Abstract
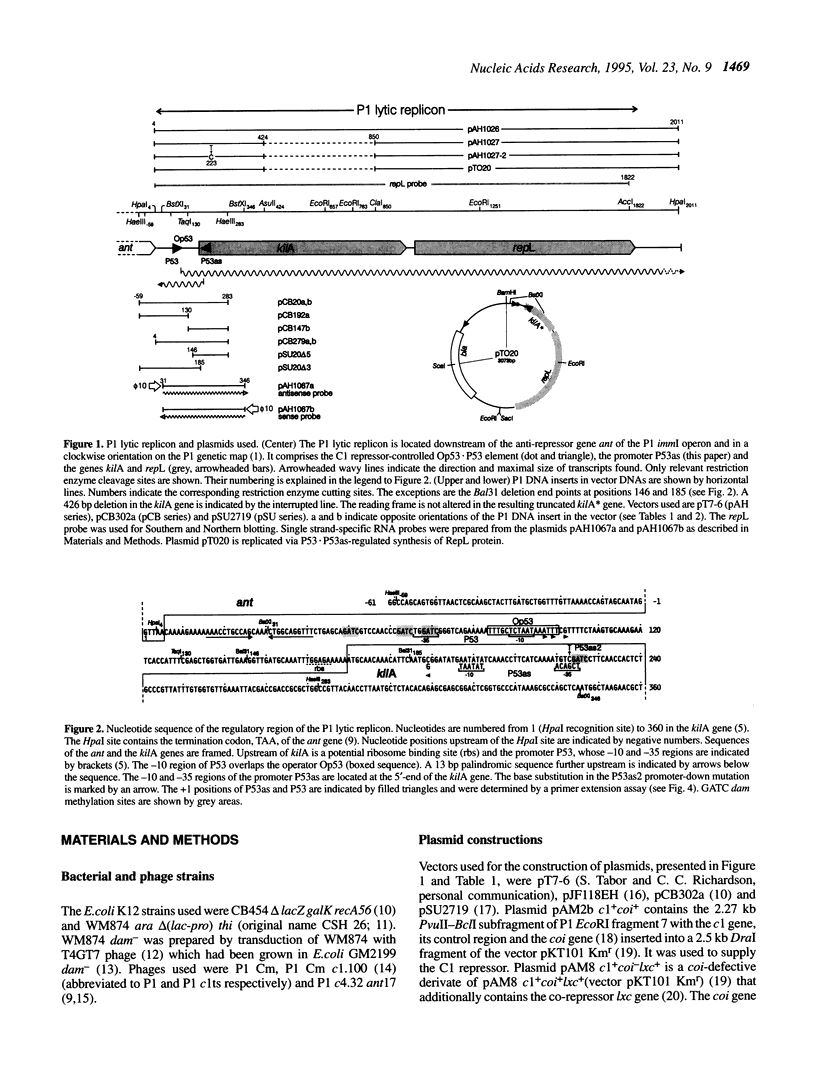
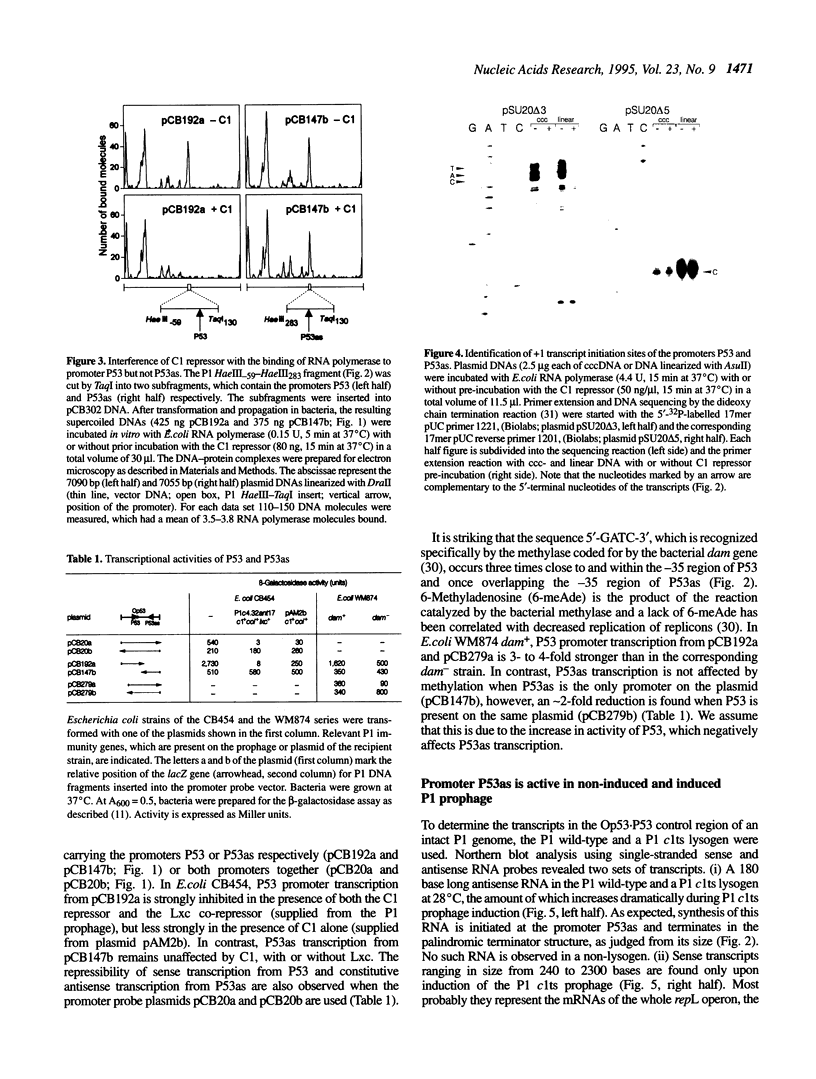
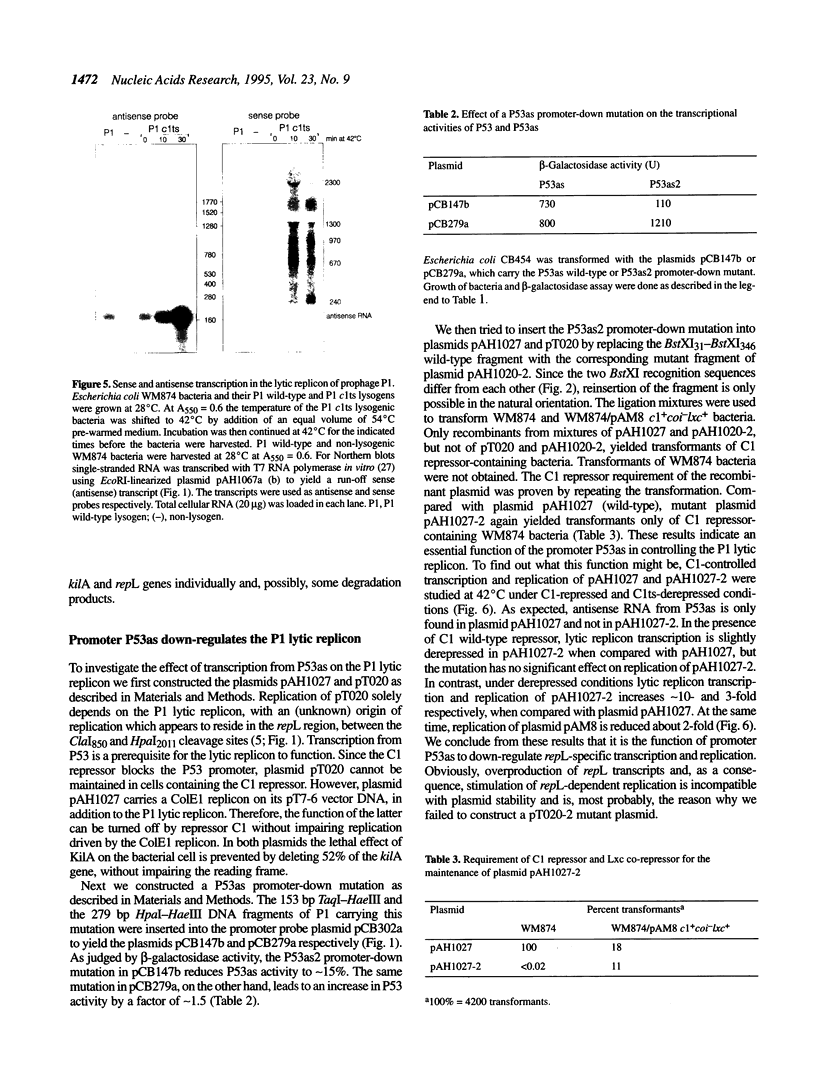
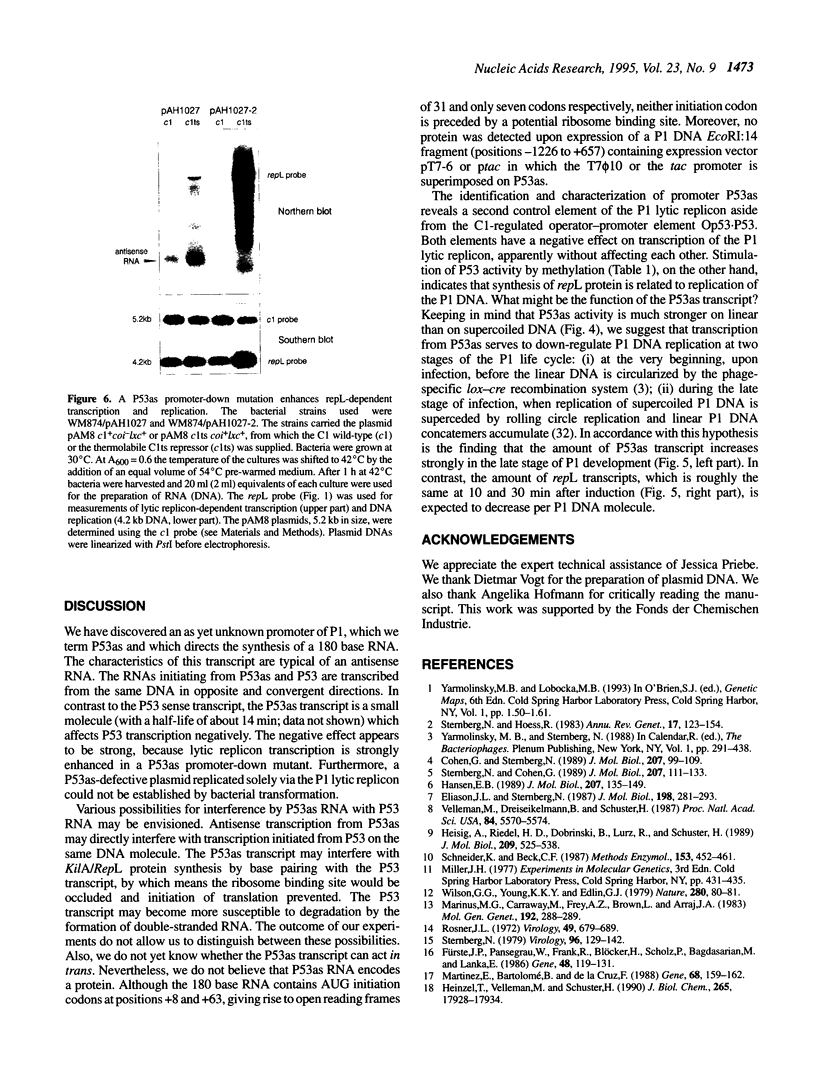
The lytic replicon of phage P1 is used for DNA replication during the lytic cycle. It comprises about 2% of the P1 genome and contains the P1 C1 repressor-controlled operator-promoter element Op53.P53 and the kilA and the repL genes, in that order. Transcription of the lytic replicon of P53 and synthesis of the product of repL, but not kilA, are required for replicon function. We have identified an additional promoter, termed P53as (antisense), at the 5'-end of the kilA gene from which a 180 base transcript is constitutively synthesized and in the opposite direction to the P53 transcript. By using a promoter probe plasmid we show that transcription from P53 is strongly repressed by the C1 repressor, whereas that of P53as remains unaffected. Accordingly, the C1 repressor inhibits binding of Escherichia coli RNA polymerase to P53, but not to P53as, as shown by electron microscopy. Under non-repressed conditions transcription from P53 appears to be inhibited by P53as activity and vice versa. An inhibitory effect of P53as on the P1 lytic replicon was revealed by the construction and characterization of a P53as promoter-down mutant. Under non-repressed conditions transcription of repL and, as a consequence, replication of the plasmid is strongly enhanced when P53as is inactive. The results suggest a regulatory role for P53as on the P1 lytic replicon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Cohen G. Electron microscopy study of early lytic replication forms of bacteriophage P1 DNA. Virology. 1983 Nov;131(1):159–170. doi: 10.1016/0042-6822(83)90542-1. [DOI] [PubMed] [Google Scholar]
- Cohen G., Sternberg N. Genetic analysis of the lytic replicon of bacteriophage P1. I. Isolation and partial characterization. J Mol Biol. 1989 May 5;207(1):99–109. doi: 10.1016/0022-2836(89)90443-9. [DOI] [PubMed] [Google Scholar]
- Eliason J. L., Sternberg N. Characterization of the binding sites of c1 repressor of bacteriophage P1. Evidence for multiple asymmetric sites. J Mol Biol. 1987 Nov 20;198(2):281–293. doi: 10.1016/0022-2836(87)90313-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Hansen E. B. Structure and regulation of the lytic replicon of phage P1. J Mol Biol. 1989 May 5;207(1):135–149. doi: 10.1016/0022-2836(89)90445-2. [DOI] [PubMed] [Google Scholar]
- Heinrich J., Riedel H. D., Baumstark B. R., Kimura M., Schuster H. The c1 repressor of bacteriophage P1 operator-repressor interaction of wild-type and mutant repressor proteins. Nucleic Acids Res. 1989 Oct 11;17(19):7681–7692. doi: 10.1093/nar/17.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel T., Velleman M., Schuster H. The c1 repressor inactivator protein coi of bacteriophage P1. Cloning and expression of coi and its interference with c1 repressor function. J Biol Chem. 1990 Oct 15;265(29):17928–17934. [PubMed] [Google Scholar]
- Heisig A., Riedel H. D., Dobrinski B., Lurz R., Schuster H. Organization of the immunity region immI of bacteriophage P1 and synthesis of the P1 antirepressor. J Mol Biol. 1989 Oct 20;209(4):525–538. doi: 10.1016/0022-2836(89)90591-3. [DOI] [PubMed] [Google Scholar]
- Lurz R., Heisig A., Velleman M., Dobrinski B., Schuster H. The ban operon of bacteriophage P1. Localization of the promoter controlled by P1 repressor. J Biol Chem. 1987 Dec 5;262(34):16575–16579. [PubMed] [Google Scholar]
- Marinus M. G., Carraway M., Frey A. Z., Brown L., Arraj J. A. Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet. 1983;192(1-2):288–289. doi: 10.1007/BF00327681. [DOI] [PubMed] [Google Scholar]
- Martinez E., Bartolomé B., de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988 Aug 15;68(1):159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- Olsen D. B., Eckstein F. High-efficiency oligonucleotide-directed plasmid mutagenesis. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1451–1455. doi: 10.1073/pnas.87.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Beck C. F. New expression vectors for identifying and testing signal structures for initiation and termination of transcription. Methods Enzymol. 1987;153:452–461. doi: 10.1016/0076-6879(87)53071-3. [DOI] [PubMed] [Google Scholar]
- Sternberg N. A characterization of bacteriophage P1 DNA fragments cloned in a lambda vector. Virology. 1979 Jul 15;96(1):129–142. doi: 10.1016/0042-6822(79)90179-x. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Cohen G. Genetic analysis of the lytic replicon of bacteriophage P1. II. Organization of replicon elements. J Mol Biol. 1989 May 5;207(1):111–133. doi: 10.1016/0022-2836(89)90444-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Evidence that adenine methylation influences DNA-protein interactions in Escherichia coli. J Bacteriol. 1985 Oct;164(1):490–493. doi: 10.1128/jb.164.1.490-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Hoess R. The molecular genetics of bacteriophage P1. Annu Rev Genet. 1983;17:123–154. doi: 10.1146/annurev.ge.17.120183.001011. [DOI] [PubMed] [Google Scholar]
- Velleman M., Dreiseikelmann B., Schuster H. Multiple repressor binding sites in the genome of bacteriophage P1. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5570–5574. doi: 10.1073/pnas.84.16.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman M., Heinzel T., Schuster H. The Bof protein of bacteriophage P1 exerts its modulating function by formation of a ternary complex with operator DNA and C1 repressor. J Biol Chem. 1992 Jun 15;267(17):12174–12181. [PubMed] [Google Scholar]
- Wilson G. G., Young K. Y., Edlin G. J., Konigsberg W. High-frequency generalised transduction by bacteriophage T4. Nature. 1979 Jul 5;280(5717):80–82. doi: 10.1038/280080a0. [DOI] [PubMed] [Google Scholar]