
Human coronary artery ECs (HCAECs; Lonza, Walkersville, Md., USA) were grown in EGM2-MV (Lonza) and were used between the 4th and 6th passage. HCAECs were exposed to defined amounts of shear stress using an artificial capillary system (FiberCell™ 4300-C2025) as previously described [15]. Artificial capillaries were activated according to the manufacturer's instructions and precoated with 0.2% gelatin. Cells were allowed to attach for 18 h and constant laminar flow sufficient to generate 5 or 15 dyn/cm2 of shear force was applied for 24 h. The two levels of shear stress were selected based on a large body of previously published data. Control cells (same passage and confluence) were grown in plates under static culturing conditions.
Large-Scale qRT-PCR
We used qRT-PCR to quantify the mRNA expression of a large number of ion channel transcripts, as described previously [16]. We used primers against 56 ion channel and exchanger subunits, designed with the Primer3 software [17]. The selection was made based on expression profiling data obtained from microarray data with ECs and other literature sources. Primers were designed to span two exons to avoid PCR amplification of genomic sequences. Other design considerations included a melting temperature of 60°C and a PCR amplicon of 90–120 base pairs. Total RNA was extracted from control and flow-adapted cells by the acid phenol guanidinium method (TriReagent, Sigma-Aldrich, St. Louis, Mo., USA). Total RNA was reverse transcribed using the Superscript III enzyme (Invitrogen) with random hexamer primers according to the manufacturer's guidelines. PCRs were performed with an ABI Prism 7900HT sequence detection system (Perkin-Elmer Applied Biosystems) with a SYBR green master mix (Perkin-Elmer Applied Biosystems). The thermal cycling conditions comprised an initial denaturing step at 95°C for 10 min and 40 cycles at 95°C for 5 s, 60°C for 15 s, and 72°C for 15 s, followed by a melting curve analysis.
Data Analysis
The PCR threshold cycle (the fractional cycle number at which the fluorescence level is distinguishable from the background, Ct) values were measured within the exponential phase of the PCR using SDS software (version 2.2, Applied Biosystems). Reactions with any evidence of nonspecificity (low melting temperatures or multiple peaks in melting-point analysis) were excluded from the analysis. We performed an initial characterization of the qRT-PCR assay in terms of its reproducibility and sensitivity. There was a high degree of reproducibility within plates [16]. The median value of the coefficient of variation of Ct values for triplicate reactions for the data presented in this study was 1.58% (range: 0.01–18.68%).
It is increasingly common with qRT-PCR to use a set of reference genes to estimate gene expression levels (e.g. [16,18]). We have used this technique in the present study and calculated expression values of each gene relative to the geometric mean of the three reference genes (‘housekeeping’ genes). For a given experiment, the expression of each gene was measured in triplicate (technical replicates), which was averaged to obtain a single expression value for that gene. The values plotted in figure 1 represent the average from 6 independent experiments (biological replicates). The subset of reference genes used included histone H3, hydroxymethylbilane synthase and dihydrofolate reductase, as previously described [16]. The selection of stable reference genes (i.e. that did not change their expression with flow adaptation) was made using the Normfinder algorithm [19]. The relative expression of ion channel subunits calculated for the flow-adapted cells was compared with the relative expression of the subunits calculated for the control cells (cultured under static conditions) to obtain the fold induction or suppression of the subunit expression. Statistical analysis was performed using a nonparametric test, the Significance Analysis of Microarrays (SAM) [20]. For this analysis, we used the Multi Experiment Viewer software (MEV, version 4.5.1); the parameters of the analysis included: use of all unique permutations, selection of S0 using the method of Tusher et al. [20] and cutoff Δ-value at false discovery rate zero. Data in figures 1 and 4 are presented as means ± SEM. Heat maps were produced using the MEV software.
Fig. 1.
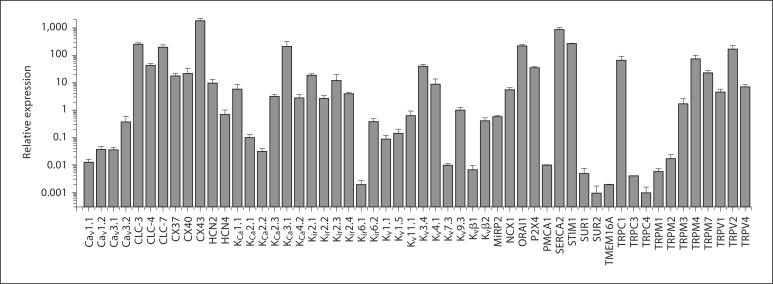
Expression of ion channel subunits and exchangers in HCAECs under basal conditions. qRT-PCR assays of 6 separate experiments were performed for 54 subunits of ion channels, exchangers and pumps. For each experiment, the expression was calculated relative to that a set of reference genes (see Methods) and depicted on a logarithmic scale. Data are presented as mean ± SEM.
Fig. 4.
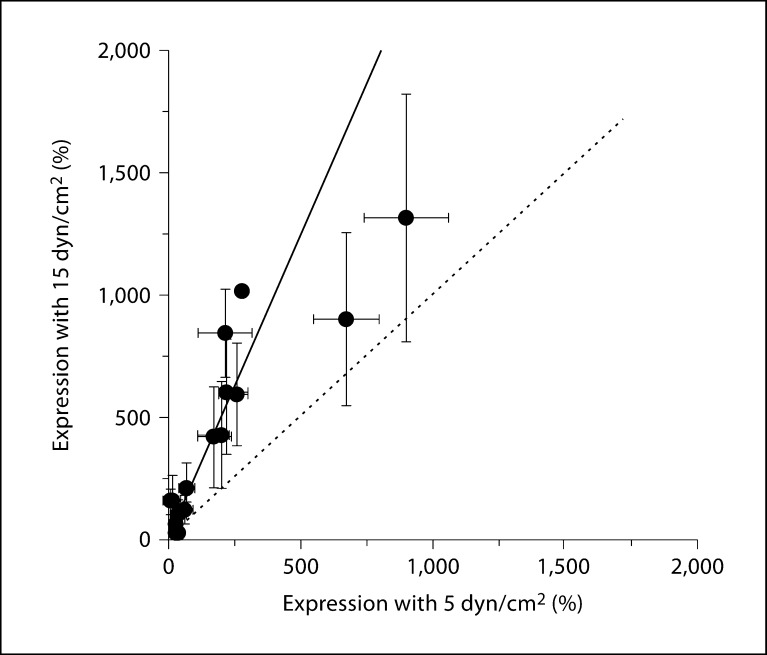
Relationship between two different applied shear forces on mRNA expression. The mRNA expression levels of 54 ion translocator subunits, relative to the reference genes, were determined at 24 h applied shear of 5 or 15 dyn/cm2 (expression values relative to reference genes after log2 transformation, same data as in fig. 3). For each subunit, the expression levels at the two shear conditions are plotted as a function of the other. The solid line represents a least-squares fitting of a straight line to the data (slope = 2.4, intercept forced through zero). For comparison, the dotted line indicates a line with unity slope.
Analysis of Microarray Data
Microarray data produced by different investigators are publicly available in Gene Expression Omnibus provided by the National Center for Biotechnology Information. We examined studies that investigated changes in endothelial gene expression levels due to changes in shear forces. We then looked for changes in ion channel and transporter gene expression. The data used were from the GSE1518 data set record. Raw data were analyzed using the Genespring GX10 software.
Results
Expression Profiles of Ion Channel Subunits in HCAECs
Our first goal was to assess the transcriptional profile of ion channel, exchanger and pump subunits in HCAECs. Microarray data have not been helpful in this regard since ion channels are generally expressed at low levels and are sometimes not readily detected with this method. We therefore used a large-scale qRT-PCR assay [16] and focused on a subset of ion transporter subunits identified from the literature and our past experience. We used a well-characterized commercial primary cell line of HCAECs. Of 55 ion channel, exchanger and pump subunits examined, we found 54 to be expressed at varying degrees in HCAECs under basal conditions (i.e. when cells were grown under static conditions). Their expression levels were calculated relative to 3 reference genes (histone H3, hydroxymethylbilane synthase, dihydrofolate reductase) and the average expression levels of 6 separate experiments are shown in figure 1. The qRT-PCRs failed for one of the ion channel subunits (Cavα2δ), which could be attributed to low expression levels or to ineffective PCR primers. In a few cases, in particular when the channel expression levels were very low, the PCRs did not result in detectable amplification product for some genes. The average expression of the respective genes was calculated from the remaining successful reactions.
K+ Channel Subunits
The expression of all 4 members of the Kir2 inward rectifying K+ (Kir) channel subfamily was relatively abundant, with Kir2.1 and Kir2.3 present at higher levels than Kir2.2 and Kir2.4. The other Kir subfamily examined (Kir6) displayed preferential expression of Kir6.2 relative to Kir6.1. The Kir6 subunit forms a tetrameric assembly with an accessory subunit, the sulfonylurea receptor (SURx). Interestingly, we found SUR1 to be expressed at higher levels relative to SUR2. We also examined expression of Ca2+-activated K+ (KCa) channel subunits. The large-conductance channel subunit (KCa1.1 or slo) was abundantly expressed. KCa4.2 (or Slo2.1) was expressed at equivalent levels. Amongst the small-conductance Ca2+-activated K+ channel subunits, KCa2.3 was expressed at higher levels relative to KCa2.1 and KCa2.2. Strong transcriptional expression was observed for the intermediate-conductance Ca2+-activated K+ channel subunit, KCa3.1 (or IKCa1). Consistent with previous reports [21], we found expression of some of the subunits of the voltage-gated K+ (Kv) family. Of note is the relatively high expression level of Kv3.4 and Kv4.1. Amongst the auxiliary subunits of Kv channels examined, Kvβ2 and MiRP2 were expressed at equivalent levels and at higher levels relative to Kvβ1.
Ca2+ Channels, Exchangers and Pumps
ECs are nonexcitable and do not depend on voltage changes for alterations in intracellular Ca2+[21]. Nevertheless, we could detect expression of all 4 of the plasmalemmal voltage-gated Ca2+ (Cav) channel subunits that we examined. Of these, Cav3.2 was the most abundantly expressed (we did not examine any of the Ca2+ channel subunit auxiliary subunits). Perhaps not surprisingly, higher expression levels were found for subunits involved in alternative pathways for plasmalemmal Ca2+ entry, including ORAI1 and the stromal interaction molecule 1 (STIM1) subunit. Possible mechanisms for plasmalemmal Ca2+ extrusion include the plasma membrane Ca2+ pump (PMCA1, which was expressed at very low levels) and the Na+/Ca2+ exchanger (NCX1, which was expressed at higher levels). Although our focus was not on intracellular channels, we examined the sarcoplasmic/endoplasmic reticulum Ca2+ pump (SERCA2), which was robustly expressed in these ECs. Extracellular ATP induces Ca2+ influx across the plasma membrane and activates Ca2+ release from intracellular pools in vascular ECs. This process is thought to be mediated by P2×4 purinergic receptors [22], which we found to be expressed relatively abundantly in HCAECs.
Transient Receptor Potential Channels
Since the transient receptor potential (TRP) channels are important in endothelial function [21], we systematically examined the expression of 12 members of this channel subunit family. Amongst the TRPCs, TRPC1 was expressed at the highest levels, whereas TRPM4, TRPM7 and TRPM3 were most abundantly expressed in the TRPM subfamily. All 3 members of the TRPV subfamily examined (TRPV1, TRPV2 and TRPV4) were found to be expressed at relatively at high levels.
Other Subunits
Other subunits examined include those involved in cation and chloride fluxes, and cell-cell communication. We examined two members of the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and found both (HCN2 and HCN4) to be expressed in HCAECs. Transcripts of all 3 of the voltage-gated chloride channel family examined (CLC-3, CLC-4 and CLC-7) were highly expressed, whereas TMEM16A (a recently discovered subunit of Ca2+-activated Cl– channels [23]) was expressed at relatively low levels. Finally, amongst the connexins (CX), we found CX43 to be expressed at higher levels relative to CX37 and CX40.
Transcriptional Remodeling of Ion Channel Subunits by Flow Adaptation
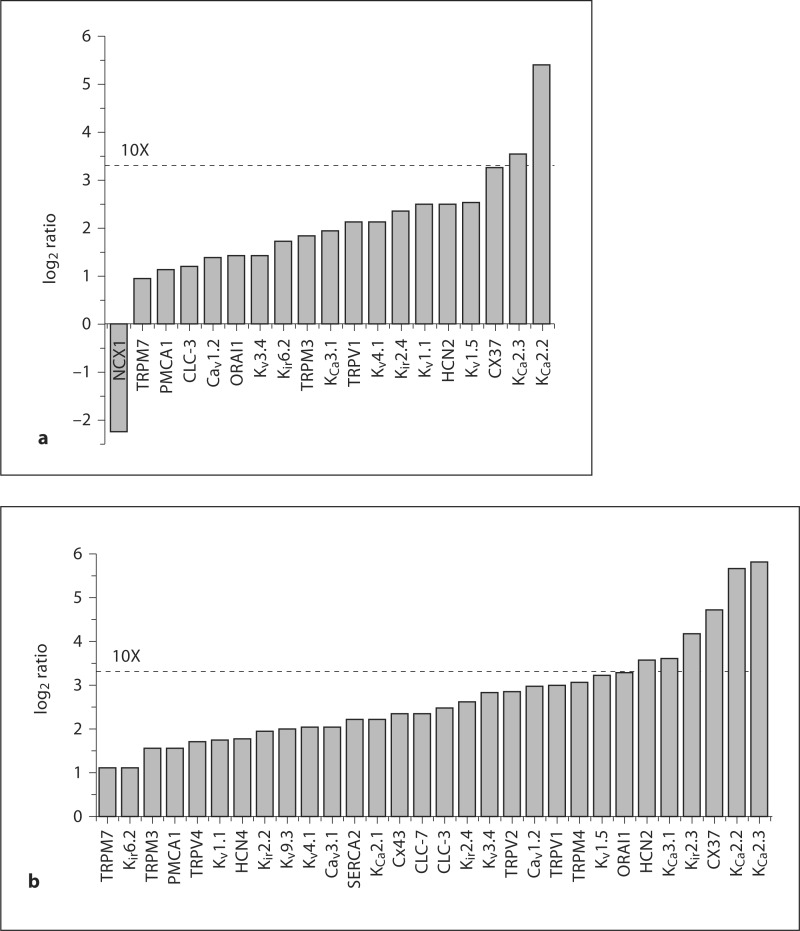
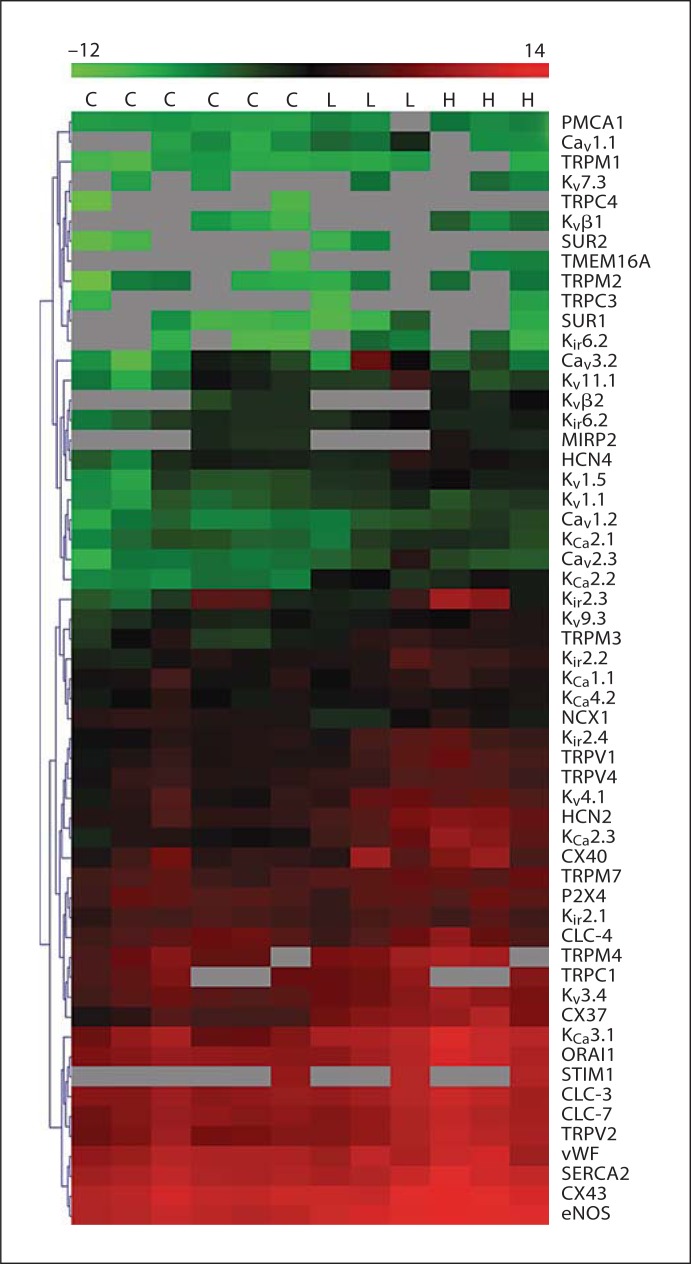
Our next goal was to examine the transcriptional remodeling of ion channel subunits when ECs adapt to increasing shear stress. We employed two levels of flow-induced shear that are commonly used in the literature (5 and 15 dyn/cm2; it should be noted that these values are less than what has been estimated as normal for in vivo shear levels [24]). Cells were grown in an artificial capillary system and subjected to two different flow rates that correspond to two different shear levels (5 or 15 dyn/cm2) for 24 h. Transcriptional analysis by qRT-PCR was performed as described above and ion channel expression levels were compared to cells grown under static (no flow) conditions. We performed the SAM test (with false discovery rate set to zero) on the data to determine differentially expressed transcripts. With 5 dyn/cm2, we found that the expression of 18 subunits was significantly upregulated and expression of 1 subunit (NCX1) was reduced (fig. 2a). Note that, with the exception of 1 subunit (TRPM7), all of these transcripts were up- or downregulated by more than 2-fold (log2 value higher than 1 or lower than −1). In some cases, e.g. with KCa2.3 and KCa2.2, the upregulation was more than an order of magnitude. The same subset of ion channel transcripts that was upregulated by 5 dyn/cm2 was also upregulated by an increased shear force of 15 dyn/cm2 (table 1). At these higher shear forces, an additional 12 transcripts demonstrated upregulated expression and many were upregulated by 10 times or more (including KCa2.3, KCa2.2, Cx37, Kir2.3, HCN2 and KCa3.1; fig. 2b). Interestingly, NCX1 was not found to be downregulated by the higher shear. It is important to note that ion channel transcriptional remodeling is not a general feature of the EC response to shear since expression of more than 40% of the transcripts examined (24/54; table 1) was unaffected by shear. Moreover, the expression levels of the endothelial marker von Willebrand factor (vWF) and of endothelial nitric oxide synthase (eNOS) were examined. These proteins represent a negative and positive control, respectively, of shear stress-induced transcriptional modulation [25,26]. In agreement with the literature, vWF was not affected by shear and eNOS was significantly upregulated by both flow levels. The data are summarized as a heat map in figure 3, which demonstrates not only the heterogeneity of ion channel transcription in HCAECs but also clusters genes with similar expression levels and alterations with shear. Finally, we examined the degree to which the two different levels of shear used in this study influenced the expression profiles of ion translocator subunits. To this end, we plotted the mRNA expression (as a percentage of the set of reference genes) measured at 15 dyn/cm2 as a function of the expression measured at 5 dyn/cm2 (fig. 4). Data were reasonably well described by a linear equation, with a slope of about 2.4. This relationship demonstrates that higher shear levels are associated with increased levels of ion translocator mRNA expression.
Fig. 2.
Shear-induced changes in ion channel subunit expression levels. Only the genes that were found to be significantly differentially expressed (by SAM analysis) are shown. HCAECs were exposed to shear force of 5 dyn/cm2 (a) or 15 dyn/cm2 (b). qRT-PCR was performed and expression levels were calculated relative to reference genes. Shown are the ratios (after log2 transformation) of the expression levels under flow compared with the expression levels under static (control) conditions.
Table 1.
Ion channel sub unit expression with two flow-induced shear conditions
| Subunits affected by 5 dyn/cm2 | Subunits affected by 15 dyn/cm2 | Subunits not affected by flow-induced shear | |||
|---|---|---|---|---|---|
| Cav1.2 | Kvl.l | Cav1.2 | Kir6.2 | Cavl.l | Kvβ2 |
| CLC-3 | Kv1.5 | Cav3.1 | Kvl.l | Cav1.3 | M1RP2 |
| CX37 | Kv3.4 | CLC-3 | Kv1.5 | Cav3.2 | P2X4 |
| HCN2 | Kv4.1 | CLC-7 | Kv3.4 | CLC-4 | STIM1 |
| KCa2.2 | ORAI1 | CX37 | Kv4.1 | CX40 | SUR1 |
| KCa2.3 | PMCA1 | CX43 | Kv9.3 | Kca1.1 | SUR2 |
| KCa3.1 | TRPM3 | HCN2 | ORAI1 | KCa4.2 | TMEM16A |
| Kir2.4 | TRPM7 | HCN4 | PMCA1 | Kir2.1 | TRPC1 |
| Kir6.2 | TRPV1 | KCa2.1 | SERCA2 | Kir6.1 | TRPC3 |
| NCX1 | Kca2.2 | TRPM3 | Kvll.l | TRPC4 | |
| KCa2.3 | TRPM4 | Kv7.3 | TRPM1 | ||
| KCa3.1 | TRPM7 | Kvβl | TRPM2 | ||
| Kir2.2 | TRPV1 | ||||
| Kir2.3 | TRPV2 | ||||
| Kir2.4 | TRPV4 | ||||
We performed a SAM test to determine genes regulated by flow: 18 subunits were statistically upregulated and one (NCX1) downregulated by 5 dyn/cm2 shear. A total of 30 subunits were upregulated by 15 dyn/cm2 shear. About 44% of the subunits (24/54) were not significantly regulated by either of the shear levels.
Fig. 3.
Heat map of expression data. Data obtained under control conditions and with externally applied shear forces were combined in a heat map. All data are depicted as log2 expression values relative to the same set of reference genes (as described in the text) within the range −12 (green) to 14 (red). Genes are grouped by hierarchical clustering (using the Euclidean distance as a distance measure). Values are shown for 6 control experiments with cells grown under static conditions (C), for 3 experiments where cells were exposed to 5 dyn/cm2 (L) and 3 experiments where cells were exposed to 15 dyn/cm2 (H) shear for 24 h. The data shown here represents the global dataset (three biological repeats for the two flow conditions, with time-matched static conditions for each of these experiments), which was used to construct figures 1, 2 and 4. The gray boxes represent missing data due to failed PCRs, which predictably mostly occurred when gene expression levels were low. Colors refer to the online version only.
Microarray Data Results
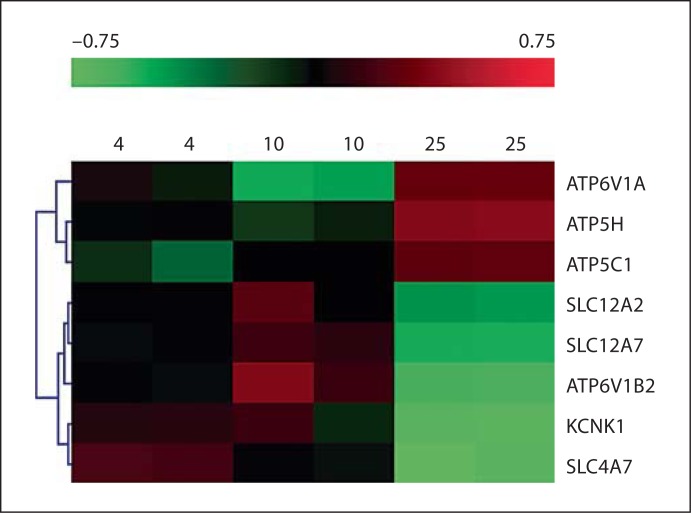
In order to obtain a different perspective of ion channel remodeling by shear forces, we examined publicly available microarray data sets. We focused on studies that compared the gene expression patterns of ECs under static conditions in comparison to different levels of applied shear forces. In one such study (GSE1518), gene expression profiles of human umbilical vein ECs (HUVECs) were examined in response to 4, 10 and 25 dyn/cm2 shear levels [27]. Using ANOVA (p ≤ 0.01), we obtained 8 ion channel and transporter genes that were differentially regulated between the three conditions (fig. 5). These include 2 lysosomal H+ ATPase subunits (ATP6V1A and ATP6V1B2), 2 mitochondrial ATP synthase subunits (ATP5H and ATP5C1), 2 potassium channel subunits (a two-pore channel, KCNK1) and 3 genes encoding proteins of the solute carrier family; the Na+ bicarbonate co-transporter isoform 3 (SLC4A7), the Na+-K+-Cl– co-transporter (SLC12A2) and K+-Cl– co-transporter 4 (SLC12A7).
Fig. 5.
Microarray analysis of alterations in ion channel and transporter gene expression under different levels of flow. HUVECs were exposed to flow levels of 4, 10 or 25 dyn/cm2 and gene expression was examined using microarray analysis [27]. Following log2 transformation, data (ranging from −0.75 to 0.75) were subjected to hierarchical clustering and a heat map was generated.
Discussion
The vascular endothelium is sensitive to alterations in shear forces resulting from alterations in blood flow. The ECs respond by alterations in function, which has important consequences for the regulation of vascular function and blood flow. Acute changes in shear forces cause transient adjustments in vessel diameter that are mediated by the release of vasoactive substances and changes in smooth muscle tone. However, changes in shear forces are often chronic, which causes important adaptive alterations in the shape and composition of the blood vessel wall, a process referred to as vascular remodeling. The endothelial events occurring with vascular remodeling may include alterations in the barrier function (cellular permeability), the inflammatory response and alterations in cell proliferation. Genome-wide assays have been performed to identify the expression profiles of endothelial genes in response to shear forces and have identified genes with diverse biological functions, including those encoding growth factors, adhesion molecules, coagulation molecules, chemoattractants, proto-oncogenes and vasoactive substances [27,28,29,30,31,32]. Ion channel subunits can be subject to chronic transcriptional remodeling (e.g. during perinatal development [16]). However, despite a known role for ion channels in endothelial function and sensing alterations in flow-induced shear [21], the effects of shear forces on ion channel transcriptional remodeling are largely unknown. Our study provides insight into the transcriptional remodeling of a large subset of ion channel subunits that are expressed in the coronary vascular endothelium.
Endothelial Electrical Activity and Shear Forces
ECs are nonexcitable and as such do not generate conducting action potentials in response to electrical or chemical stimuli. Nevertheless, ion channels, pumps and exchangers play a fundamentally important role in the function of ECs [21]. For example, Kir channels act to stabilize the membrane potential. We examined only 2 of the 7 Kir subfamilies. The Kir2 subfamily is thought to play a predominant role in stabilizing the membrane potential [33] and is represented by 4 members (Kir2.1–Kir2.4). We found all 4 of the Kir2 members to be abundantly expressed in ECs. Although Kir2.1 is the best characterized member of this family in the endothelium, the presence of all members has been previously reported in human aortic ECs [34]. Furthermore, the expression of 3 of these (Kir2.2–2.4) was upregulated by flow-induced shear, which raises the possibility that shear-adapted ECs may have a more negative membrane potential. We also examined expression levels of another Kir subfamily, namely the Kir6 subfamily members (Kir6.1 and Kir6.2), which form an octameric complex with sulfonylurea receptors (SUR1 and SUR2) to form the KATP channel [33]. The latter channel is particularly important in linking alterations in the cellular metabolic status with its electrical activity, but there is also evidence for a role in mechanotransduction [35]. Similar to our previous report [36], we found expression of both Kir6.1 and Kir6.2 in HCAEC, with Kir6.2 being expressed at higher levels. SUR1 was expressed at higher levels than SUR2, which may partly explain the exquisite sensitivity of ECs to some pharmacological KATP channel openers, such as diazoxide [37]. The shear-induced upregulation of some of these KATP channel subunits observed in our experiments suggests the possibility of an enhanced response of ECs to conditions that impair energy metabolism (e.g. hypoxia) and/or an increased response to mechanotransduction. Indeed, one of the earliest and most rapid responses of ECs to alterations in hemodynamic forces is the activation of a K+ conductance [11], which has recently been identified as a KATP channel current [38]. Thus, upregulation of KATP channel subunits suggests an adaptive response of the EC to further changes in shear forces.
Another important role for K+ channels in ECs is to maintain a negative membrane potential upon agonist-induced Ca2+ influx [21]. Although the K+ channels discussed above participate in this response, ECs express a specialized class of Ca2+-activated K+ (KCa) channels that are particularly suited for this role. We examined several members of the KCa family. In keeping with the literature [21], we found strong expression of subunits of members with large conductance (BKCa; KCa1.1 or slo) and intermediate conductance (IKCa; KCa3.1), the latter being strongly upregulated by both shear levels examined. Shear-induced upregulation of KCa3.1 expression has also been noted by others [12]. We could also detect the expression of small-conductance (SKCa or KCa2.x subfamily) Ca2+-activated K+ channel subunits, which were amongst those subunits that were most upregulated by shear forces (fig. 2, 3). This suggests that transcriptional plasticity of the small-conductance Ca2+-activated K+ channels might have a previously unidentified role in the adaptive response of the EC to chronic changes in blood flow.
Ion channels that are selectively permeable to Cl– ions are ubiquitously expressed, and the EC is no exception [21]. These channels are categorized into several functional groups, which include those activated by voltage, swelling, changes in cytosolic Ca2+, and cyclic nucleotides [39]. The molecular correlates of these functional groups have not been completely resolved. We examined a relatively small subset of Cl– channel subunits and found strong expression of CLC-3, CLC-4 and CLC-7, which are Cl–/H+ exchangers [40]. In contrast, we found low expression levels of TMEM16A, a recently identified subunit of a Ca2+-activated Cl– channel. Only CLC-3 and CLC-7 were upregulated significantly by flow-induced shear, suggesting a role for Cl– channels in the adaptive response of an EC to flow changes. Indeed, application of shear stress induces rapid development of a Cl– current in ECs [41,42].
Since ECs are nonexcitable, the expression of voltage-gated channels may seem paradoxical. Nevertheless, a variety of voltage-gated channels that are nonselective, or specifically conduct Na+, Ca2+ or K+ ions, are expressed in vascular ECs [21]. Voltage-gated K+ channels can have very important roles in nonexcitable cells (for example in controlling T lymphocyte activation by Kv1.3 channels [43]). We examined 7 voltage-activated K+ (Kv) channel subunits and found relatively abundant expression of several of these, in particular Kv3.4, Kv4.1 and Kv9.3. It would be of great interest to extend our screen to comprehensively examine the large family of Kv channel subunits [33]. An important potential role for Kv channels in nonexcitable cells is to prevent excessive depolarization. As the membrane potential depolarizes into the voltage range where the Kv channel activates, their opening will minimize further depolarization and therefore clamp the voltage to the activation threshold. In this regard, it is interesting to note that the Kv channels expressed in ECs have very different activation voltages (Kv4 and Kv3 channels respectively activate at around −60 and −10 mV [33]), which raises the possibility that these channels may serve different roles in controlling the EC membrane potential. It is of further interest to note that Kv4.1 has been described as molecular correlate of the oxygen-sensitive K+ channel in chemoreceptor cells of the rabbit carotid body [44]. Its expression, and upregulation with shear forces, suggests a similar role in the vascular endothelium. In addition to the Kv channel subunits, we also found subunits of the hyperpolarization-activated, nonselective cation channel (HCN2 and HCN4), which in other tissues are involved in events such as pacemaking activity. These channels are also important in a variety of other cellular responses (e.g. in neurons they participate in motor learning, spatial learning and memory [45]) and their roles in ECs remain to be elucidated. In addition to our qRT-PCR data, the microarray data analysis revealed the electrogenic Na+ bicarbonate co-transporter 1 (NBC1; co-transport of 2 HCO3+ per 1 Na+) to be shear-regulated. Furthermore, the Na+-K+-Cl– co-transporter (previously also reported to be regulated by shear in HUVECs by 10 dyn/cm2 of laminar shear [46]) and the K+-Cl– co-transporter 4 expression levels were modified by shear. The two former subunits are electrically neutral; however, it seems that the transporters that regulate the electrochemical gradient of Na+, K+ and Cl– are, at least to some extent, coregulated under different shear stress conditions.
Endothelial Ca2+ Homeostasis
One of the first, and perhaps the most important, response of an EC when challenged with agonists or changes in shear forces is an increase in cytosolic Ca2+, which in turn controls events such as activation of proteins (e.g. of eNOS), the release of vasoactive and immunoreactive substances and activation of gene transcription programs. The control of cytosolic Ca2+ is therefore of paramount importance and is orchestrated by ion channels, exchangers and pumps. In addition to the ion channels already discussed above, a rich variety of transport proteins maintain cytosolic Ca2+, both directly and indirectly. Of the limited set of subunits examined, we found significant expression of those that mediate agonist-induced Ca2+ entry (such as TRP channels), those that are involved in store-operated Ca2+ entry (ORAI1 and STIM1), and those that allow Ca2+ entry when the membrane potential is depolarized into their threshold potential (Cav channels). Additionally, we found strong expression of a bidirectional Ca2+ transporter, the Na+-Ca2+ exchanger (NCX1). It is interesting and informative that many of the subunits involved in Ca2+ transport pathways were upregulated by shear forces. This occurred in concert with the upregulation of Ca2+-activated K+ channel subunits that coregulate Ca2+ influx (see above), which suggests coordination of the transcriptional mechanisms involved in controlling the levels of proteins implicated in Ca2+ handling.
As with the Kv channels discussed above, many have questioned the presence or relevance of voltage-activated Ca2+ channels in ECs. Our study provides no additional clues in answering this question. Nonetheless, it is clear that some ECs (e.g. from bovine capillary and adrenal medulla) express both T- and L-type Ca2+ transient currents that have the ability to mediate Ca2+ entry [47,48]. There is evidence that T-type Ca2+ channels participate in Ca2+ influx resulting from shear stress in pulmonary ECs [14,49]. The latter result is interesting when considering that the T-type Ca2+ channel subunit Cav3.1 was not only detected but also upregulated by 15 dyn/cm2 shear. Future studies must be directed at unraveling the link between shear and Cav channels.
The TRP channel family is of particular interest given the important role that it has in regulating EC membrane potential and Ca2+ homeostasis [50]. We found several members of the TRP channel families to be expressed in HCAECs. Furthermore, the expression levels of many of these were strongly affected by shear. This finding is in keeping with the literature. For example, TRPV4 (a Ca2+-permeable nonselective cation channel) is activated by various environmental factors, including flow (3–20 dyn/cm2) [51]. We found TRPV4 expression to be increased by shear. TRPM7, a nonselective cation channel with predominant permeability for Ca2+ and Mg2+, rapidly translocates to the plasma membrane in response to changes in flow [52]. Interestingly, TRPM7 expression was also increased by shear in our experiments. Our data point to a potential role for other TRP channels in the adaptive response of an EC to shear.
Channels Involved in Atherosclerosis
Continuous exposure to a physiologic range of shear stress, especially to unidirectional laminar flow, promotes the establishment of important physiological characteristics of the artery wall, leading to an anti-inflammatory, antithrombotic, anticoagulative and antihypertrophic state, while it is involved in flow-mediated dilation [5]. Alteration of the shear forces alters the biology of the endothelial monolayer and subsequently the susceptibility of vessels to atherosclerosis. Ion channels that are directly implicated in the development of atherosclerosis have not been identified. However, channels that are involved in flow-mediated artery vasodilation, such as TRPV4 [8] and KCa3.1 [53] (which are upregulated in our study by 5 dyn/cm2 or both 5 and 15 dyn/cm2, respectively) or inhibition of EC proliferation, such as TRPM7 [54] (upregulated by both shear levels), could be correlated with atheroprotective effects.
Limitations
A technical limitation inherent in large-scale qRT-PCR is that the analysis often does not correct for differences in primer efficiency. Although this is possible (e.g. by producing standard curves for each transcript and primer pair), this procedure is impractical at large scale. Thus, the relative expression of the various subunits (fig. 1) should be interpreted with this limitation in mind. Another obvious limitation of the present study is that we measured transcriptional levels of ion channel subunits, which may not correspond to the functional states of the various ion channels, exchangers and pumps. Translational and post-translational processes may skew this relationship. Nevertheless, our data provide valuable information regarding the profiles and plasticity of groups of ion channels expressed in ECs as the cells adapt to changes in shear forces. Although we examined the expression profiles of a relatively large number (about 60) channel subunits, the reader should note that this represents only a very small subset of the genes identified to date that encode subunits of channels, pumps and exchangers. The total number of transcripts is even larger if one includes events such as alternative splicing and RNA editing. Finally, although we used a relatively well established EC primary line (HCAEC), there may well be important differences when comparing these cells to freshly isolated cells or to ECs in vivo.
Conclusions
In conclusion, this study provides for the first time a large-scale analysis of ion channel subunit expression in ECs and how this expression is modified by shear stress. The ion channel remodeling observed is substantial and suggests that altered ion channel and transporter expression may significantly contribute to vascular pathology associated with flow-induced alterations. Taken together, results presented here contribute to the understanding of endothelial ion channel regulation and could aid in elucidating their roles in flow-associated diseases.
Acknowledgements
We are grateful to Dr. Aron B. Fisher and Dr. Shampa Chatterjee (Institute for Environmental Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pa., USA) for kindly providing support and training for the adaptation of ECs to flow. We also thank the NYU Cancer Institute Genomics Facility for access to the high-capacity real-time PCR methodology. The study was supported by a grant from the National Institutes of Health 5R01HL085820-02 and partial support was provided by the 7th Masonic District.
References
- 1.White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L529–L533. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 3.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 6.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med. 2009;41:19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- 7.Adams DJ, Hill MA. Potassium channels and membrane potential in the modulation of intracellular calcium in vascular endothelial cells. J Cardiovasc Electrophysiol. 2004;15:598–610. doi: 10.1046/j.1540-8167.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- 8.Folgering JH, Sharif-Naeini R, Dedman A, Patel A, Delmas P, Honore E. Molecular basis of the mammalian pressure-sensitive ion channels: focus on vascular mechanotransduction. Prog Biophys Mol Biol. 2008;97:180–195. doi: 10.1016/j.pbiomolbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Gautam M, Gojova A, Barakat AI. Flow-activated ion channels in vascular endothelium. Cell Biochem Biophys. 2006;46:277–284. doi: 10.1385/CBB:46:3:277. [DOI] [PubMed] [Google Scholar]
- 10.Nakache M, Gaub HE. Hydrodynamic hyperpolarization of endothelial cells. Proc Natl Acad Sci USA. 1988;85:1841–1843. doi: 10.1073/pnas.85.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 12.Brakemeier S, Kersten A, Eichler I, Grgic I, Zakrzewicz A, Hopp H, Kohler R, Hoyer J. Shear stress-induced up-regulation of the intermediate-conductance Ca2+-activated K+ channel in human endothelium. Cardiovasc Res. 2003;60:488–496. doi: 10.1016/j.cardiores.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol. 2003;285:C959–C967. doi: 10.1152/ajpcell.00511.2002. [DOI] [PubMed] [Google Scholar]
- 14.Wei Z, Manevich Y, Al-Mehdi AB, Chatterjee S, Fisher AB. Ca2+ flux through voltage-gated channels with flow cessation in pulmonary microvascular endothelial cells. Microcirculation. 2004;11:517–526. doi: 10.1080/10739680490476367. [DOI] [PubMed] [Google Scholar]
- 15.Milovanova T, Manevich Y, Haddad A, Chatterjee S, Moore JS, Fisher AB. Endothelial cell proliferation associated with abrupt reduction in shear stress is dependent on reactive oxygen species. Antioxid Redox Signal. 2004;6:245–258. doi: 10.1089/152308604322899314. [DOI] [PubMed] [Google Scholar]
- 16.Harrell MD, Harbi S, Hoffman JF, Zavadil J, Coetzee WA. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol Genomics. 2007;28:273–283. doi: 10.1152/physiolgenomics.00163.2006. [DOI] [PubMed] [Google Scholar]
- 17.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 18.Ohl F, Jung M, Xu C, Stephan C, Rabien A, Burkhardt M, Nitsche A, Kristiansen G, Loening SA, Radonic A, Jung K. Gene expression studies in prostate cancer tissue: which reference gene should be selected for normalization? J Mol Med. 2005;83:1014–1024. doi: 10.1007/s00109-005-0703-z. [DOI] [PubMed] [Google Scholar]
- 19.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 20.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 23.Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J. 2009;97:3047–3053. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saihara K, Hamasaki S, Okui H, Biro S, Ishida S, Yoshikawa A, Kataoka T, Ninomiya Y, Mizoguchi E, Ichiki T, Otsuji Y, Tei C. Association of coronary shear stress with endothelial function and vascular remodeling in patients with normal or mildly diseased coronary arteries. Coron Artery Dis. 2006;17:401–407. doi: 10.1097/00019501-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Galbusera M, Zoja C, Donadelli R, Paris S, Morigi M, Benigni A, Figliuzzi M, Remuzzi G, Remuzzi A. Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood. 1997;90:1558–1564. [PubMed] [Google Scholar]
- 26.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 27.Andersson M, Karlsson L, Svensson PA, Ulfhammer E, Ekman M, Jernas M, Carlsson LM, Jern S. Differential global gene expression response patterns of human endothelium exposed to shear stress and intraluminal pressure. J Vasc Res. 2005;42:441–452. doi: 10.1159/000087983. [DOI] [PubMed] [Google Scholar]
- 28.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb. 2003;10:304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- 29.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu TJ, Peters DG. Serial analysis of the vascular endothelial transcriptome under static and shear stress conditions. Physiol Genomics. 2008;34:185–192. doi: 10.1152/physiolgenomics.90201.2008. [DOI] [PubMed] [Google Scholar]
- 31.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 32.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 33.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de ME, Rudy B. Molecular diversity of K+ channels. Ann NY AcadSci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 34.Fang Y, Schram G, Romanenko VG, Shi C, Conti L, Vandenberg CA, Davies PF, Nattel S, Levitan I. Functional expression of Kir2.x in human aortic endothelial cells: the dominant role of Kir2.2. Am J Physiol Cell Physiol. 2005;289:C1134–C1144. doi: 10.1152/ajpcell.00077.2005. [DOI] [PubMed] [Google Scholar]
- 35.Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. K ATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37:857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Langheinrich U, Daut J. Hyperpolarization of isolated capillaries from guinea-pig heart induced by K+ channel openers and glucose deprivation. J Physiol. 1997;502(Pt 2):397–408. doi: 10.1111/j.1469-7793.1997.397bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation. 2006;13:633–644. doi: 10.1080/10739680600930255. [DOI] [PubMed] [Google Scholar]
- 39.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 40.Plans V, Rickheit G, Jentsch TJ. Physiological roles of CLC Cl–/H+ exchangers in renal proximal tubules. Pflügers Arch. 2009;458:23–37. doi: 10.1007/s00424-008-0597-z. [DOI] [PubMed] [Google Scholar]
- 41.Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ Res. 1999;85:820–828. doi: 10.1161/01.res.85.9.820. [DOI] [PubMed] [Google Scholar]
- 42.Gautam M, Shen Y, Thirkill TL, Douglas GC, Barakat AI. Flow-activated chloride channels in vascular endothelium. Shear stress sensitivity, desensitization dynamics, and physiological implications. J Biol Chem. 2006;281:36492–36500. doi: 10.1074/jbc.M605866200. [DOI] [PubMed] [Google Scholar]
- 43.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez D, Lopez-Lopez JR, Perez-Garcia MT, Sanz-Alfayate G, Obeso A, Ganfornina MD, Gonzalez C. Molecular identification of Kvα subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid body. J Physiol. 2002;542:369–382. doi: 10.1113/jphysiol.2002.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrmann S, Stieber J, Ludwig A. Pathophysiology of HCN channels. Pflügers Arch. 2007;454:517–522. doi: 10.1007/s00424-007-0224-4. [DOI] [PubMed] [Google Scholar]
- 46.Topper JN, Wasserman SM, Anderson KR, Cai J, Falb D, Gimbrone MA., Jr Expression of the bumetanide-sensitive Na-K-Cl cotransporter BSC2 is differentially regulated by fluid mechanical and inflammatory cytokine stimuli in vascular endothelium. J Clin Invest. 1997;99:2941–2949. doi: 10.1172/JCI119489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bossu JL, Elhamdani A, Feltz A. Voltage-dependent calcium entry in confluent bovine capillary endothelial cells. FEBS Lett. 1992;299:239–242. doi: 10.1016/0014-5793(92)80123-x. [DOI] [PubMed] [Google Scholar]
- 48.Vinet R, Vargas FF. L- and T-type voltage-gated Ca2+ currents in adrenal medulla endothelial cells. Am J Physiol. 1999;276:H1313–H1322. doi: 10.1152/ajpheart.1999.276.4.H1313. [DOI] [PubMed] [Google Scholar]
- 49.Fisher AB, Al-Mehdi AB, Manevich Y. Shear stress and endothelial cell activation. Crit Care Med. 2002;30:S192–S197. doi: 10.1097/00003246-200205001-00004. [DOI] [PubMed] [Google Scholar]
- 50.Nilius B, Voets T. Diversity of TRP channel activation. Novartis Found Symp. 2004;258:140–149. discussion 149–159, 263–146. [PubMed] [Google Scholar]
- 51.Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 52.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 53.Tharp DL, Bowles DK. The intermediate-conductance Ca2+-activated K+ channel (KCa3.1) in vascular disease. Cardiovasc Hematol Agents Med Chem. 2009;7:1–11. doi: 10.2174/187152509787047649. [DOI] [PubMed] [Google Scholar]
- 54.Inoue K, Xiong ZG. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc Res. 2009;83:547–557. doi: 10.1093/cvr/cvp153. [DOI] [PMC free article] [PubMed] [Google Scholar]