Abstract
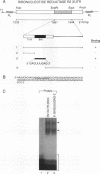
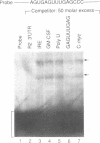
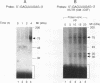
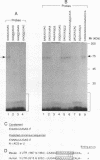
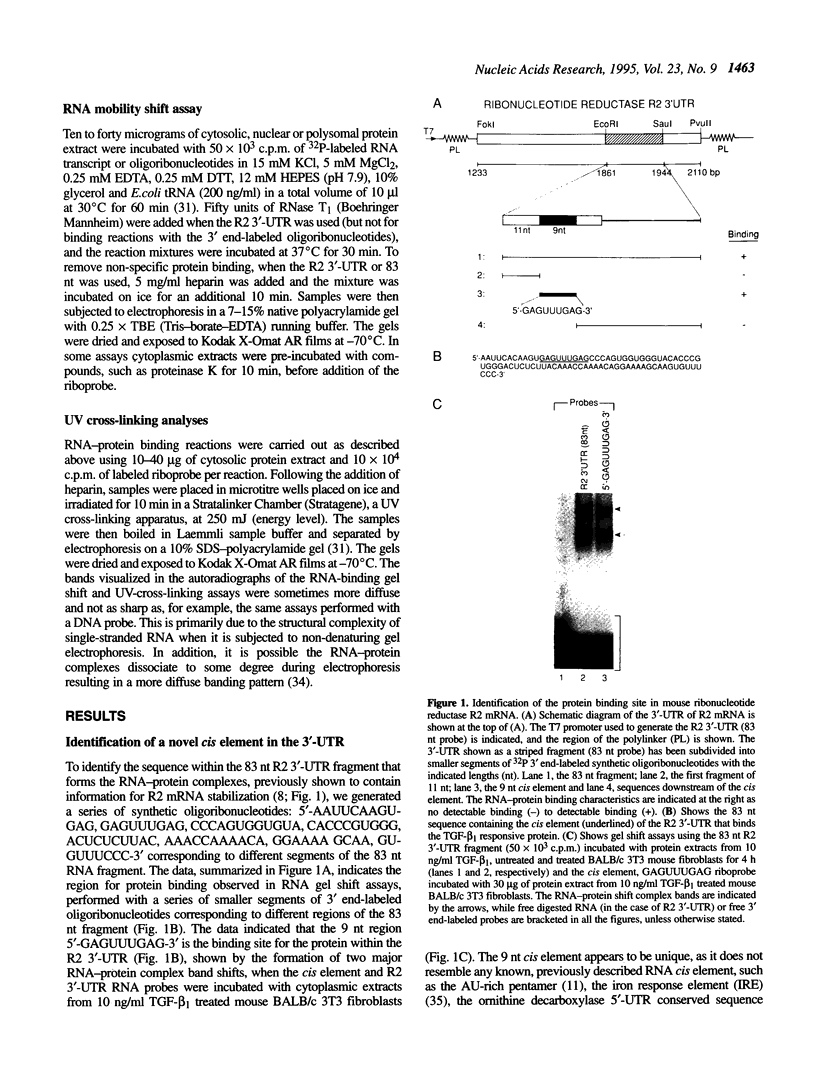
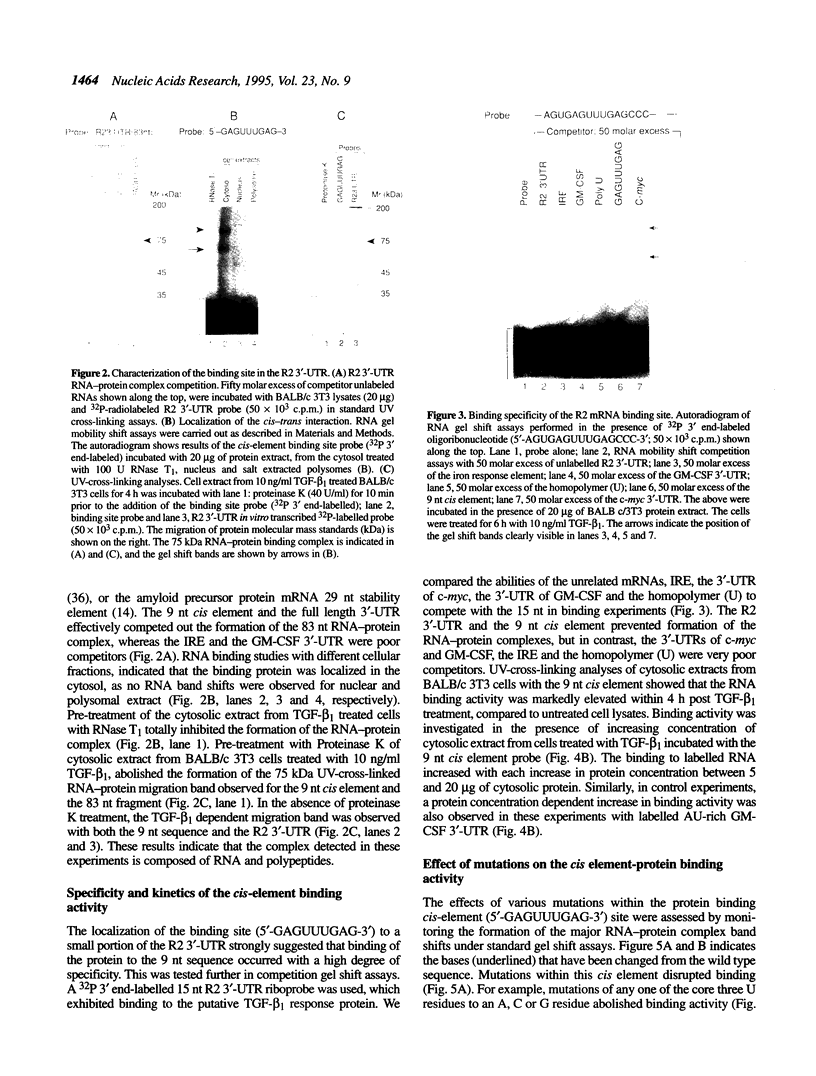
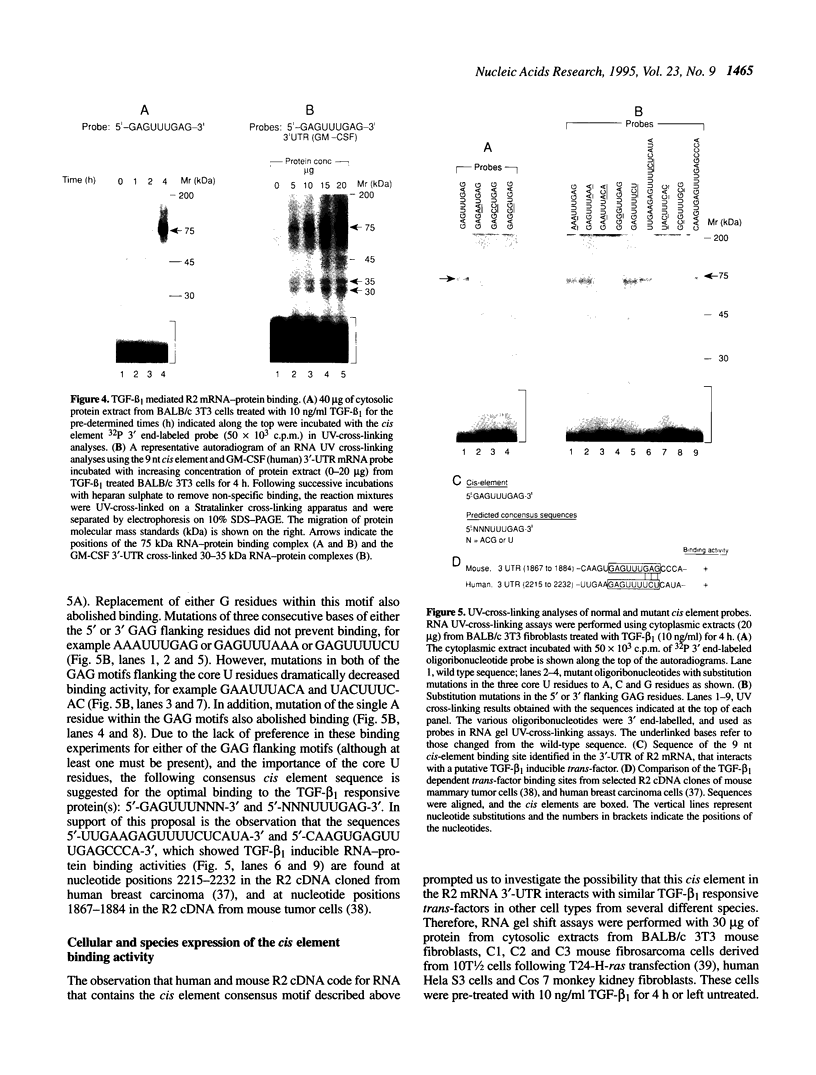
Ribonucleotide reductase R2 gene expression is elevated in BALB/c 3T3 fibroblasts treated with transforming growth factor beta 1. We investigated the possibility that the 3'-UTR of ribonucleotide reductase R2 mRNA contains regulatory information for TGF-beta 1 induced message stability. Using end-labeled RNA fragments in gel shift assays and UV cross-linking analyses, we detected in the 3'-UTR a novel 9 nucleotide (nt) cis element, 5'-GAGUUUGAG-3' site, which interacted specifically with a cytosolic protease sensitive factor to form a 75 kDa complex. The cis element protein binding activity was inducible and markedly up-regulated cross-link 4 h after TGF-beta 1 treatment of mouse BALB/c 3T3 cells. Other 3'-UTRs [IRE, GM-CSF, c-myc and homopolymer (U)] were poor competitors to the cis element with regard to forming the TGF-beta 1 dependent RNA-protein complex. However, the cis element effectively competed out the formation of the R2 3'-UTR protein complex. Cytosolic extracts from a variety of mammalian cell lines (monkey Cos7, several mouse fibrosarcomas and human HeLa S3) demonstrated similar TGF-beta 1 dependent RNA-protein band shifts as cell extract from BALB/c 3T3 mouse fibroblasts. Binding was completely prevented by several different mutations within the cis element, and by substitution mutagenesis, we were able to predict the consensus sequences, 5'-GAGUUUNNN-3' and 5'-NNNUUUGAG-3' for optimal protein binding. These results support a model in which the 9 nt region functions in cis to destabilize R2 mRNA in cells; and upon activation, a TGF-beta 1 responsive protein is induced and interacts with the 9 nt cis element in a mechanism that leads to stabilization of the mRNA. This appears to be the first example of a mRNA binding site that is involved in TGF-beta 1-mediated effects.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberta J. A., Rundell K., Stiles C. D. Identification of an activity that interacts with the 3'-untranslated region of c-myc mRNA and the role of its target sequence in mediating rapid mRNA degradation. J Biol Chem. 1994 Feb 11;269(6):4532–4538. [PubMed] [Google Scholar]
- Amara F. M., Chen F. Y., Wright J. A. A novel transforming growth factor-beta 1 responsive cytoplasmic trans-acting factor binds selectively to the 3'-untranslated region of mammalian ribonucleotide reductase R2 mRNA: role in message stability. Nucleic Acids Res. 1993 Oct 11;21(20):4803–4809. doi: 10.1093/nar/21.20.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara F. M., Chen F. Y., Wright J. A. Phorbol ester modulation of a novel cytoplasmic protein binding activity at the 3'-untranslated region of mammalian ribonucleotide reductase R2 mRNA and role in message stability. J Biol Chem. 1994 Mar 4;269(9):6709–6715. [PubMed] [Google Scholar]
- Binder R., Hwang S. P., Ratnasabapathy R., Williams D. L. Degradation of apolipoprotein II mRNA occurs via endonucleolytic cleavage at 5'-AAU-3'/5'-UAA-3' elements in single-stranded loop domains of the 3'-noncoding region. J Biol Chem. 1989 Oct 5;264(28):16910–16918. [PubMed] [Google Scholar]
- Blosmanis R., Wright J. A., Goldenberg G. J. Sensitivity to melphalan as a function of transport activity and proliferative rate in BALB/c 3T3 fibroblasts. Cancer Res. 1987 Mar 1;47(5):1273–1277. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Di Jeso B., Rao K., Klausner R. D., Harford J. B. Two genetic loci participate in the regulation by iron of the gene for the human transferrin receptor. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1787–1791. doi: 10.1073/pnas.85.6.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. Y., Amara F. M., Wright J. A. Mammalian ribonucleotide reductase R1 mRNA stability under normal and phorbol ester stimulating conditions: involvement of a cis-trans interaction at the 3' untranslated region. EMBO J. 1993 Oct;12(10):3977–3986. doi: 10.1002/j.1460-2075.1993.tb06075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. Y., Amara F. M., Wright J. A. Regulation of mammalian ribonucleotide reductase R1 mRNA stability is mediated by a ribonucleotide reductase R1 mRNA 3'-untranslated region cis-trans interaction through a protein kinase C-controlled pathway. Biochem J. 1994 Aug 15;302(Pt 1):125–132. doi: 10.1042/bj3020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerch L. B., Massaro D. Oxidation-reduction-sensitive binding of lung protein to rat catalase mRNA. J Biol Chem. 1992 Feb 15;267(5):2853–2855. [PubMed] [Google Scholar]
- Dodson R. E., Shapiro D. J. An estrogen-inducible protein binds specifically to a sequence in the 3' untranslated region of estrogen-stabilized vitellogenin mRNA. Mol Cell Biol. 1994 May;14(5):3130–3138. doi: 10.1128/mcb.14.5.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S. E., McClarty G. A., Jarolim L., Wright J. A., Spiro I., Hager G., Greenberg A. H. Expression of H-ras correlates with metastatic potential: evidence for direct regulation of the metastatic phenotype in 10T1/2 and NIH 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):830–837. doi: 10.1128/mcb.7.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton B., Decker C., Muhlrad D., Donahue J., Jacobson A., Parker R. Analysis of chimeric mRNAs derived from the STE3 mRNA identifies multiple regions within yeast mRNAs that modulate mRNA decay. Nucleic Acids Res. 1992 Oct 25;20(20):5365–5373. doi: 10.1093/nar/20.20.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurta R. A., Samuel S. K., Greenberg A. H., Wright J. A. Early induction of ribonucleotide reductase gene expression by transforming growth factor beta 1 in malignant H-ras transformed cell lines. J Biol Chem. 1991 Dec 15;266(35):24097–24100. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Alterations in the activity and regulation of mammalian ribonucleotide reductase by chlorambucil, a DNA damaging agent. J Biol Chem. 1992 Apr 5;267(10):7066–7071. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Malignant transformation by H-ras results in aberrant regulation of ribonucleotide reductase gene expression by transforming growth factor-beta 1. J Cell Biochem. 1995 Mar;57(3):543–556. doi: 10.1002/jcb.240570319. [DOI] [PubMed] [Google Scholar]
- Juliusson G., Liliemark J. High complete remission rate from 2-chloro-2'-deoxyadenosine in previously treated patients with B-cell chronic lymphocytic leukemia: response predicted by rapid decrease of blood lymphocyte count. J Clin Oncol. 1993 Apr;11(4):679–689. doi: 10.1200/JCO.1993.11.4.679. [DOI] [PubMed] [Google Scholar]
- Kataoka R., Sherlock J., Lanier S. M. Signaling events initiated by transforming growth factor-beta 1 that require Gi alpha 1. J Biol Chem. 1993 Sep 15;268(26):19851–19857. [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Horowitz J. A., Casey J. L., Klausner R. D., Harford J. B. Translation and the stability of mRNAs encoding the transferrin receptor and c-fos. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7778–7782. doi: 10.1073/pnas.88.17.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Manzella J. M., Blackshear P. J. Specific protein binding to a conserved region of the ornithine decarboxylase mRNA 5'-untranslated region. J Biol Chem. 1992 Apr 5;267(10):7077–7082. [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991 Jul 25;266(21):14064–14071. [PubMed] [Google Scholar]
- Pavloff N., Rivard D., Masson S., Shen S. H., Mes-Masson A. M. Sequence analysis of the large and small subunits of human ribonucleotide reductase. DNA Seq. 1992;2(4):227–234. doi: 10.3109/10425179209020807. [DOI] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokipcak R. D., Herrick D. J., Ross J. Purification and properties of a protein that binds to the C-terminal coding region of human c-myc mRNA. J Biol Chem. 1994 Mar 25;269(12):9261–9269. [PubMed] [Google Scholar]
- Roberts A. B., Kim S. J., Noma T., Glick A. B., Lafyatis R., Lechleider R., Jakowlew S. B., Geiser A., O'Reilly M. A., Danielpour D. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp. 1991;157:7–28. doi: 10.1002/9780470514061.ch2. [DOI] [PubMed] [Google Scholar]
- Rondon I. J., MacMillan L. A., Beckman B. S., Goldberg M. A., Schneider T., Bunn H. F., Malter J. S. Hypoxia up-regulates the activity of a novel erythropoietin mRNA binding protein. J Biol Chem. 1991 Sep 5;266(25):16594–16598. [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Schwarz L. C., Gingras M. C., Goldberg G., Greenberg A. H., Wright J. A. Loss of growth factor dependence and conversion of transforming growth factor-beta 1 inhibition to stimulation in metastatic H-ras-transformed murine fibroblasts. Cancer Res. 1988 Dec 15;48(24 Pt 1):6999–7003. [PubMed] [Google Scholar]
- Schwarz L. C., Wright J. A., Gingras M. C., Kondaiah P., Danielpour D., Pimentel M., Sporn M. B., Greenberg A. H. Aberrant TGF-beta production and regulation in metastatic malignancy. Growth Factors. 1990;3(2):115–127. doi: 10.3109/08977199009108274. [DOI] [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Chan A. K., Choy B. K., Hurta R. A., McClarty G. A., Tagger A. Y. Regulation and drug resistance mechanisms of mammalian ribonucleotide reductase, and the significance to DNA synthesis. Biochem Cell Biol. 1990 Dec;68(12):1364–1371. doi: 10.1139/o90-199. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Turley E. A., Greenberg A. H. Transforming growth factor beta and fibroblast growth factor as promoters of tumor progression to malignancy. Crit Rev Oncog. 1993;4(5):473–492. [PubMed] [Google Scholar]
- Zaidi S. H., Denman R., Malter J. S. Multiple proteins interact at a unique cis-element in the 3'-untranslated region of amyloid precursor protein mRNA. J Biol Chem. 1994 Sep 30;269(39):24000–24006. [PubMed] [Google Scholar]
- Zaidi S. H., Malter J. S. Amyloid precursor protein mRNA stability is controlled by a 29-base element in the 3'-untranslated region. J Biol Chem. 1994 Sep 30;269(39):24007–24013. [PubMed] [Google Scholar]