Abstract
In both humans and animals, the hippocampus is critical to memory across modalities of information (e.g., spatial and nonspatial memory) and plays a critical role in the organization and flexible expression of memories. Recent studies have advanced our understanding of cellular basis of hippocampal function, showing that N-methyl-d-aspartate (NMDA) receptors in area CA1 are required in both the spatial and nonspatial domains of learning. Here we examined whether CA1 NMDA receptors are specifically required for the acquisition and flexible expression of nonspatial memory. Mice lacking CA1 NMDA receptors were impaired in solving a transverse patterning problem that required the simultaneous acquisition of three overlapping odor discriminations, and their impairment was related to an abnormal strategy by which they failed to adequately sample and compare the critical odor stimuli. By contrast, they performed normally, and used normal stimulus sampling strategies, in the concurrent learning of three nonoverlapping concurrent odor discriminations. These results suggest that CA1 NMDA receptors play a crucial role in the encoding and flexible expression of stimulus relations in nonspatial memory.
In humans, it is widely accepted that structures within the hippocampus (i.e., CA1-CA3, dentate gyrus, and subiculum) play a critical and selective role in declarative memory. This kind of memory involves creating functional neural networks that mediate our ability to express memory “flexibly,” that is, to use information in ambiguous and novel situations (1). Recent studies on humans have shown that the hippocampus proper (CA1-CA2 and CA3) is critical to declarative memory. Indeed, damage largely limited to this region (2), and even that restricted to area CA1 (3), results in a significant declarative memory deficit. The scope of this hippocampus-dependant memory is “global” in that it includes all sensory modalities and extends to both spatial and nonspatial information (4).
Animal models of amnesia can provide further insights into the nature of the memory dependent on the hippocampus, the specific roles of particular hippocampal areas, and the critical physiological mechanisms within the hippocampus. There is abundant evidence that damage to the hippocampus in animals results in an impairment in a particular type of spatial learning and memory (5–7). Damage to the hippocampus produces impairments in spatial learning when the task requires the subject to acquire memory for spatial relations among salient environmental cues (6–8). By contrast, animals with hippocampal damage succeed in learning a consistent trajectory toward a spatial scene (8) and carrying out tasks that involve learning habitual responses that lead to particular locations in space (7, 9). Likewise, recent evidence has suggested that the hippocampus also plays a critical and selective role in nonspatial learning and memory (10). Rats with hippocampal disconnection can form memories for even very complex single stimuli, but they fail in learning relationships among multiple cues (11, 12). Furthermore, damage restricted to hippocampal subfields results in the same selective impairment in nonspatial memory (13).
Recently, much attention has focused on the cellular and molecular processes that underlie hippocampal memory. N-methyl-d-aspartate (NMDA)-dependent synaptic plasticity has been implicated in the cellular regulation of memory formation within hippocampal circuitry. Pharmacological blockade of NMDA receptors in the hippocampus prevents long-term potentiation, a mechanism suggested to be involved in memory storage, and results in spatial memory impairments (14). Selective deletion of the gene encoding NMDA receptors in area CA1 of the hippocampus is sufficient to cause a severe impairment in the animal's ability to learn spatial tasks relying on distal cues, whereas it does not affect the learning of a task that can be carried out by a single proximal cue (15). In addition, two recent studies have shown that the learning impairment in mice lacking CA1 NMDA receptors extends to the domain of nonspatial learning and memory (16, 17). However, the behavioral protocols used in these studies did not directly assess the capacity for the acquisition and flexible expression of nonspatial memory.
Using mice with the same selective receptor deletion (15), we assessed the role of CA1 NMDA receptors in two nonspatial, odor-guided learning tasks: transverse patterning and concurrent discrimination. These tasks involved similar stimulus materials and behavioral protocols but differed in their demands for formation and flexible expression of memory: indeed, only the transverse patterning task requires the acquisition of three overlapping odor discrimination. Our results show that CA1 NMDA knockout mice use abnormal stimulus sampling strategies, and consequently show an abnormal pattern of learning, in the transverse patterning task. By contrast, their stimulus sampling strategies and learning are fully normal in the concurrent discrimination task.
Materials and Methods
Experimental Subjects.
We used mice in which the gene for the NR1 subunit of the NMDA receptor was deleted selectively in the CA1 pyramidal cells; the generation of these knockout mice is detailed elsewhere (15). CA1-NR1 knockout mice (n = 11) were heterozygous for the viral Cre recombinase gene and homozygous for the floxed NR1 gene. The control group was composed of male littermates of three genotypes: homozygous for the floxed NR1 gene (n = 10), heterozygous for Cre (n = 4), and wild type (n = 4). In situ hybridization experiments showed that after 3 months of age, the recombination extends beyond the CA1 area (Akira Kato, personal communication). We analyzed mice from 6 to 9 weeks old. Mice were food-deprived to maintain 85% of initial body weight throughout testing. All of the behavioral analyses were performed blind to the genotype of the mice, which was determined after these tests. All experiments were performed in compliance with the Massachusetts Institute of Technology animal committee.
Apparatus.
All tests were conducted in a standard (30 × 19 cm) clear Plexiglas cage. During all training phases two opaque plastic cups were located at one end of the cage, about 3 cm apart. During the transverse patterning and the concurrent discrimination tasks, cups were filled with sterilized playground sand. The odor stimuli were common spices mixed at 0.1% concentration by weight in sand. Spices used were: A = pepper, B = paprika, C = ginger, D = coriander, E = onion, and F = kelp. Cups were baited with small (about 15 mg) pieces of chocolate.
Shaping.
Before transverse patterning training, mice were trained to dig in sand-filled cups to obtain rewards. On day 1, each mouse was placed in a cage and a single baited cup was presented at one end of the cage. Rewards were dispersed throughout the sand, and some were visible from the surface. The mouse was allowed a maximum of 1 h to retrieve all of the rewards. This procedure was repeated three times. On day 2 the mouse was presented with one cup containing several hidden rewards. On days 3 and 4 the mouse was presented with two cups, only one of which was baited with a hidden reward, for three trials. On each trial the animal was given 10 min to retrieve the reward; if it did not dig, a reward was placed on top of the baited cup. On day 5, the procedure was the same as on day 4, and two scents were introduced. These scents (fennel and turmeric) were different from the ones used during the transverse patterning and the concurrent discrimination tasks. If a mouse did not consistently dig by the end of shaping, it was excluded from the study.
Behavioral Testing.
The transverse patterning and the concurrent discrimination tasks.
Two days after the final shaping, testing on transverse patterning began. In this test subjects concurrently solved three overlapping discrimination problems each of which involved a different pairing of stimuli from a set of three (A, B, and C): stimulus A was rewarded when presented with stimulus B (A+B−); B was rewarded when presented with C (B+C−); and C was rewarded when presented with A (C+A−). The mice were given nine trials per day for 5 consecutive days. Inter-trial interval included the time for an animal to perform the task and the time to fill the cups with fresh scented sand; it ranged between 10 and 15 min in both transverse patterning and concurrent discrimination tasks. On the first day the animal was presented with the odor pairs in an orderly series—AB, then BC, then CA. This order was repeated three times with the location of the baited cup selected according to a pseudorandom schedule. The amount of time spent sniffing over each odor cup, the number of stimulus samplings at each cup, and the first cup in which animals dug into were recorded for each trial. Mice were allowed to make a correct choice on each trial even if they had already made an error. Animals were given a maximum of 10 min to make the first choice and were removed once a correct choice was made. If no choice was made within 10 min a reward was placed on the top of the correct cup and the mouse was allowed to retrieve it. The same protocol was used on the next 4 days, except that the odor pairs were presented in a pseudorandom order. After 2 days of rest, mice were trained on the concurrent discrimination problem. The protocol was identical, except the odors pairs were changed to A+B−, C+D−, E+F−, presented in pseudorandom order, for 5 days of testing.
The motivation task.
The test was conducted in a standard (30 × 19 cm) clear Plexiglas cage. Two opaque plastic cups were located at one end of the cage, about 3 cm apart. Both cups were, respectively, filled with chocolate and cinnamon cereals mixed with 30% cocoa or 10% cinnamon. Mice spent 2 h in the cage. The total number of cups visited was recorded through a video monitor. The amount of food eaten was assessed for each mouse after the completion of the experiment by comparing the weight of each cup before and after the 2-h session.
The activity chamber task.
We used a computer-controlled system (digipro software; AccuScan Instruments, Columbus, OH). Each animal was placed in a clear Plexiglas cage (42 × 42 cm). Motor activity was monitored via a grid of invisible infrared light beams. The digipro software allowed the analyzer to collect the beam status information from the activity monitor, and all analyses were directly fed into the computer. The motor activity parameters (i.e., time moving, time resting, distance run, and stereotypy number) thereafter were analyzed with a statistical software (statview 4.01).
Results
CA1-NR1 Mice Are Abnormal in Solving the Transverse Patterning Task.
A characterization of cognitive processes in learning is optimally accomplished by using behavioral protocols in which performance can be mediated by multiple, dissociable strategies. Transverse patterning is one of several tests of nonspatial memory that can be solved by multiple strategies that differ in how stimulus relations are encoded (1, 12). This problem involving three different pairs of stimuli (A+B−, B+C−, and C+A−) could be partially solved by adopting a specific response for two of the individual stimulus elements (the elemental strategy). For example, subjects could learn to always choose A and never choose C. This would be expected to lead to accurate performance on two of the problems (A+B− and B+C−), but especially poor (less than chance) performance on the third problem (C+A− in this case) because the reward associations of this problem are contrary to the acquired responses for both A and C. Alternatively, fully successful performance could be achieved by encoding each stimulus pairing as a unique configuration and adopting a particular response for each configuration (the configural strategy; ref. 18). Thus, subjects can learn to approach A when the configural cue AB is presented, to approach B when BC is presented, and to approach C when CA is presented. Yet another solution is to memorize each of the stimuli in terms of its relationship to the others in the set, in a “circular” organization of stimulus relations: A is to be selected over B, which is to be selected over C, which is to be selected over A (the relational strategy). The latter two strategies may be distinguished by examination of the subjects' stimulus sampling behavior. Thus, recognition of the configural cue presented on each pairing might be expected to require minimal sampling of each stimulus, whereas judging the relationships between stimulus elements might be expected to involve repetitive sampling and comparisons between the cues. We expected that normal subjects would have all three strategies available, but that subjects with compromised hippocampal function may not be able to adopt the relational strategy to learn the task. Additional clues about strategies available to the subjects can be obtained by comparing both performance and stimulus sampling behavior in transverse patterning to those in concurrent discrimination problems that put no demands on the memory of stimulus configurations or relationships. Without such a demand one might expect fully intact performance and normal stimulus sampling behavior even in subjects with compromised hippocampal function.
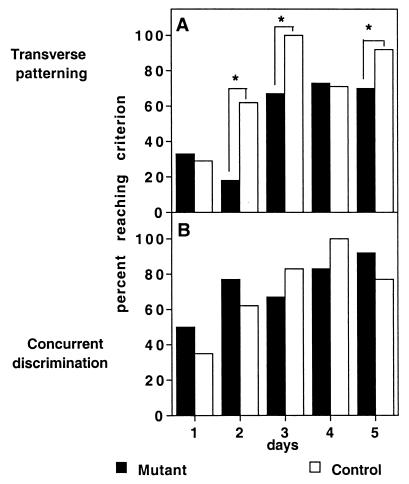
For training in transverse patterning and concurrent discrimination, we examined both the pattern of acquisition and the nature of stimulus sampling in mice in which the NMDA receptor in area CA1 had been deleted (CA1-NR1 mice) and in control mice. Control mice very rapidly solved the transverse patterning problem (Fig. 1A). Even on day 2, the first day in which the stimulus pairs were presented in random order, more than 60% of the control mice reached the behavioral criterion of eight of nine (89%) correct responses, and on the third session all of the controls reached the criterion. By contrast, CA1-NR1 mice showed an overall impairment in reaching the criterion (χ2 = 20.397, df = 4, P = 0.0004). They were severely impaired in the early training sessions, such that fewer than 20% of the mutants reached the criterion on session 2 (χ2 = 40.333, df = 1, P < 0.0001). In addition, whereas the majority of the CA1-NR1 mice eventually were successful in reaching the performance criterion, they remained significantly impaired on session 3 (χ2 = 39.521, df = 1, P < 0.0001) and session 5 (χ2 = 15.724, df = 1, P < 0.0001).
Figure 1.
Percent of subjects reaching the performance criterion (eight of nine correct choices) on each training session in (A) transverse patterning and (B) concurrent discrimination. *, χ2; P < 0.001.
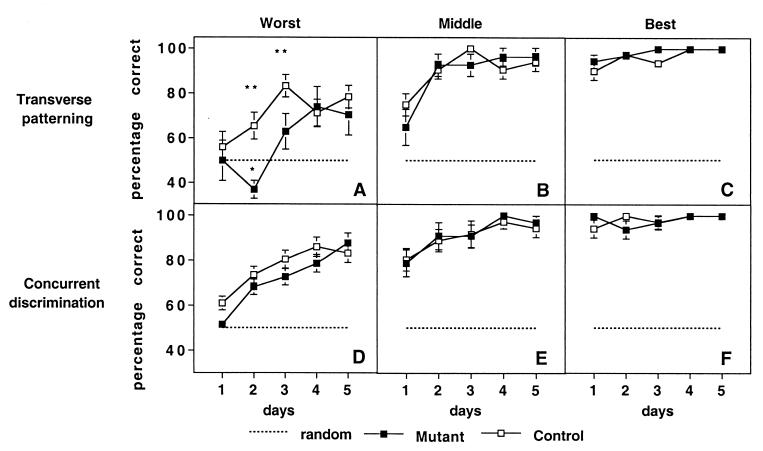
To better understand the nature of the initially severe impairment and later partial success in transverse patterning, we examined the course of learning on each of the three odor pairs over successive training sessions (Fig. 2). Choice accuracy was assessed separately for the pair in which each animal had the least accurate performance (worst pair), for the pair in which performance was intermediate (middle pair), and for the pair in which performance was the most accurate (best pair) (12). Performance of control and CA1-NR1 mice differed across the five training sessions [F(1,28) = 11.01; P < 0.0003]. Control mice performed above chance on all three pairs throughout test sessions beyond the initial training day (Fig. 2D). Even at their worst, control mice succeeded in reaching above-chance performance on the second day of training (post hoc analysis, P < 0.0003; Fig. 2A). CA1-NR1 mice performed as well as control mice on their best and middle pairs (P > 0.05). In contrast to control mice, CA1-NR1 mice performed poorly on their worst pair early in training (P < 0.0005). During the first day of training, CA1-NR1 mice performed at chance for the worst pair (P > 0.05), as did control mice. However, in the second training session, whereas control mice performed significantly above chance level, CA1-NR1 mice performed even less well than expected by chance (post hoc analysis, P < 0.05). Subsequently, the response accuracy of CA1-NR1 mice improved such that it did not differ significantly from that of control mice on the fourth and fifth sessions (post hoc analysis, P > 0.05).
Figure 2.
Mean percent correct responses on each training session for odor pairs associated with worst (A and D), middle (B and E), and best (C and F) accurate performance. (A–C) The transverse patterning problem. (D–F) The concurrent discrimination problem. *, Significantly different from chance; ANOVA; P < 0.05. **, Significantly different from both chance level and the score for CA1-NR1 mice; ANOVA; P < 0.001.
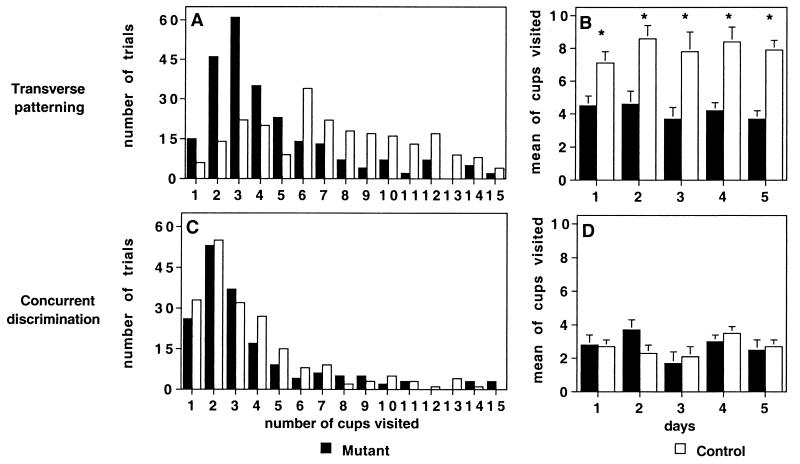
In addition, we examined the behavior of animals in sampling the two odor stimuli that were presented in separated cups. This was done by two independent observers. An interrater reliability of 98% was found for the measurement. Control mice repeatedly interleaved brief (<1 s) sniffing bouts at each odor cup with longer bouts of general exploration throughout the test chamber. Although the number of visits to the two odor cups varied widely in controls, the modal number of visits was six (three per cup) before a choice response (Fig. 3A). CA1-NR1 mice exhibited the same general behavioral pattern, composed of brief stimulus sampling bouts interleaved with general exploration. However, their modal number of visits was three (1.5 per cup), only half that of controls (F = 71.065, P < 0.0001; Fig. 3A). The pattern of diminished stimulus sampling in CA1-NR1 mice was apparent throughout training, even when most of the animals eventually reached the performance criterion; they had fewer bouts of stimulus sampling than control mice across all sessions (P = 0.007 to P = 0.0004; Fig. 3B).
Figure 3.
Distribution of the number of odor cups visited per trial combined across all sessions of (A) transverse patterning and (C) concurrent discrimination, and the mean number of odor cups visited on individual training sessions in (B) transverse patterning and (D) concurrent discrimination. *, ANOVA, P < 0.007.
CA1-NR1 Mice Are Normal in Solving the Concurrent Discrimination Task.
All animals were tested further on a concurrent odor discrimination task that also involved three odor pairs, but without any overlapping stimuli among the pairs. Concurrent discrimination has the same format of odor presentations, and a larger number of presented odors, but puts no demand on learning stimulus configurations or relations among the full set of odor cues.
Animals were trained for five sessions, each of which involved a set of three nonoverlapping odor pairs (A+B−, C+D−, E+F−). Animals should be able to acquire this set of discriminations by learning to approach or avoid each odor element (e.g., approach A, avoid B, etc.; elemental strategy), or by adopting a particular response selection for each of the three stimulus-pair configurations (if AB then choose A, etc.; configural strategy). Because the odor pairs presented in this problem have no overlap, this task minimizes any demand on comparing the odors among the set of pairs. Accordingly, spared learning of the concurrent discrimination would strongly indicate that CA1-NR1 mutants are not impaired in odor discrimination learning in a general way.
Control and CA1-NR1 mice readily succeeded at the concurrent odor discrimination task (Fig. 1B). On day 2 more than 60% of both control and CA1-NR1 mice reached the criterion of eight of nine trials correct, and on day 5 approximately 80% of the subjects in both groups reached the criterion; there was no significant difference in the number of subjects per group reaching criterion in any session (χ2, P > 0.05). In addition, both CA1-NR1 and control mice rapidly achieved above-chance performance on all three pairs (ANOVA, P < 0.0005; Fig. 2 Lower), and there were no significant group differences in performance [F(1,28); P > 0.05].
Correspondingly, the stimulus sampling strategies were similar in the two groups. The controls showed a striking decrease in the numbers of odor cups sampled, compared with those in the transverse patterning task, reducing them to a modal number of two (one visit per cup) on the concurrent discrimination task (compare Fig. 3 A and C; t test, t = 9.498, P < 0.0001). Mutant mice also sampled slightly fewer odor cups than they had in the transverse patterning task, reducing the modal number of visits to two (one per cup) as compared with three in the transverse patterning task, although this difference was not statistically reliable (t test, t = −0.34, P > 0.05). The distribution of number of odor cup visits did not statistically differ between control and CA1-NR1 mice (ANOVA, P > 0.05; Fig. 3C), nor did the mean number of visits to odor cups differ statistically over the training sessions (ANOVA, P > 0.05; Fig. 3D).
CA1-NR1 Mice Are Normal in the Motivation to Ease Hunger and in General Motor Activity.
The reduction in the number of cups visits observed in CA1-NR1 mice could arise from a general deficit such as a decrease in activity or diminished motivation to ease hunger. To test the motivation to eat, food-deprived mice were placed in a cage with two cups full of food (chocolate and cinnamon cereals). The motivation of the mice to go to the cups was assessed by analyzing the number of cups visited. The amount of food eaten was an indication of how hungry the mice were. No learning was necessary in this task; animals had only to go to the cups and to eat whenever they were hungry. Mutant mice visited the cups as many times and ate as much as control mice did (Table 1). No difference was observed between mutant and control mice (ANOVA, P > 0.05).
Table 1.
Motivation to ease the hunger
| Mice | Number of visits | Amount of food eaten, g |
|---|---|---|
| Mutant (n = 15) | 196 ± 20 | 0.39 ± 0.04 |
| Control (n = 14) | 202 ± 30 | 0.44 ± 0.03 |
Mice were food-deprived and placed 2 h in a cage with two cups full of food (chocolate and cinnamon cereals). The number of cups visited and the amount of food eaten are indicated above. No difference (ANOVA, P > 0.05) was observed between mutant and controls.
Motor activity was monitored by using the activity chamber task. The time mice were moving or resting, the total distance run, and the number of times the animal broke the same beam repeatedly as a measure of stereotypy (for example, grooming, head bobbing) were automatically quantified. CA1-NR1 mice presented the same pattern of activity as controls (Table 2). These results demonstrate that CA1-NR1 mice have no deficit in motivation to ease hunger or motor activity.
Table 2.
General motor activity
| Mice | Total distance, cm | Moving time, sec | Resting time, sec | Stereotypy number |
|---|---|---|---|---|
| Mutant (n = 9) | 131 ± 9 | 207 ± 31 | 393 ± 31 | 81 ± 7 |
| Control (n = 8) | 128 ± 7 | 183 ± 24 | 417 ± 25 | 87 ± 4 |
Mice were put 10 min in a square cage (42 × 42 cm) and the time they were moving, resting, the total distance run, and the number of stereotypy were quantified (digipro software; AccuScan Instruments). No difference was observed between mutants and controls (ANOVA, P > 0.05).
Discussion
The performance and sampling behavior displayed by the CA1-NR1 KO mice (mutants) and the control mice in the transverse patterning task provide clues about the distinct natures of memory acquired by the two types of mice. Furthermore, the role (or lack of it) of NMDA receptors in the CA1 pyramidal cells in each type of memory can be assessed.
Control mice typically sampled each odor three or more times before making a choice and improved the performance quickly on all three pairs of odors. As the training advanced, control mice continued to sample each odor back and forth repeatedly, suggesting that they compared and contrasted the individual odors throughout training. This strategy, and the associated high-frequency success in the performance on all three stimulus pairs, suggests that they were learning the relationship of the entire set of stimuli and the association of the reward with the particular stimulus in the context of stimulus pairing. In contrast to the control mice, CA1-NR1 mutant mice typically sampled each odor only once or twice throughout the training. On the two of three pairs of odors (best pair and intermediate pair) the mutant mice improved their performance as fast as the control mice. However, on the third pair of odors (worst pair), the mutant mice performed much more poorly than the control mice during the first half of the entire training period (days 2 and 3). Particularly revealing was the observation that during this period the mutants performed significantly worse than the level that would have been expected from a random choice. These observations, combined with the low numbers of cup visits, strongly suggest that the mutants adopted an elemental strategy in which they associated a particular odor with the reward (e.g., A+) or a particular odor with no reward (e.g., C−) and acted with no consideration to the specific odor pair. This strategy demands less time and labor but will permit a correct choice only in two (i.e., best pair and intermediate pair) of the three pair. Indeed, 87% of errors made by the mutants on the worst pair trials in day 2 accompanied the trials where the animals sampled only one cup and immediately selected that odor or the alternative in the other cup. This finding suggests that the animals were simply in search of a particular odor to take or avoid, independent of the odor in the other cup.
There can be several possibilities as to why the NMDA receptor knockout in the CA1 area might have led to the lack of adoption of the sample-comparing strategy. Because the sample-comparing strategy requires on average more time and labor one must consider the possibility that reduced motivation or reduced general motor activity may have prevented mutants to adopt this strategy. However, the results shown in Tables 1 and 2 suggest that the mutants are as motivated and as active as the control mice. Another possibility that mutants are impaired generally in olfactory perception or adoption of the cup-digging response is also unlikely because these animals performed perfectly well on the best and intermediate trials. Furthermore, the mutant mice performed as well as the control animals on the olfaction-based concurrent discrimination task (see Figs. 1B and 2).
Given that the mutants are not impaired in motivation, general activity, olfactory perception, or specific responses, the most likely reason they did not adopt the sample-comparing, high-number visit strategy is that they are impaired in acquiring the memory of the complex relationships among the odors and the reward. This type of memory is an effective means by which the transverse patterning problem would be solved and the one that seems to be used by control mice. The possibility that the central nervous system directly encodes the ability to adopt the multivisit strategy per se is unlikely. Even more unlikely is the possibility that NMDA receptors in the CA1 area of the hippocampus are responsible for such hypothetical ability. Thus, the most likely interpretation of our observations are as follows. Control animals adopt the sample-comparing strategy because they are capable of acquiring the memory of the stimulus-reward associations where the relations among the three stimuli are circular (relational memory). The relational memory gives the animal the highest rate of success. The mutant mice are incapable of acquiring this type of memory because of the knockout of CA1 NR1 and therefore there is no incentive to adopt the more time- and labor-consuming stimuli-comparing strategy. They initially (day 2 and part of day 3) resort to the elemental strategy that gives them a partial solution: successes in best and intermediate trials but failures in worst trials. But, as the mutants undergo more trials, they seem to realize the usefulness of the third strategy, the configural strategy (18), that gives them a higher rate of success and switch to this strategy as the training advances from day 3 to day 4 to day 5. It is important to note that this improvement in performance does not reflect a slower acquisition of relational memory. This analysis is suggested strongly by the observation that the numbers of cups visited by CA1-NR1 mice remained low in the advanced stage of the training (see Fig. 3B).
Such an interpretation of our results is in agreement with the fact that the type of memory underlying either the elemental or configural strategy can be supported by structures outside the hippocampus (19). This interpretation was demonstrated again in this study by the lack of impairment displayed in the CA1-NR1 KO mice in the concurrent discrimination task. This task, in contrast to the transverse patterning task, demands only memory of a simple inflexible association. The normal performance on the concurrent discrimination task by the CA1-NR1 KO mice confirmed that they are intact in olfactory perception and in the adoption of specific responses to individual odors or odor compounds.
The transverse patterning task previously has been used in the analysis of learning by animals with hippocampal damage, with mixed results. In some studies rats with hippocampal damage were severely impaired (20) whereas in others the impairment was more subtle (12), and in yet another study the lesion resulted in facilitation of learning (20). An in-depth examination of these findings suggests that whether or not an impairment is observed depends on the cognitive strategies used to solve the problem. Alvarado and Rudy (20) trained rats in three phases of increasing complexity (phase 1, A+ versus B−; phase 2, A+ versus B− and B+ versus C−; and phase 3, A+ versus B−, B+ versus C−, and C+ versus A−) and found that rats with hippocampal formation damage performed well on the first two phases of the task, but not on the third. Dusek and Eichenbaum (12) trained rats in a succession of stages in which they initially were presented with blocks of trials on each pair in an odor, and finally were presented with all of the pairs concurrently in a random order. Dusek and Eichenbaum found that rats with hippocampal damage succeeded in the initial training stages but failed in performing the concurrent random presentations. Bussey et al. (21) trained animals in a protocol similar to that of Alvarado and Rudy (20), but presented the stimuli on a touch screen, and found that both normal rats and rats with hippocampal damage gradually acquired the task. One interpretation of these findings is that animals with hippocampal damage can partially succeed or even outperform normal animals whenever they adopt specific response to individual stimuli or stimulus compounds. Thus, in the Alvarado and Rudy study (20) animals with hippocampal damage may have solved the first two pairs by using this strategy, but this was insufficient to resolve the ambiguities that arose when the third pair was introduced. In the Dusek and Eichenbaum study (12), animals with hippocampal damage might have used other nonrelational strategies that were effective for the pairs presented in an orderly sequence (i.e., always chose the odor that was not previously rewarded, for example), but these strategies did not support performance when the order of pairs was random. In the Bussey et al. study (21), the presentation of two-dimensional stimuli on a screen may have encouraged the acquisition of stimulus compounds, and that strategy may have been especially well used by animals with hippocampal damage.
Our findings on the transverse patterning task are consistent with these previous studies that indicated that this task can be solved by multiple strategies. Similar to Alvarado and Rudy's findings on rats with hippocampal damage (20), our findings show that CA1-NR1 mice could adopt specific responses to two of the stimulus elements early in training. Later improvement by some CA1-NR1 mice may have been mediated by adopting specific responses to each stimulus configuration, a strategy consistent with other observations of spared transverse patterning in rats with hippocampal damage trained under circumstances that encouraged configural strategy (21) (see discussion in refs. 1 and 12).
Several studies have been aimed at characterizing the fundamental nature of memory dependent on the hippocampus (10, 22, 23). These studies indicated that learning is impaired by hippocampal damage in those situations where normal animals would acquire a memory of relationships among multiple stimuli and where they express this memory flexibly by responding differently to particular stimuli, depending on their relationship to others. By contrast, animals with hippocampal damage perform as well as intact animals in the acquisition of consistent responses or biases toward individual stimuli or stimulus configurations. For example, in the Morris water maze task, CA1-NR1 mice were severely impaired in acquiring the memory of relationship among multiple distal cues and in navigating flexibly to find the hidden platform. By contrast, they performed normally in learning to approach a specific landmark in the environment (15). Other recent studies with the same mutant mice (16, 17) indicated that CA1 NMDA receptors play a crucial role in the acquisition of memory on a broad range of nonspatial tasks. However, these studies on nonspatial memory did not use tasks that demand memory of relationships among multiple stimuli and its flexible expression. Our present study examined whether the requirement of NMDA receptor-mediated function in area CA1 of hippocampus extend to “relational memory” of nonspatial type. Our results, when combined with those previously reported on spatial tasks (15), suggest that NMDA receptors in CA1 plays a crucial role in the general capacity for relational memory.
Acknowledgments
We thank Dennis King, Beryl Fung, Candice Carr, Jayson Derwin, Sean Montgomery, Amy Baughman, and Audrey Chang for their technical help and Earl Miller and Michela Gallagher for critical reading of the manuscript. The work was in part supported by National Institute of Mental Health Grant 1-P30-MH58880. L.R.-R. was supported by a European Molecular Biology Organization fellowship.
Abbreviation
- NMDA
N-methyl-d-aspartate
References
- 1.Reed J, Squire L. Behav Neurosci. 1999;113:3–9. doi: 10.1037//0735-7044.113.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Vargha-Khadem F, Gadian D G, Watkins K E, Connelly A, Van Paesschen W, Mishkin M. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 3.Zola-Morgan S, Squire L R, Amaral D. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng E, Squire L R. Nature (London) 1999;12:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 6.Morris R G M, Garrud P, Rawlins J N P, O'Keefe I. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 7.Whishaw I Q, Cassel J C, Jarrard J E. J Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenbaum H E, Stewart C, Morris R G M. J Neurosci. 1990;10:331–339. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packard M G, McGaugh J L. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 10.Eichenbaum H, Shoenbaum G, Young B, Bunsey M. Proc Natl Acad Sci USA. 1996;93:13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dusek J A, Eichenbaum H. Proc Natl Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dusek J A, Eichenbaum H. Behav Neurosci. 1998;4:762–771. doi: 10.1037//0735-7044.112.4.762. [DOI] [PubMed] [Google Scholar]
- 13.Bunsey M, Eichenbaum H. Nature (London) 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 14.Morris R G, Frey U. Trans R Soc London Biol Sci. 1997;352:1489–1503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsien J Z, Huerta P T, Tonegawa S. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 16.Huerta P T, Sun L D, Wilson M A, Tonegawa S. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- 17.Rampon C, Tang Y P, Goodhouse J, Shimizu E, Kyin M, Tsien J Z. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 18.Rudy J W, Sutherland R J. Behav Brain Res. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- 19.Eichenbaum H, Mathews P, Cohen N J. Behav Neurosci. 1989;103:1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- 20.Alvarado M C, Rudy J W. Behav Neurosci. 1995;109:204–211. doi: 10.1037//0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- 21.Bussey T J, Clea Warburton E, Aggleton J P, Muir J L. J Neurosci. 1998;18:1622–1631. doi: 10.1523/JNEUROSCI.18-04-01622.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen N, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 23.Knowlton B J, Fanselow M S. Curr Opin Neurobiol. 1998;8:293–296. doi: 10.1016/s0959-4388(98)80154-2. [DOI] [PubMed] [Google Scholar]