Abstract
CD8+ T cells restricted to MHC class Ib molecules other than H2-M3 have been shown to recognize bacterial antigens. However, the contribution of these T cells to immune responses against bacterial infection is not well-defined. To investigate the immune potential of MHC class Ib-restricted CD8+ T cells, we have generated mice that lack both MHC class Ia and H2-M3 molecules (Kb−/−D b−/−M3−/−). The CD8+ T cells present in Kb−/−D b−/−M3−/− mice display an activated surface phenotype and are able to rapidly secrete IFN-γ upon anti-CD3 and anti-CD28 stimulation. While the CD8+ T cell population is reduced in Kb−/−D b−/−M3−/− mice as compared to Kb−/−D b−/− mice, this population retains the capacity to expand significantly in response to primary infection with the bacteria Listeria monocytogenes. However, Kb−/−D b−/−M3−/− CD8+ T cells do not expand upon secondary infection, similar to what has been observed for H2-M3-restricted T cells. CD8+ T cells isolated from Listeria-infected Kb−/−D b−/−M3−/− mice exhibit cytotoxicity and secrete proinflammatory cytokines in response to Listeria-infected antigen-presenting cells. These T cells are protective against primary Listeria infection, as Listeria-infected Kb−/−D b−/−M3−/− mice exhibit reduced bacterial burden as compared to infected β2-microglobulin-deficient mice that lack MHC class Ib-restricted CD8+ T cells altogether. In addition, adoptive transfer of Listeria-experienced Kb−/−D b−/−M3−/− splenocytes protects recipient mice against subsequent Listeria infection in a CD8+ T cell-dependent manner. These data demonstrate that other MHC class Ib-restricted CD8+ T cells, in addition to H2-M3-restricted T cells, contribute to anti-listerial immunity and may contribute to immune responses against other intracellular bacteria.
Keywords: Listeria infection, MHC class Ib, CD8 T cells, H2-M3, knockout mice
INTRODUCTION
Effector CD8+ T cells restricted to the classical MHC class I (MHC class Ia) antigen-presenting molecules have been shown to play critical roles in the clearance of bacterial and viral infection. MHC class Ib molecules are structurally-related to MHC class Ia and are likewise composed of three immunoglobulin-like domains that non-covalently associate with β2-microglobulin (β2m) (1). While the mammalian genome encodes many more MHC class Ib molecules than MHC class Ia molecules, comparatively little is known regarding their immunological function. However, the conservation of these molecules in mammals indicates that they play important roles that are non-redundant to those of MHC class Ia (1–4). Genes encoding MHC class Ib molecules can be found linked to the MHC (e.g. H2-M3, Qa-1/HLA-E, Qa-2) on chromosome 6 in humans and on chromosome 17 in mice, as well as elsewhere in the genome (e.g. CD1, MR1) (1). In general, MHC class Ib molecules are significantly less polymorphic, are more restricted in their tissue distribution, and have lower cell surface expression than MHC class Ia (1, 5), although in some cases these expression levels can be increased in the presence of antigen (6, 7). Importantly, over the last decade, emerging studies have found that MHC class Ib molecules can contribute to host immune responses through the presentation of microbial antigens to T cells (1, 8, 9).
Some MHC class Ib molecules, such as CD1 and H2-M3, have antigen-binding regions specialized to accommodate antigens that are unique in structure, perhaps positioning them to recognize hallmarks of microbial infection. The hydrophobic binding cleft of CD1 allows it to accommodate and present bacterial lipid antigens to T cells (10–17), while H2-M3 preferentially binds peptides that have N-terminal formylation, a signature of bacterial peptide synthesis, with up to a thousand-fold stronger affinity than non-formylated peptides (18, 19). H2-M3-restricted T cells have been shown to recognize peptides derived from many bacteria, including Listeria monocytogenes (LM), Mycobacterium tuberculosis (Mtb), Salmonella typhimurium and Chlamydia pneumoniae (20–25). We have previously demonstrated that H2-M3-restricted CD8+ T cells play a non-redundant role in host responses against LM and that mice lacking H2-M3 (M3−/−) have an increased susceptibility to LM infection (26). In addition to H2-M3, there is some evidence that Qa-1 can present listerial antigens (27–30). Qa-1 and its human homologue, HLA-E, have been shown to present peptides derived from Salmonella to CD8+ T cells (8, 31, 32). HLA-E-restricted T cells can also respond to antigens derived from Mtb (33) and have been isolated from Mtb-infected patients (34). Recent studies have demonstrated that mucosal-associated invariant T (MAIT) cells can be activated by MR1-expressing antigen-presenting cells that have been cultured with various bacteria, indicating that they recognize bacterial antigens presented by MR1 (35, 36). In addition to bacterial peptides, both HLA-E and Qa-2 have been shown to present peptides of viral origin to CD8+ T cells, suggesting that MHC class Ib molecules are also involved in anti-viral immune responses (9, 37).
Like MHC class Ia-restricted CD8+ T cells, most MHC class Ib-restricted T cells are cytotoxic and can secrete inflammatory cytokines such as IFN-γ upon stimulation with antigen (31, 38–40). However, other characteristics of MHC class Ib-restricted T cells distinguish them from conventional T cells. While the majority of T cells restricted by the MHC-linked MHC class Ib molecules studied thus far express the CD8 co-receptor (2, 9, 19, 20, 22, 24, 25, 33), the T cells restricted by MHC-unlinked MHC class Ib molecules are predominantly CD8− (38, 41). Many MHC class Ib-restricted T cells, including H2-M3-restricted CD8+ T cells, CD1d-restricted NKT cells and MAIT cells display an activated cell surface phenotype in the absence of infection (41–45). This pre-activated status may contribute to the unique kinetics of MHC class Ib-restricted T cell responses, as H2-M3-restricted T cells, NKT cells and MAIT cells all respond more rapidly to antigenic stimulation than do conventional T cells (40, 42, 43, 45–47). Interestingly, although their responses to primary stimuli are rapid, H2-M3-restricted T cell and NKT cell responses to secondary stimulation lack the accelerated responses and significant expansion that characterize secondary conventional T cell responses (42, 43, 46–50). It is not clear whether this limited responsiveness is a general feature of MHC class Ib-restricted T cells upon secondary stimulation.
LM infection has long been utilized to study conventional CD8+ T cell responses to intracellular bacteria (51–54), but has also proven a useful model for studying MHC class Ib-restricted responses. Studies performed using MHC class Ia-deficient (Kb−/−Db−/−) mice have demonstrated that MHC class Ib-restricted CD8+ T cells are protective against listerial infection, and that anti-listerial responses are not limited to H2-M3-restricted T cells (30, 42, 55). As listerial antigens that bind H2-M3 have been identified, it has been easiest to examine H2-M3-restricted CD8+ T cell responses to LM infection using LM antigen-loaded H2-M3 tetramers (46). However, due to the magnitude of H2-M3-restricted immune responses to LM infection and the limited availability of reagents that can detect other MHC class Ib-restricted CD8+ T cells, it has not been possible to investigate the relative contribution of non-H2-M3 MHC class Ib-restricted CD8+ T cell responses even in Kb−/−Db−/− mice.
To address this issue, we have generated mice that lack MHC class Ia as well as H2-M3 (Kb−/−Db−/−M3−/−). Using this novel animal model, we found that the non-H2-M3 MHC class Ib-restricted CD8+ T cell population exhibits an activated phenotype and responds to both primary and secondary LM infection with kinetics that resemble H2-M3-restricted T cell responses. In addition, we demonstrate that although non-H2-M3 MHC class Ib-restricted CD8+ T cells are few in number, they are cytotoxic, secrete proinflammatory cytokines, and can protect against LM infection. Given that MHC class Ib-restricted T cells display significantly less polymorphism than MHC class Ia, these new findings position MHC class Ib molecules and their bound bacterial antigens as attractive vaccine targets that could be widely recognized across the general population to protect against bacterial infection.
MATERIALS AND METHODS
Mice
C57BL/6 and β2m−/− mice were purchased from the Jackson Laboratory. M3−/− and Kb−/−Db−/− mice (backcrossed with B6 mice for at least ten generations) were generated or maintained in house as previously described (26). Kb−/−Db−/−M3−/− were generated by crossing Kb−/−Db−/− mice with M3−/− mice. F1 offspring were intercrossed and all resulting F2 progeny which lacked surface expression of H2-Kb and -Db on peripheral blood lymphocytes were screened for an intra-H2 recombinant as previously described using PCR analysis and the following primer set: M3 forward (5′-CAGCGATGGAACCCACCCACAATGA-3′), M3 reverse (5′-AGACTAGCAACGATGACCATGATGAC-3′), and Neo (5′-GATTCGCAGCGCATCGCCTTCTA-3′) (26). Of 165 F2 offspring tested, one male mouse was found to carry the desired intra-H2 recombination (Kb−/−Db−/−M3+/−). We bred this male with Kb−/−Db−/− females to produce Kb−/−Db−/−M3+/− offspring, which were then intercrossed to generate Kb−/−Db−/−M3−/− mice. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of Northwestern University.
Primary cell preparation and dendritic cell generation
Single cell suspensions were prepared from whole tissues by mechanical disruption in HBSS/2% FBS, or as described (56). T cells were purified from splenic lymphocytes using a Pan T Cell Isolation Kit (Miltenyi Biotec). To enrich for CD8+ T cells, splenocytes were labeled with biotin-conjugated anti-B220, anti-CD4, anti-CD11b, anti-CD11c, anti-CD49b and anti-NK1.1 antibodies (eBioscience) followed by anti-biotin-conjugated magnetic beads. CD8+ T cells were then isolated to a purity of ~95% by negative selection according to the manufacture’s instruction (Miltenyi Biotec). BMDC were derived from mouse bone marrow progenitors using GM-CSF and IL-4 (PeproTech) as previously described (57).
Antibodies and flow cytometry
FITC-conjugated mAbs specific for CD8β, CD44, CD62L, hamster IgG, H2-Kb, Vβ2, Vβ 5.1/5.2, Vβ 6, Vβ8.1/8.2, Vβ8.3, Vβ12 and Vβ13, FITC-conjugated streptavidin, PE-conjugated Abs specific for B220, CD8α, CD8β, Ly6C, Vβ3, and Vβ11, PerCP-conjugated mAb specific for CD4 and TCRβ and biotinylated mAb specific for H2-Db were purchased from BD Pharmingen. Cells were incubated with 2.4G2 Fcγ RII/RIII blocking mAb (hybridoma supernatant) for 15 min, then stained in HBSS containing 2% FBS for 30 min at 4 °C. For detection of H2-M3 surface expression, splenocytes from B6 and Kb−/−Db−/−M3−/− mice were cultured overnight at 37 °C in RPMI-10 containing 10 μM LemA peptide (f-MIGWII). Cells were stained first with the anti-H2-M3 antibody 130 (7) followed by staining with anti-hamster IgG. Flow cytometric analysis was performed using a FACSCantoII (BD Biosciences) and FlowJo software (Tree Star).
Cytokine assay
For polyclonal TCR stimulation, enriched CD8+ T cells (5 × 105 cells/well) from naïve WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice were stimulated with anti-CD3 (3 μg/ml) and anti-CD28 (3 μg/ml) mAb and cultured in RPMI-10. After 12 h in culture, intracellular staining for IFN-γ was performed as described below. For LM-specific cytokine responses, BMDC were pulsed with heat-killed Listeria monocytogenes (HKLM) or infected with LM as described below. Responders (5 × 106 CD8+ T cells/well) were stimulated with various BMDC (1 × 105) in 200 μl RPMI-10 for 12 h (intracellular staining) or 48 h (ELISA and Cytometric Bead Array). IFN-γ production was measured by intracellular staining and flow cytometry. IL-17A levels were quantitated by sandwich ELISA using anti-IL-17A mAb pairs (eBioscience) whereas other cytokines were measured using an Inflammatory and Th1/Th2 Cytometric Bead Array Kit (BD Bioscience) according to the manufacturer’s instructions. To generate HKLM, 1 × 1010 CFU/ml LM culture in PBS was incubated at 70 °C for 3 h, then stored at −20 °C.
Intracellular cytokine staining
Splenocytes or hepatic leukocytes from naïve or LM-infected mice were cultured in 96-well plates and either stimulated or left unstimulated for 7 h at 37 °C. For the last 2 h of stimulation, 10 μM monensin (Sigma) was added to block cytokine secretion. Cells were washed and stained for the cell surface markers CD8β, CD4 and TCRβ. After fixation with 4% paraformaldehyde and permeabilization with 0.15% saponin (Sigma), cells were stained with an APC-conjugated anti-IFN-γ antibody (eBioscience) for 30 min in PBS containing 1% bovine serum albumin and 0.1% saponin. Flow cytometry was performed as described above.
Listeria infection and CFU assay
The LM EGD strain was provided by R. Kurlander (National Institutes of Health). Bacteria were grown in brain-heart infusion broth (Difco Laboratories) and virulent stocks were maintained by repeated passage through B6 mice. For primary infection, mice were i.v. infected with 2 × 103 CFU (1/10 LD50) of LM. For rechallenge with LM, mice were rested for 1 month and then infected with 5 × 104 CFU of LM. Bacterial CFU in the spleen and liver were determined at indicated time points after infection. Briefly, organs were homogenized in sterile water with 0.2% NP-40 and serial dilutions were plated onto BHI agar plates. CFU were counted after incubation at 37°C for 24 h.
Adoptive transfer study
Donor WT and Kb−/−Db−/−M3−/− mice were immunized with 2 × 103 CFU of LM. Seven days later, splenocytes from LM immune mice were isolated and were divided into two groups. One donor group was incubated with anti-CD8α mAb (3.155) at 4 °C for 30 min. Cells were then washed and incubated with 10% rabbit complement (Cedarane Labs) at 37 °C for 30 min to deplete CD8+ T cells. All donor cells from naïve spleen, CD8+ T cell-depleted immune spleen and non-depleted immune spleen were washed twice with PBS. Naïve WT mice received an i.v. injection of 2 × 107 donor splenocytes and were then challenged with 5 × 104 CFU of LM 30–60 min after cell transfer. Three days after infection, spleen and liver were removed from the recipients and the bacterial CFU per organ was determined as described above.
ELISPOT assay
Multiscreen-IP plates (Millipore) were coated with anti-IFN-γ mAb (eBioscience) at 5 μg/ml in PBS. BMDC were infected with LM 4 h before each assay as described above. For blocking experiments, LM-infected BMDC were preincubated with mouse IgG or mAb against CD1d (3H3, in house)(58), H2-M3 (130, in house) (7), MR1 (26.5) (4), Qa-1b (6A8.6F10.1A6, ATCC) (31, 59), and Qa-2 (M46) (60) for 30 min at 37 °C prior to assay setup (42). Enriched CD8+ T cells (104–105) were mixed with BMDC stimulator cells (5 × 104/well) in RPMI-10 medium and plated in triplicate wells. After 18 h incubation at 37 °C, plates were washed free of cells using PBS-Tween (PBS and 0.05% Tween-20) and incubated overnight at 4 °C with biotinylated anti-IFN-γ mAb (eBioscience) at 1μg/ml. Plates were washed and incubated with streptavidin-conjugated alkaline phosphatase (Jackson ImmunoResearch). After 1 h incubation at room temperature, plates were developed with a BCIP/NBT substrate kit (Bio-Rad) according to the manufacturer’s instruction. Spots were counted using an ImmunoSpot reader (Cellular Technology Ltd).
CTL assay
To examine LM-specific CTL ex vivo, splenocytes from LM-infected mice (at day 7 post-infection) were enriched for CD8+ T cells and cultured in RPMI-10 with 1 μg/ml ConA. After 3 d in culture, cells were used as effectors in a 51Cr release CTL assay. Monolayers of the macrophage cell line J774 were grown in antibiotic-free medium and infected with LM for 1 h at a multiplicity of infection 5:1. Cells were then washed with warm PBS, and cultured in DMEM containing 40 μg/ml gentamicin for an additional 3 h. Both uninfected and LM-infected J774 target cells were labeled with 51Cr for 1 h at 37°C. To examine cytotoxicity in the T2 CTL line, BMDC were labeled with 51Cr and used as target cells. BMDC targets were derived from β2m−/− or Kb−/−Db−/−M3−/− mice and either left untreated or treated overnight with HKLM. Some target cells were additionally pretreated with blocking mAb against MR1, Qa-1b or Qa-2 before labeling as described above. Target cells (104) were added to a round-bottom 96-well plate containing varying concentrations of effector cells. Four hours after incubation, 100 μl of supernatant was collected from each well and the amount of 51Cr release was determined using a TopCount scintillation counter. The percentage of specific lysis was calculated as 100 × (experimental cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm).
Generation of Kb−/−Db−/−M3−/− CTL lines
To generate the T2 CTL line, Kb−/−Db−/−M3−/− mice were immunized with 1 × 106 HKLM-pulsed BMDC. 7 days post-immunization, splenocytes were harvested and placed in culture for one week in RPMI-10. Cells were subsequently cultured in supplemented Mischell Dutton Medium with 20 U/ml IL-2 (partially purified from EL4.IL2 cell supernatant) and 20 ng/ml IL-7 (PeproTech). Cells were restimulated weekly with HKLM-pulsed irradiated Kb−/−Db−/−M3−/− BMDC.
Statistical Analysis
Mean values were compared using unpaired Student’s t tests. All statistical analyses were performed with the PRISM program (GraphPad).
RESULTS
Generation and characterization of Kb−/−Db−/−M3−/− mice
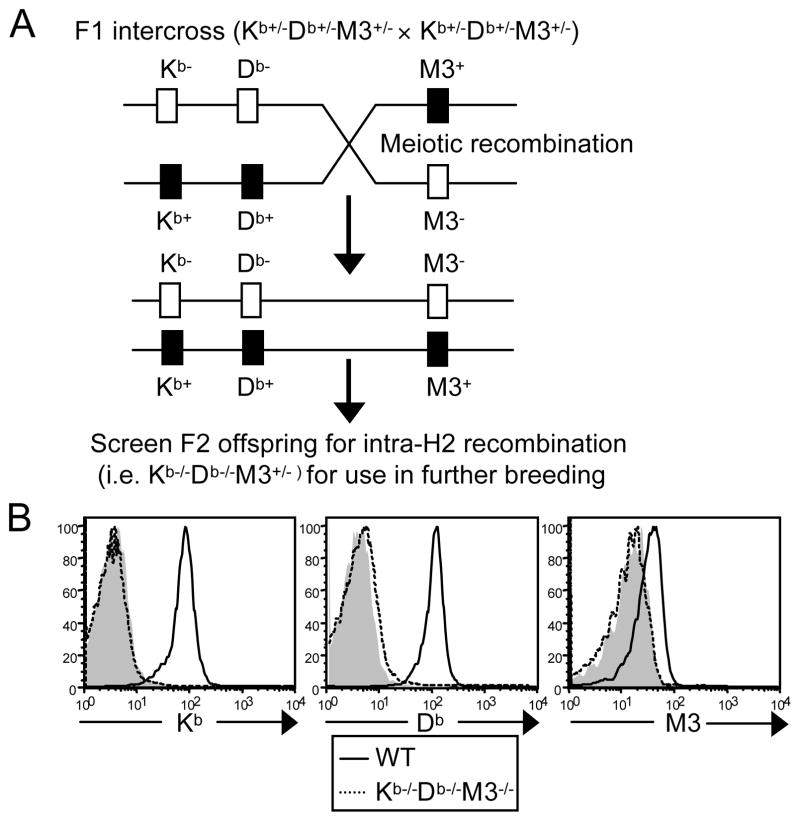
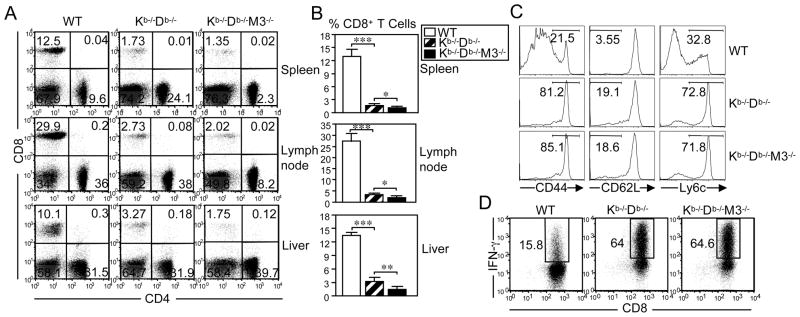
To investigate the relative contribution of H2-M3 and other MHC class Ib molecules to CD8+ T cell development and associated responses to bacterial infection, we generated Kb−/−Db−/−M3−/− mice by crossing Kb−/−Db−/− and M3−/− mice (26). F1 offspring were intercrossed and the resulting F2 offspring were then screened for intra-H2-recombination using FACS and PCR analysis (Figure 1A). The genetic distance between H2-D and H2-M3 is approximately 0.7 cM (61), necessitating extensive screening. Of 165 F2 offspring tested, one mouse was found to carry each targeted locus on the same chromosome (Kb−/−Db−/−M3+/−) and was selected for further breeding to establish Kb−/−Db−/−M3−/− mice. Flow cytometry confirmed that Kb−/−Db−/−M3−/− mice do not express H2-Kb, H2-Db or H2-M3 molecules on the cell surface (Figure 1B). To determine the respective role of H2-M3 and other MHC class Ib molecules in the development of CD8+ T cells, we compared the CD8+ T cell populations in the spleen, liver and lymph nodes of WT, Kb−/−Db−/−, and Kb−/−Db−/−M3−/− mice. Total lymphocyte numbers were comparable between these three genotypes (data not shown). However, compared with WT mice, the percentage of CD8+ T cells was profoundly reduced in the spleen and lymph nodes of Kb−/−Db−/− mice and was further reduced, albeit modestly, in Kb−/−Db−/−M3−/− mice (Figure 2A, B). Interestingly, the reduction in CD8+ T cell percentage was less profound in the liver of Kb−/−Db−/− mice compared to that of WT mice, while Kb−/−Db−/−M3−/− mice exhibited a 2–3 fold reduction in the percentage of hepatic CD8+ T cells when compared with Kb−/−Db−/− mice (Figure 2A, B). Judging from the enumeration of CD8+ T cell populations in Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice, H2-M3-restricted CD8+ T cells constitute approximately 20%-30% of the MHC class Ib-restricted CD8+ T cells found in the peripheral lymphoid tissues of naïve animals but contribute 50%-75% of the MHC class Ib-restricted CD8+ T cell population in the liver. These data indicate that CD8+ T cells restricted by different MHC class Ib molecules may have distinct tissue distributions, with H2-M3-restricted CD8+ T cells being particularly enriched in the liver.
Figure 1. Generation of Kb−/−Db−/−M3−/− mice.
(A) Schematic detailing the meiotic intra-H2 recombination required to generate Kb−/−Db−/−M3+/− mice. (B) Flow cytometric analysis of H2-Kb, H2-Db and H2-M3 cell surface expression on B220+ splenocytes isolated from WT (thick line) and Kb−/−Db−/−M3−/− (dotted line) mice. Isotype controls are shown for comparison as shaded histograms. To detect H2-M3 expression, splenocytes from indicated mice were incubated overnight with 10 μM LemA peptide.
Figure 2. Characterization of CD8+ T cells in naïve Kb−/−Db−/−M3−/− mice.
(A–C) Flow cytometric analysis of CD4+ and CD8β+ T cell populations in WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice. Data shown are representative of three independent experiments. (A) Lymphocytes were isolated from the spleen, liver and lymph nodes. Numbers indicate the percentage of cells in each quadrant in the lymphocyte gate. (B) Bar graphs indicate the percentage of CD8+ T cells. Data are presented as the mean ± SEM using six mice per genotype. *, p < 0.05; **, p < 0.01; ***, p < 0.001. (C) Cell surface expression of activation markers on TCRβ+CD8+ splenocytes in naïve WT, Kb−/−Db−/−, and Kb−/−Db−/−M3−/− mice. (D) Ex vivo anti-CD3 and anti-CD28 antibody stimulation of CD8+ T cells enriched from the spleen of WT, Kb−/−Db−/−, and Kb−/−Db−/−M3−/− mice. Intracellular staining for IFN-γ was performed at 12 h post-stimulation. Data shown are representative of three experiments.
Phenotypic and functional analysis of residual CD8+ T cells in naïve Kb−/−Db−/−M3−/− mice
Two unconventional subsets of MHC class Ib-restricted T cells, namely CD1d-restricted invariant NKT cells and MR1-restricted MAIT cells, exhibit restricted TCR usage (45, 62). To examine the diversity of the TCR Vβ region expressed by H2-M3- and non-H2-M3-restricted MHC class Ib-restricted CD8+ T cells, splenocytes from WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice were stained with antibodies against various TCR Vβ chains. Although the CD8+ T cell population found in Kb−/−Db−/− M3−/− mice exhibited a decreased representation of Vβ2, Vβ5, Vβ6, Vβ8.3, Vβ11 and Vβ13 as compared to the CD8+ T cell population found in WT mice, residual Kb−/−Db−/− M3−/− CD8+ T cells still exhibit a large diversity in TCRβ usage (Figure S1). This observation could indicate that the CD8+ T cell population found in Kb−/−Db−/− M3−/− mice does not exhibit restricted TCR Vβ chain usage, or could merely be reflective of a polyclonal population of CD8+ T cells recognizing diverse restriction elements. However, the residual CD8+ T cells found in Kb−/−Db−/− mice exhibit an increase in the proportion of Vβ8.1/8.2 and Vβ13 usage which is not observed in Kb−/−Db−/−M3−/− mice, suggesting that H2-M3-restricted CD8+ T cells may display a preferential usage for these Vβ chains.
It has been shown that a large proportion of CD8+ T cells in naïve Kb−/−Db−/− mice display an activated/memory-like phenotype (42–44). However, it is not clear whether H2-M3- and/or other MHC class Ib-restricted CD8+ T cells contribute to this phenotype. To address this question, we compared the expression of various activation markers on CD8+ T cells isolated from WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice. Similar to Kb−/−Db−/− mice, the majority of CD8+ T cells in Kb−/−Db−/−M3−/− mice exhibit an activated T cell phenotype that is CD44high and Ly6Chigh (Figure 2C). In addition, the percentages of CD62Llow cells are also increased in both Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice compared to WT mice. These data indicate that, in the absence of MHC class Ia-restricted CD8+ T cells, most H2-M3-restricted as well as other MHC class Ib-restricted CD8+ T cells are activated in naïve mice.
To examine the functional properties of non-H2-M3 MHC class Ib-restricted CD8+ T cells, CD8+ T cells were enriched from WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice and stimulated with anti-CD3 and anti-CD28 antibodies. Following in vitro stimulation, intracellular staining revealed that the majority of MHC class Ib-restricted Kb−/−Db−/− and Kb−/−Db−/−M3−/− CD8+ T cells robustly secreted IFN-γ, while a significantly smaller proportion of WT CD8+ T cells was able to do so (Figure 2D). These data indicate that, consistent with their activated phenotype, MHC class Ib-restricted CD8+ T cells more readily produce proinflammatory cytokines as compared to MHC class Ia-restricted CD8+ T cells.
Non-H2-M3 MHC class Ib-restricted CD8+ T cells expand upon primary infection with Listeria
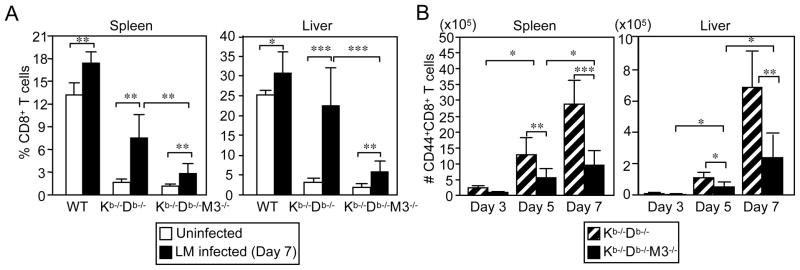
To assess whether residual CD8+ T cells from Kb−/−Db−/−M3−/− mice expand in response to bacterial infection, WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice were infected with a sublethal dose of LM. Seven days following infection, splenocytes and hepatic leukocytes were harvested and the CD4+ and CD8+ T cell populations were analyzed by flow cytometry. Compared to naïve mice, a significant increase in overall cellularity (~2-fold in spleen and ~3-5-fold in liver) was detected in all three LM-infected mouse strains (data not shown). In addition, the percentages of CD8+ T cells increased by 2- to 4-fold and 5- to 7-fold in LM-infected Kb−/−Db−/−M3−/− and Kb−/−Db−/− mice, respectively (Figure 3A). These data indicate that, similar to H2-M3-restricted CD8+ T cells, CD8+ T cells restricted to other MHC class Ib molecules are able to undergo extensive proliferation following primary infection with LM.
Figure 3. Expansion of CD8+ T cells in Kb−/−Db−/−M3−/− mice during LM infection.
(A) WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice were infected with 2 × 103 CFU of LM. 7 days following infection, splenocytes and hepatic leukocytes were harvested and stained with antibodies against CD8β and TCRβ. Bar graphs depict the mean ± SEM for the percentage of CD8+ T cells in the lymphocyte gate for uninfected and LM-infected WT, Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001. (B) Splenocytes and hepatic leukocytes were harvested from LM-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice at the indicated time points and stained with antibodies against CD8α, TCRβ and CD44. Bar graphs depict the mean ± SEM for the absolute number of CD44highCD8+ T cells for each indicated genotype. Results from 3–9 mice per genotype are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To compare the kinetics of H2-M3-restricted and non-H2-M3 MHC class Ib-restricted CD8+ effector T cells during primary LM infection, we infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice with LM and examined the CD44highCD8+ T cell populations in these mice at various time points following infection. Both LM-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice exhibited a steady increase in the total number of CD44highCD8+ T cells during the first week of infection even though the number of CD44highCD8+ T cells was significantly greater in Kb−/−Db−/− mice than in Kb−/−Db−/−M3−/− mice (Figure 3B). The number of CD44highCD8+ T cells in LM-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice was significantly increased in the spleen (~5-fold) and liver (~17-fold) at day 5 post-infection as compared with day 3 post-infection. At day 7 post-infection, the number of CD44highCD8+ T cells was further increased in both the spleen (~1.5-fold) and liver (~4.5-fold) of Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice. These data suggest that the kinetics of non-H2-M3 MHC class Ib-restricted CD8+ T cells are similar to those of H2-M3-restricted CD8+ T cells in response to primary LM infection. Interestingly, as observed in LM-infected Kb−/−Db−/− mice, hepatic CD8+ effector T cells in LM-infected Kb−/−Db−/−M3−/− mice underwent a more vigorous expansion than did splenic CD8+ effector T cells. These data indicate that, similar to H2-M3-restricted T cells, non-H2-M3 class Ib-restricted effector CD8+ T cells may preferentially expand in the liver or may be preferentially recruited to the liver during LM infection.
CD8+ T cells in Kb−/−Db−/−M3−/− mice protect against Listeria infection
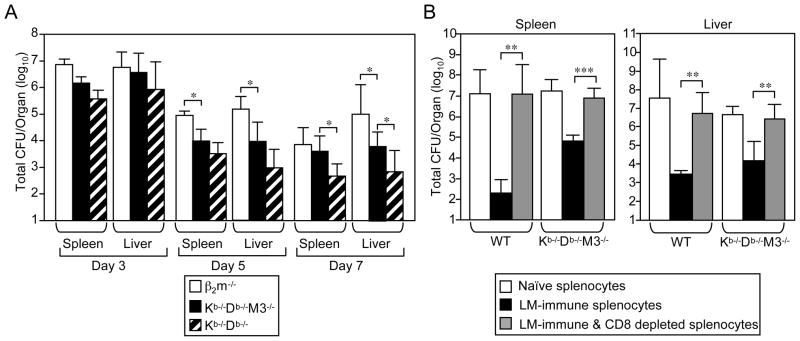
To examine the protective capacity of non-H2-M3 class Ib-restricted CD8+ T cells during LM infection, we compared the bacterial burden in both the spleen and liver of infected Kb−/−Db−/−and Kb−/−Db−/−M3−/− mice with that of infected β2m−/− mice that lack most MHC class Ib-restricted CD8+ T cells. At 5 and 7 days post-infection, the bacterial burden was significantly lower in both the spleen and liver of Kb−/−Db−/−M3−/− mice as compared with β2m−/− mice (Figure 4A), suggesting that non-H2-M3 MHC class Ib-restricted CD8+ T cells contribute to bacterial clearance. At day 7 post-infection, bacterial burdens were further reduced in Kb−/−Db−/− mice as compared to those in Kb−/−Db−/−M3−/− mice (Figure 4A), confirming a protective role for H2-M3-restricted T cells against LM infection (26).
Figure 4. Protective role of residual CD8+ T cells in Kb−/−Db−/−M3−/− mice.
(A) Kb−/−Db−/−, Kb−/−Db−/−M3−/− and β2m−/− mice were infected with 2 × 103 CFU of LM. Bacterial burden in the spleen and liver was determined at indicated time points post-infection. Data are presented as the mean ± SEM from 3–6 mice per genotype for each time point. *, p < 0.05. (B) WT recipient mice were adoptively transferred with 2 × 107 splenocytes isolated from naïve WT or Kb−/−Db−/−M3−/− mice or from WT or Kb−/−Db−/−M3−/− mice that had been infected with 2 × 103 LM 7 days prior. In some cases, splenocytes from infected donor mice were depleted of CD8+ T cells. 30–60 min following cell transfer, recipient mice were infected with 5 × 104 LM. Three days post-infection, spleen and liver were harvested and the bacterial burden was determined. Data shown are the mean ± SEM from six mice for each transfer group. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To demonstrate that the protective effect observed in LM-infected Kb−/−Db−/−M3−/− mice is mediated by CD8+ T cells, we adoptively transferred either splenocytes or CD8+ T cell-depleted splenocytes isolated from naïve or LM-infected WT or Kb−/−Db−/−M3−/− mice into naïve WT recipient mice. The recipient mice were then challenged with a lethal dose of LM and protective immunity was evaluated on day 3 post-infection by determining the bacterial burden in the spleen and liver. Transfer of splenocytes from LM-vaccinated WT or Kb−/−Db−/−M3−/− mice provided significant protection to recipient mice against subsequent LM challenge, compared to transfer of naïve splenocytes (Figure 4B). However, this protective effect was abolished when CD8+ T cells were depleted from WT or Kb−/−Db−/−M3−/− splenocytes mice prior to transfer. Collectively, these data indicate that residual CD8+ T cells in Kb−/−Db−/−M3−/− mice can contribute to protective immunity against LM infection.
Non-H2-M3 MHC class Ib-restricted CD8+ T cells are cytotoxic and secrete proinflammatory cytokines in response to Listeria infection
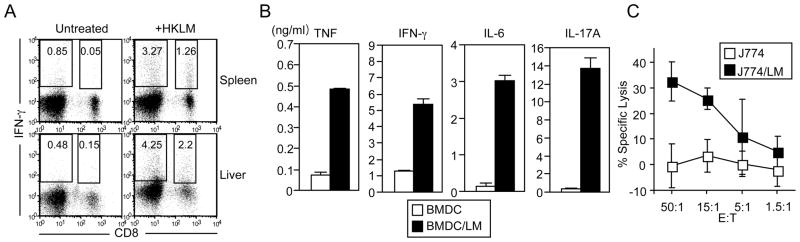
To determine the effector function of non-H2-M3 MHC class Ib-restricted CD8+ T cells during LM infection, we harvested splenocytes and hepatic leukocytes from LM-infected Kb−/−Db−/−M3−/− mice and stimulated them ex vivo with heat-killed LM (HKLM). Consistent with the kinetics of total CD8+ effector T cells upon LM infection, the number of LM-specific non-H2-M3 MHC class Ib-restricted CD8+ T cells was significantly increased at day 5 following infection and was further increased by day 7 (Figure S2). A significant proportion of Kb−/−Db−/−M3−/− CD8+ T cells from LM-infected mice were able to produce IFN-γ when stimulated with HKLM, whereas unstimulated CD8+ T cells did not (Figures 5A and S3).
Figure 5. Effector function of LM-specific CD8+ T cells in Kb−/−Db−/−M3−/− mice.
(A–C) Splenocytes and hepatic leukocytes were harvested from Kb−/−Db−/−M3−/− mice 7 days post LM infection. (A) Cells were stimulated with HKLM for 7 h and stained with antibodies against CD8β and TCRβ. Cells were then intracellularly stained for IFN-γ and analyzed by flow cytometry. The percentages of CD8+IFN-γ+ and CD8−IFN-γ+ cells within the TCRβ+ gate are indicated. Results are representative of three experiments. (B) Splenocytes were harvested from Kb−/−Db−/−M3−/− mice 7 days post LM infection and enriched for CD8+ T cells. These T cells were then cultured with LM-infected BMDC for 48 h. Culture supernatants were then harvested to determine the presence of cytokines using a Cytometric Bead Array kit (for TNF, IFN-γ, and IL-6) or by ELISA (for IL-17A). Bar graphs depict means of duplicate wells ± SEM from two representative experiments pooling two mice per genotype. (C) Splenocytes were isolated from Kb−/−Db−/−M3−/− mice 7 days post LM infection, enriched for CD8+ T cells, and activated with ConA. After 3 days of ConA stimulation, splenocytes were used as effectors in a 51Cr release CTL assay at the indicated effector:target cell ratios. Uninfected or LM-infected J774 cells were labeled with 51Cr and used as targets. Graph depicts the mean ± SEM for the percentage of LM-specific killing pooling two mice per genotype. Data are representative of three independent experiments.
To further characterize the cytokines produced by the CD8+ T cells found in LM-infected Kb−/−Db−/−M3−/− mice, CD8+ T cells were enriched from the spleen of Kb−/−Db−/−M3−/− mice at 7 days post-LM infection and stimulated ex vivo with LM-infected bone marrow-derived dendritic cells (BMDC). We found that CD8+ T cells isolated from LM-infected Kb−/−Db−/−M3−/− mice were able to produce significant amounts of TNF-α, IFN-γ, IL-6 and IL-17A in response to stimulation with LM-infected BMDC (Figure 5B).
To investigate the cytolytic capacity of non-H2-M3 MHC class Ib-restricted CD8+ T cells during LM infection, we performed an in vitro CTL assay using ConA-activated CD8+ effector T cells enriched from LM-infected Kb−/−Db−/−M3−/− mice. Effector T cells enriched from LM-infected Kb−/−Db−/−M3−/− mice preferentially lysed LM-infected J774 target cells but not uninfected targets, indicating that the residual CD8+ T cell population found in Kb−/−Db−/−M3−/− mice contains CTL specific to LM (Figure 5C). The combined abilities of non-H2-M3 MHC class Ib-restricted LM-specific CD8+ cells to lyse LM-infected cells and to produce proinflammatory cytokines in response to listerial antigens likely contribute to their ability to protect against LM infection.
Anti-listerial CD8+ T cells from Kb−/−Db−/−M3−/− mice do not recognize previously characterized MHC class Ib molecules
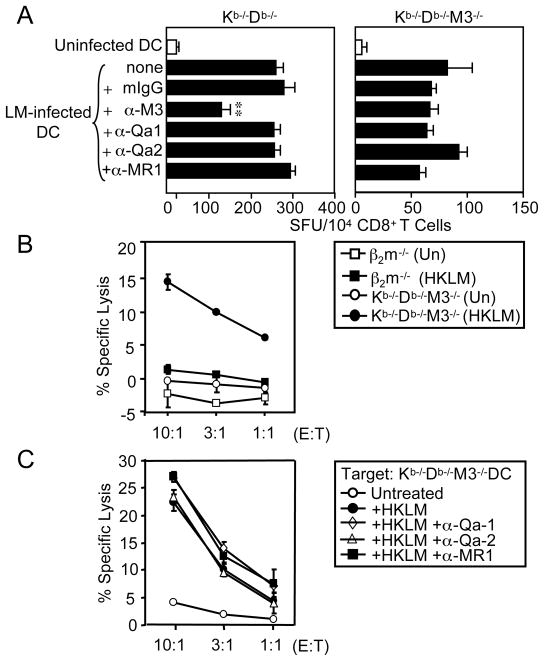
While more than 40 MHC class Ib molecules are encoded in the murine genome, only H2-M3 and Qa-1 have been shown to function as restriction elements for LM-specific CTL (20–22, 27–30). However, H2-M3- and Qa-1-restricted T cell responses can account for only a fraction of the MHC class Ib-restricted responses observed during LM infection. To investigate which MHC class Ib molecules might serve as restriction elements for the CD8+ T cells found in LM-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice, we enriched splenic CD8+ T cells from these mice and cultured them with LM-infected BMDC that had been pretreated with blocking antibodies against CD1d, H2-M3, Qa-1b, Qa-2 or MR1 in an ELISPOT assay. LM-specific IFN-γ secretion by CD8+ T cells isolated from Kb−/−Db−/− mice was significantly reduced in the presence of anti-H2-M3 antibody, confirming that a substantial fraction of LM-specific CD8+ T cells in Kb−/−Db−/− mice are restricted to H2-M3 (Figures 6A and S4). However, the presence of blocking antibodies against H2-M3, Qa-1b, Qa-2 and MR1 had no effect on LM-specific responses produced by CD8+ T cells isolated from Kb−/−Db−/−M3−/− mice. These data suggest that neither Qa-1b, Qa-2 nor MR1 serve as major restriction elements for the MHC class Ib-restricted anti-listerial CTL found in Kb−/−Db−/−M3−/− mice.
Figure 6. LM-specific Kb−/−Db−/−M3−/− CD8+ T cells are not restricted to Qa-1b, Qa2 or MR1.
(A) Splenocytes were harvested from LM-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice at 7 days post-infection and enriched for CD8+ T cells. These T cells were then used as effectors in an IFN-γ ELISPOT assay. Uninfected or LM-infected BMDC were used as stimulators and were incubated with CD8+ T cells in medium alone, in the presence of isotype control Ab, or with mAb against either H2-M3, Qa-1b, Qa-2 or MR1. Results are presented as the mean ± SEM of the number of IFN-γ spot-forming units from two pooled animals per genotype and are representative of three independent experiments. **, p < 0.01. (B, C) T2 CTL, a CD8+ T cell line derived from LM-infected Kb−/−Db−/−M3−/− mice, were used as effector cells in a 51Cr release CTL assay. Results are representative of three independent experiments. (B) BMDC derived from Kb−/−Db−/−M3−/− or β2m−/− mice were labeled with 51Cr and used as targets at the indicated effector: target cell ratios. Some target cells were incubated overnight with HKLM prior to assay setup. (C) BMDC derived from Kb−/−Db−/−M3−/− mice were labeled with 51Cr and used as targets at the indicated effector: target cell ratios. Some target cells were incubated overnight with HKLM prior to assay setup. In addition, some target cells were incubated with blocking mAb against Qa-1b, Qa-2, or MR1 30 min prior to labeling.
To attempt to identify novel MHC class Ib molecules that are capable of presenting bacterial antigens to CD8+ T cells, we established T cell lines from HKLM-immunized Kb−/−Db−/−M3−/− mice. One of these CD8+ T cell lines, T2 CTL, preferentially lysed HKLM-pulsed BMDC derived from Kb−/−Db−/−M3−/− mice, but not BMDC derived from β2m−/− mice, suggesting that T2 CTL recognizes a β2m-associated MHC class Ib molecule (Figure 6B). In addition, blocking antibodies against Qa-1b, Qa-2, and MR1 did not inhibit the reactivity of T2 CTL, suggesting that this T cell line recognizes a novel MHC class Ib molecule (Figure 6C).
Non-H2-M3 MHC class Ib-restricted memory T cells do not exhibit enhanced recall responses to LM
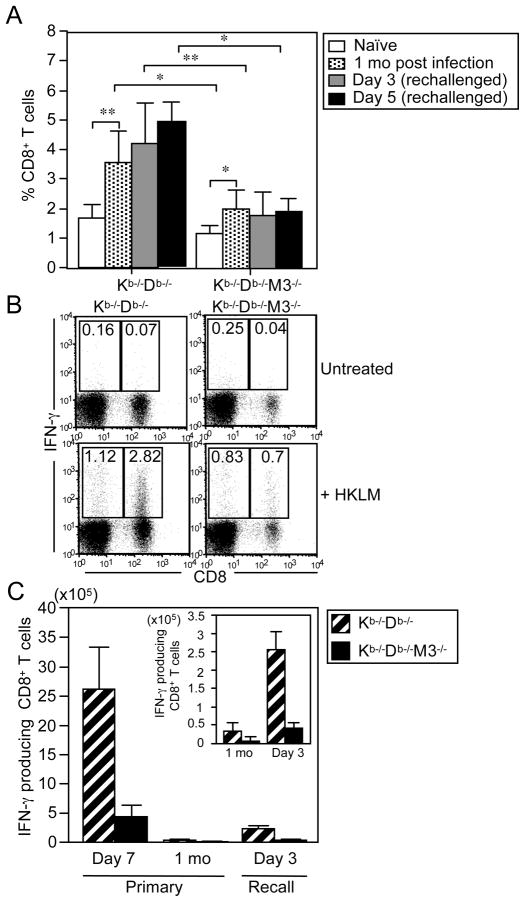
Previous studies have shown that H2-M3-restricted CD8+ T cells do not undergo significant expansion following secondary infection with LM (42, 43, 46, 47). To determine whether non-H2-M3 MHC class Ib-restricted CD8+ T cells can persist as long-lasting memory T cells and expand in response to secondary LM infection, we compared the percentage of CD8+ T cells in the spleens of naïve Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice with those in mice that had been infected one month previously with LM and had been either allowed to recover or had received a secondary lethal dose of LM. The proportion of CD8+ T cells in Kb−/−Db−/− mice was increased 2-fold at one month post-primary LM infection, as compared to that in naïve mice, but did not increase further upon secondary challenge (Figure 7A). Compared to naïve Kb−/−Db−/−M3−/− mice, Kb−/−Db−/−M3−/− mice that had been infected one month previously also exhibited a 2-fold increase in the proportion of CD8+ T cells (Figure 7A). However, neither the percentage nor the total number of Kb−/−Db−/−M3−/− CD8+ T cells changed significantly upon rechallenge with LM (Figure 7A, data not shown), suggesting that similar to H2-M3-restricted CD8+ T cells, non-H2-M3 MHC class Ib-restricted CD8+ T cells do not proliferate extensively during recall responses to LM infection. Compared to the analogous CD8+ T cell population in rechallenged Kb−/−Db−/− mice, the percentage of CD8+ T cells in rechallenged Kb−/−Db−/−M3−/− mice is substantially lower, suggesting that H2-M3-restricted CD8+ T cells remain the dominant MHC class Ib-restricted T cell population during secondary LM infection (Figure 7A). However, no differences in bacterial burden were observed between Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice upon rechallenge with LM (Figure S5).
Figure 7. Non-M3 MHC class Ib-restricted CD8+ T cell responses to secondary LM infection.
(A) Splenocytes were harvested from naïve Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice, from mice that had been infected with 2×103 CFU of LM 1 mo previously, and from mice that had been infected 1 mo previously and subsequently rechallenged with 5×104 CFU of LM. Cells were stained with antibodies against CD8β and TCRβ at 3 and 5 days post-rechallenge and flow cytometry was performed to determine the proportion of CD8+ T cells in the TCRβ+ gate. Results shown are presented as the mean ± SEM from 3–5 mice per experimental group and are representative of two experiments. *, p < 0.05; **, p < 0.01. (B) Splenocytes isolated from Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice at 3 days after secondary LM infection were stimulated ex vivo with HKLM. The proportion of IFN-γ-producing CD8+ T cells in the TCRβ+ gate was determined by intracellular staining. Results are representative of three individual experiments. (C) Bar graphs depict the mean ± SEM for the number of LM-specific IFN-γ-producing CD8+ T cells in spleens of Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice 7 days after primary LM infection, 1 mo after primary infection or 3 days after secondary infection. Results shown are from 3–5 mice per experimental group are representative of two individual experiments.
In spite of their lack of expansion, CD8+ T cells isolated from both Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice at 3 days post-secondary LM infection are capable of secreting IFN-γ upon ex vivo stimulation with listerial antigens (Figure 7B), suggesting that both H2-M3- and non-H2-M3 MHC class Ib-restricted CD8+ T cells can survive for extended periods of time post-infection and maintain effector function. Although secondary LM infection led to an increase in total numbers of LM-specific IFN-γproducing CD8+ T cells in both Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice, the magnitude of LM-specific CD8+ T cell responses to secondary infection was significantly lower when compared to CD8+ T cell responses in Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice after primary infection (Figure 7C). These data indicate that the lack of ability to undergo memory cell expansion may be a common feature of MHC class Ib-restricted CD8+ T cell responses to LM infection.
DISCUSSION
Emerging evidence suggests that MHC class Ib molecules can contribute to host immune responses through the presentation of unusual bacterial antigens to T cells. H2-M3 exemplifies this strategy by presenting N-formylated bacterial peptides to CD8+ cytotoxic T cells (20–22). Recent studies by our lab have shown that H2-M3-restricted CD8+ T cells play a significant and non-redundant role during LM infection that bridges innate and adaptive immunity (26, 42). However, the relative contribution of other MHC class Ib-restricted CD8+ T cells to bacterial infection has yet to be defined. In this report, we have demonstrated an important role for non-H2-M3 MHC class Ib-restricted CD8+ T cells in the protection of mice from LM infection. We have shown that the CD8+ T cells found in Kb−/−Db−/−M3−/− mice can expand rapidly, secrete inflammatory cytokines, and exert antigen-specific cytolytic activities in response to LM infection. By comparing the CD8+ T cell responses observed in LM-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice, we found that H2-M3 and non-H2-M3 MHC class Ib-restricted CD8+ T cells respond with similar kinetics during LM infection, characterized by rapid and extensive proliferation during primary infection and limited expansion during secondary infection.
H2-M3-restricted T cells make up a large proportion of MHC class Ib-restricted CD8+ T cells during both primary and secondary LM infection, which is likely reflective of a significant H2-M3-restricted T cell pool in naïve mice. Indeed, a comparison of the residual CD8+ T cell population in naïve Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice revealed that H2-M3-restricted CD8+ T cells constitute approximately 20–30% and 50–75% of the MHC class Ib-restricted CD8+ T cell population in the spleen and liver, respectively. The enrichment of H2-M3-restricted CD8+ T cells in the liver is reminiscent of CD1d-restricted NKT cells, and might be due at least in part to their unique developmental requirement that positive selection be mediated by hematopoietic-derived cells in the thymus (63). The fact that we observed significantly fewer CD8+ T cells in the periphery of Kb−/−Db−/−M3−/− mice than that of Kb−/−Db−/− mice demonstrates that T cells restricted by non-H2-M3-restricted MHC class Ib molecules are not be able to fill the CD8+ T cell compartment, even in the absence of MHC class Ia-restricted and H2-M3-restricted CD8+ T cells. These data suggest that non-H2-M3 MHC class Ib-restricted CD8+ T cells may be limited in their ability to undergo homeostatic expansion.
The majority of LM-specific MHC class Ib-restricted CD8+ T cells isolated thus far are restricted to H2-M3, which appears to play a dominant role in anti-listerial immunity (26, 42). Qa-1 is the only other MHC class Ib molecule known to present LM antigens (27–30). However, the LM-specific CD8+ T cell responses observed in infected Kb−/−Db−/−M3−/− mice were not noticeably inhibited by antibodies specific to several known MHC class Ib molecules, including Qa-1b, Qa-2, MR1, and CD1d (Figure 6A and Figure S3), suggesting that these LM-specific CD8+ T cells are restricted by a previously uncharacterized MHC class Ib molecule(s). Indeed, CD8+ T cells isolated from infected Kb−/−Db−/−M3−/− mice failed to lyse Qa-1b-transfectants (Figure S6). Of the more than 40 MHC-linked MHC class Ib genes present in the mouse, only three definitively present microbial antigens to CD8+ T cells while little is known regarding the expression status and function of the remaining MHC class Ib genes. Thus, T cell lines derived from LM-infected Kb−/−Db−/−M3−/− mice would be useful tools for identifying novel antigen-presenting MHC class Ib molecules.
Analysis of the surface phenotype and functional properties of the residual CD8+ T cell populations found in the peripheral lymphoid organs of Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice indicated that most H2-M3- and non-H2-M3 MHC class Ib-restricted CD8+ T cells display an activated/memory phenotype. However, most of CD8+ T cells in the thymus of Kb−/−Db−/−M3−/− mice are CD44low (data not shown), suggesting that the acquisition of a memory phenotype by non-H2-M3 MHC class Ib-restricted CD8+ T cells is mainly a post-thymic event. It is possible that these non-H2-M3 MHC class Ib-restricted CD8+ T cells recognize commensal antigens and thereby become primed once they enter the periphery. The residual CD8+ T cells in Kb−/−Db−/−M3−/− mice can rapidly produce IFN-γ following TCR stimulation. In addition, culture of Kb−/−Db−/−M3−/− CD8+ T cells with IL-12 and IL-18, cytokines induced by LM infection as well as by infection with other pathogens, can induce IFN-γ secretion (data not shown). These data suggest that non-H2-M3 MHC class Ib-restricted CD8+ T cells may respond to LM infection in an antigen- and cytokine-dependent manner to contribute to the early phase of acquired immune responses against infection. Indeed, Kb−/−Db−/−M3−/− mice are more resistant to primary LM infection as compared to β2m−/− mice that lack all MHC class Ib-restricted CD8+ T cells. We cannot eliminate the possibility that the observed differences in susceptibility to LM infection between β2m−/− and Kb−/−Db−/−M3−/− mice may in part be due to differential NK cell activity, as it has been shown that ligand expression during NK cell development can affect their functional activity (64, 65). However, adoptive transfer of splenocytes isolated from LM-immunized Kb−/−Db−/−M3−/− mice confers significant protection upon recipient mice against lethal LM infection in a CD8+ T cell-dependent manner. These data provide direct evidence that CD8+ T cells restricted by MHC class Ib molecules other than H2-M3 can contribute to protective immunity against bacterial pathogens. Although H2-M3 appears to be the dominant restriction element for MHC class Ib-restricted responses during LM infection, our preliminary studies suggest that other MHC class Ib-restricted CD8+ T cells may play a more prominent role during Mtb infection, as we do not detect significant differences in CD8+ T cell expansion when comparing Mtb-infected Kb−/−Db−/− and Kb−/−Db−/−M3−/− mice (data not shown). Thus, CD8+ T cells restricted by distinct MHC class Ib molecules may play differential roles in host defense against different bacterial infections.
A recent study has shown that HLA-E can present a panel of Mtb-derived peptides to CD8+ T cells that have both cytotoxic and immunoregulatory activity (34). In addition, Mtb-reactive MR1-restricted CD8+ T cells were found to be enriched in the lungs of patients with active tuberculosis and responded to Mtb-infected MR1-expressing lung epithelial cells (35). These studies suggest that MHC class Ib-restricted CD8+ T cells may participate in immune responses against bacterial infection in humans. Further studies of the in vivo function of MHC class Ib-restricted CD8+ T cells during microbial infection using mice deficient in MHC class Ia and various MHC class Ib molecules would provide insight into the relative contribution of different MHC class Ib-restricted T cell populations to anti-microbial immunity. In addition, they may lead to the identification of novel antigen-presenting MHC class Ib molecules, which could be further explored as targets for vaccines against intracellular bacteria. While the highly polymorphic nature of MHC Ia molecules complicates vaccine design, vaccines that induce MHC class Ib-restricted T cell responses by targeting the relatively non-polymorphic MHC class Ib molecules would likely be effective in most human individuals.
Supplementary Material
Acknowledgments
We thank Dr. James Forman for providing us with the HeLa cell line and HeLa-Qa-1b transfectant, Dr. Ted Hansen for providing us with anti-MR1 antibody, and Dr. Iwana Stroynowski for providing us with anti-Qa-2 antibody. We also acknowledge Jessica Rojas, Ashley Rohr, Sharmila Shanmuganad, Stephen Wood and Chunting Yang for technical assistance.
ABBREVIATIONS USED IN THIS PAPER
- BMDC
bone marrow-derived dendritic cell
- B6
C57BL/6
- HKLM
Heat-killed Listeria monocytogenes
- iNKT cell
invariant NKT cell
- LM
Listeria monocytogenes
- MAIT cell
mucosal-associated invariant T cell
- Mtb
Mycobacterium tuberculosis
Footnotes
This work was supported by National Institute of Health grants AI40310 and AI57460 (to C.-R.W.).
References
- 1.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 2.Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA. Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol Res. 2004;29:81–92. doi: 10.1385/IR:29:1-3:081. [DOI] [PubMed] [Google Scholar]
- 3.Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. 2007;314:3–26. doi: 10.1007/978-3-540-69511-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howcroft TK, Singer DS. Expression of nonclassical MHC class Ib genes: comparison of regulatory elements. Immunol Res. 2003;27:1–30. doi: 10.1385/IR:27:1:1. [DOI] [PubMed] [Google Scholar]
- 6.Imani F, Soloski MJ. Heat shock proteins can regulate expression of the Tla region-encoded class Ib molecule Qa-1. Proc Natl Acad Sci U S A. 1991;88:10475–10479. doi: 10.1073/pnas.88.23.10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu NM, Chun T, Fay M, Mandal M, Wang CR. The majority of H2-M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J Exp Med. 1999;190:423–434. doi: 10.1084/jem.190.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, Soloski MJ. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 9.Swanson PA, 2nd, Pack CD, Hadley A, Wang CR, Stroynowski I, Jensen PE, Lukacher AE. An MHC class Ib-restricted CD8 T cell response confers antiviral immunity. J Exp Med. 2008;205:1647–1657. doi: 10.1084/jem.20080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, Schaible UE. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Bohmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 14.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 15.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson HL, Crossley EC, Thirumalapura N, Walker DH, Ismail N. Regulatory roles of CD1d-restricted NKT cells in the induction of toxic shock-like syndrome in an animal model of fatal ehrlichiosis. Infect Immun. 2008;76:1434–1444. doi: 10.1128/IAI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, Gilleron M. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol. 2009;16:82–92. doi: 10.1016/j.chembiol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Shawar SM, Vyas JM, Rodgers JR, Cook RG, Rich RR. Specialized functions of major histocompatibility complex class I molecules. II. Hmt binds N-formylated peptides of mitochondrial and prokaryotic origin. J Exp Med. 1991;174:941–944. doi: 10.1084/jem.174.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith GP, V, Dabhi M, Pamer EG, Lindahl KF. Peptide presentation by the MHC class Ib molecule, H2-M3. Int Immunol. 1994;6:1917–1926. doi: 10.1093/intimm/6.12.1917. [DOI] [PubMed] [Google Scholar]
- 20.Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 21.Gulden PH, Fischer P, 3rd, Sherman NE, Wang W, Engelhard VH, Shabanowitz J, Hunt DF, Pamer EG. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity. 1996;5:73–79. doi: 10.1016/s1074-7613(00)80311-8. [DOI] [PubMed] [Google Scholar]
- 22.Nataraj C, Brown ML, Poston RM, Shawar SM, Rich RR, Lindahl KF, Kurlander RJ. H2-M3wt-restricted, Listeria monocytogenes-specific CD8 T cells recognize a novel, hydrophobic, protease-resistant, periodate-sensitive antigen. Int Immunol. 1996;8:367–378. doi: 10.1093/intimm/8.3.367. [DOI] [PubMed] [Google Scholar]
- 23.Chun T, Serbina NV, Nolt D, Wang B, Chiu NM, Flynn JL, Wang CR. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J Exp Med. 2001;193:1213–1220. doi: 10.1084/jem.193.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ugrinovic S, Brooks CG, Robson J, Blacklaws BA, Hormaeche CE, Robinson JH. H2-M3 major histocompatibility complex class Ib-restricted CD8 T cells induced by Salmonella enterica serovar Typhimurium infection recognize proteins released by Salmonella serovar Typhimurium. Infect Immun. 2005;73:8002–8008. doi: 10.1128/IAI.73.12.8002-8008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tvinnereim A, Wizel B. CD8+ T cell protective immunity against Chlamydia pneumoniae includes an H2-M3-restricted response that is largely CD4+ T cell-independent. J Immunol. 2007;179:3947–3957. doi: 10.4049/jimmunol.179.6.3947. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouwer HG, Lindahl KF, Baldridge JR, Wagner CR, Barry RA, Hinrichs DJ. An H2-T MHC class Ib molecule presents Listeria monocytogenes-derived antigen to immune CD8+ cytotoxic T cells. J Immunol. 1994;152:5352–5360. [PubMed] [Google Scholar]
- 28.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ib-restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J Immunol. 1997;159:2795–2801. [PubMed] [Google Scholar]
- 29.Bouwer HG, Bai A, Forman J, Gregory SH, Wing EJ, Barry RA, Hinrichs DJ. Listeria monocytogenes-infected hepatocytes are targets of major histocompatibility complex class Ib-restricted antilisterial cytotoxic T lymphocytes. Infect Immun. 1998;66:2814–2817. doi: 10.1128/iai.66.6.2814-2817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaman MS, Perarnau B, Lindahl KF, Lemonnier FA, Forman J. Response to Listeria monocytogenes in mice lacking MHC class Ia molecules. J Immunol. 1999;162:5429–5436. [PubMed] [Google Scholar]
- 31.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 32.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 33.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196:1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joosten SA, van Meijgaarden KE, van Weeren PC, Kazi F, Geluk A, Savage ND, Drijfhout JW, Flower DR, Hanekom WA, Klein MR, Ottenhoff TH. Mycobacterium tuberculosis Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity. PLoS Pathog. 2010;6:e1000782. doi: 10.1371/journal.ppat.1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 37.Pietra G, Romagnani C, Mazzarino P, Falco M, Millo E, Moretta A, Moretta L, Mingari MC. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 2003;100:10896–10901. doi: 10.1073/pnas.1834449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 39.Colmone A, Wang CR. H2-M3-restricted T cell response to infection. Microbes Infect. 2006;8:2277–2283. doi: 10.1016/j.micinf.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol. 2006;176:1618–1627. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 41.Treiner E, Duban L, Moura IC, Hansen T, Gilfillan S, Lantz O. Mucosal-associated invariant T (MAIT) cells: an evolutionarily conserved T cell subset. Microbes Infect. 2005;7:552–559. doi: 10.1016/j.micinf.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Seaman MS, Wang CR, Forman J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J Immunol. 2000;165:5192–5201. doi: 10.4049/jimmunol.165.9.5192. [DOI] [PubMed] [Google Scholar]
- 43.Kerksiek KM, Ploss A, Leiner I, Busch DH, Pamer EG. H2-M3-restricted memory T cells: persistence and activation without expansion. J Immunol. 2003;170:1862–1869. doi: 10.4049/jimmunol.170.4.1862. [DOI] [PubMed] [Google Scholar]
- 44.Kurepa Z, Su J, Forman J. Memory phenotype of CD8+ T cells in MHC class Ia-deficient mice. J Immunol. 2003;170:5414–5420. doi: 10.4049/jimmunol.170.11.5414. [DOI] [PubMed] [Google Scholar]
- 45.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 46.Kerksiek KM, Busch DH, Pilip IM, Allen SE, Pamer EG. H2-M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J Exp Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton SE, Porter BB, Messingham KA, Badovinac VP, Harty JT. MHC class Ia-restricted memory T cells inhibit expansion of a nonprotective MHC class Ib (H2-M3)-restricted memory response. Nat Immunol. 2004;5:159–168. doi: 10.1038/ni1026. [DOI] [PubMed] [Google Scholar]
- 48.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 49.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane FC, Unanue ER. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972;135:1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busch DH, I, Pilip M, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 53.Mielke ME, Ehlers S, Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988;56:1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baldridge JR, Barry RA, Hinrichs DJ. Expression of systemic protection and delayed-type hypersensitivity to Listeria monocytogenes is mediated by different T-cell subsets. Infect Immun. 1990;58:654–658. doi: 10.1128/iai.58.3.654-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Orazio SE, Halme DG, Ploegh HL, Starnbach MN. Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection. J Immunol. 2003;171:291–298. doi: 10.4049/jimmunol.171.1.291. [DOI] [PubMed] [Google Scholar]
- 56.Goossens PL, Jouin H, Marchal G, Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990;132:137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- 57.Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandal M, Chen XR, Alegre ML, Chiu NM, Chen YH, Castano AR, Wang CR. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35:525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira CC, van Veelen PA, Querido B, de Ru A, Sluijter M, Laban S, Drijfhout JW, van der Burg SH, Offringa R, van Hall T. The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigen-processing defects. J Exp Med. 2010;207:207–221. doi: 10.1084/jem.20091429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiang EY, Stroynowski I. The role of structurally conserved class I MHC in tumor rejection: contribution of the Q8 locus. J Immunol. 2006;177:2123–2130. doi: 10.4049/jimmunol.177.4.2123. [DOI] [PubMed] [Google Scholar]
- 61.Uematsu Y, Kiefer H, Schulze R, Fischer-Lindahl K, Steinmetz M. Molecular characterization of a meiotic recombinational hotspot enhancing homologous equal crossing-over. EMBO J. 1986;5:2123–2129. doi: 10.1002/j.1460-2075.1986.tb04475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 63.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 65.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.