Abstract
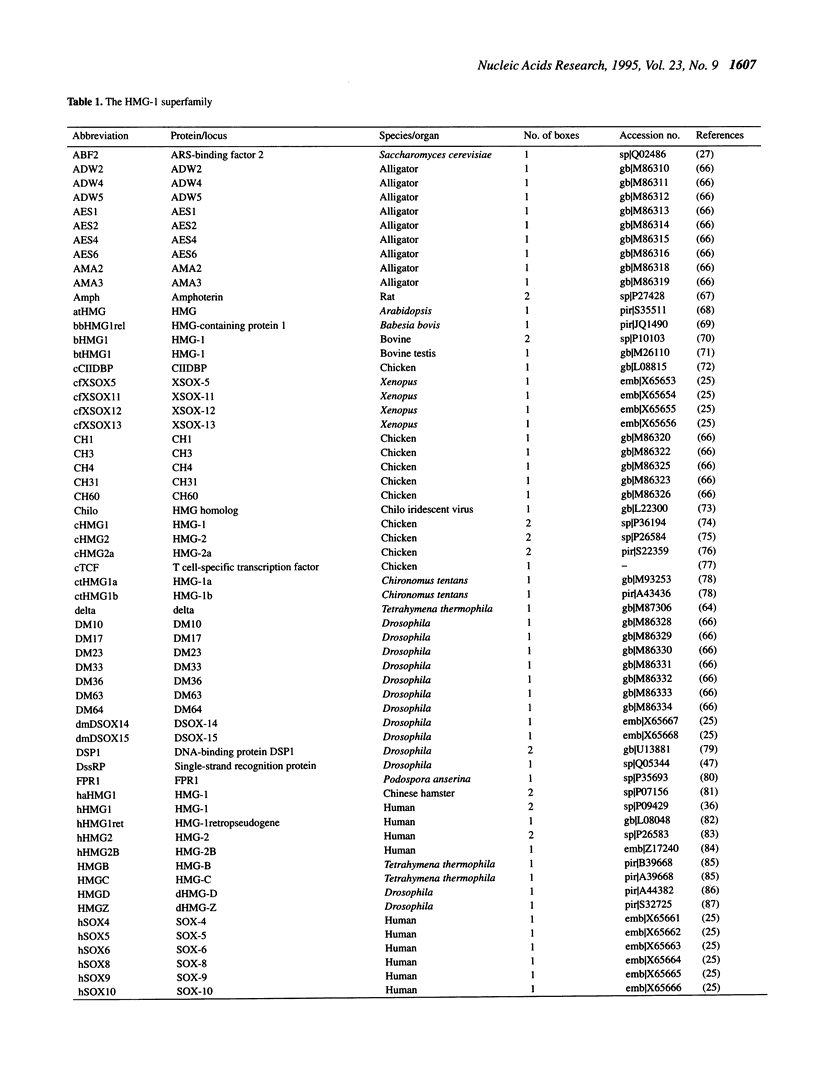
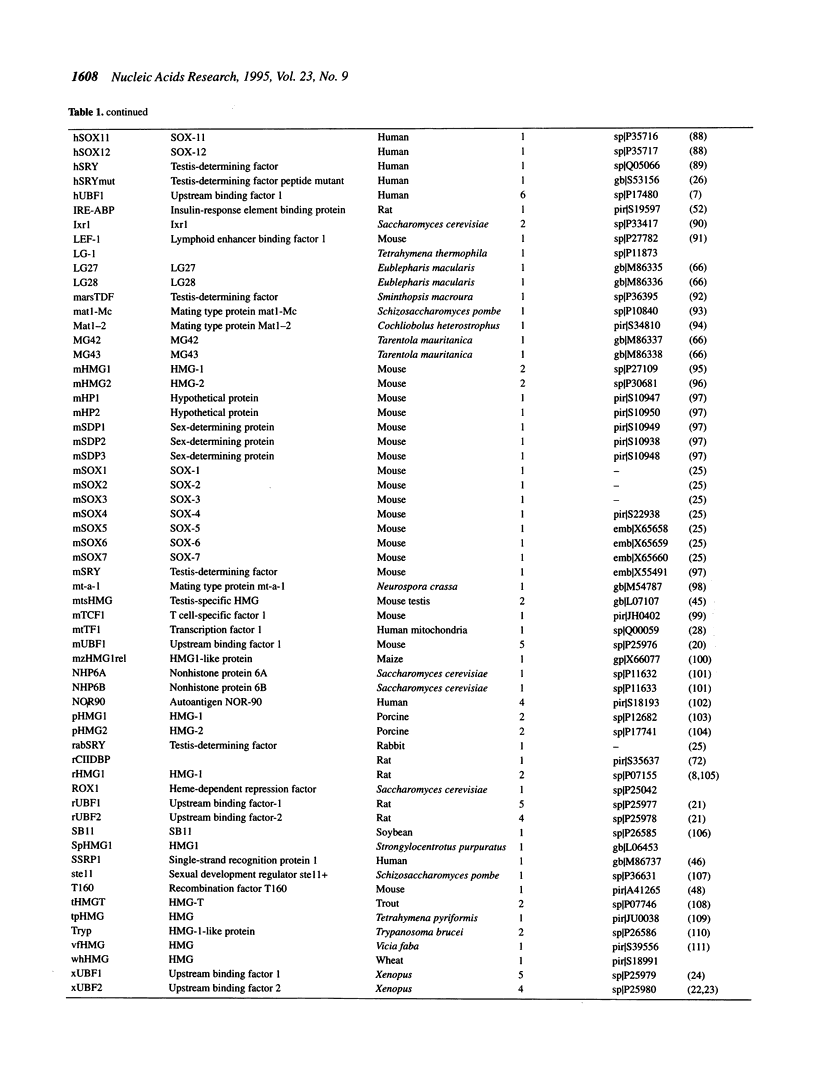
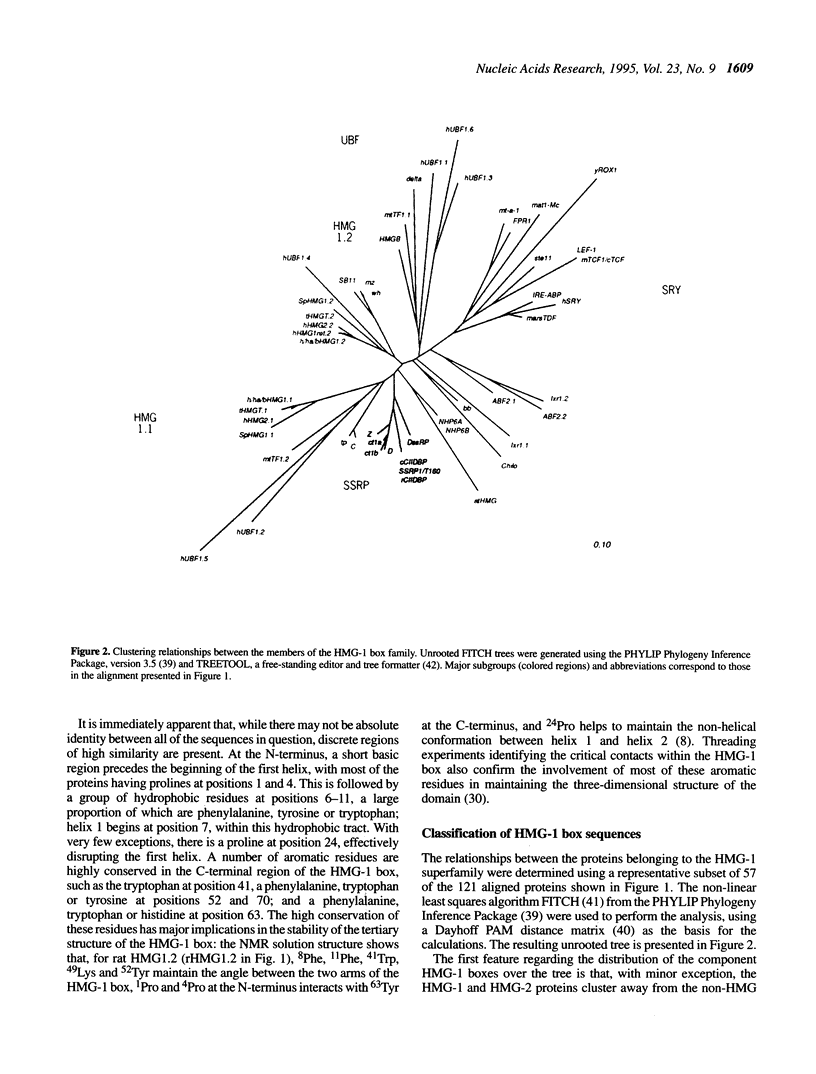
The abundant and highly-conserved nucleoproteins comprising the high mobility group-1/2 (HMG-1/2) family contains two homologous basic domains of about 75 amino acids. These basic domains, termed HMG-1 boxes, are highly structured and facilitate HMG-DNA interactions. Many proteins that regulate various cellular functions involving DNA binding and whose target DNA sequences share common structural characteristics have been identified as having an HMG-1 box; these proteins include the RNA polymerase I transcription factor UBF, the mammalian testis-determining factor SRY and the mitochondrial transcription factors ABF2 and mtTF1, among others. The sequences of 121 HMG-1 boxes have been compiled and aligned in accordance with thermodynamic results from homology model building (threading) experiments, basing the alignment on structure rather than by using traditional sequence homology methods. The classification of a representative subset of these proteins was then determined using standard least-squares distance methods. The proteins segregate into two groups, the first consisting of HMG-1/2 proteins and the second consisting of proteins containing the HMG-1 box but which are not canonical HMG proteins. The proteins in the second group further segregate based on their function, their ability to bind specific sequences of DNA, or their ability to recognize discrete non-B-DNA structures. The HMG-1 box provides an excellent example of how a specific protein motif, with slight alteration, can be used to recognize DNA in a variety of functional contexts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre S., Li W. W., Lee A. S. A human HMG2 cDNA with a novel 3'-untranslated region. Nucleic Acids Res. 1992 Dec 11;20(23):6413–6413. doi: 10.1093/nar/20.23.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bachvarov D., Moss T. The RNA polymerase I transcription factor xUBF contains 5 tandemly repeated HMG homology boxes. Nucleic Acids Res. 1991 May 11;19(9):2331–2335. doi: 10.1093/nar/19.9.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarov D., Normandeau M., Moss T. Heterogeneity in the Xenopus ribosomal transcription factor xUBF has a molecular basis distinct from that in mammals. FEBS Lett. 1991 Aug 19;288(1-2):55–59. doi: 10.1016/0014-5793(91)81002-p. [DOI] [PubMed] [Google Scholar]
- Bairoch A., Boeckmann B. The SWISS-PROT protein sequence data bank, recent developments. Nucleic Acids Res. 1993 Jul 1;21(13):3093–3096. doi: 10.1093/nar/21.13.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker W. C., George D. G., Mewes H. W., Pfeiffer F., Tsugita A. The PIR-International databases. Nucleic Acids Res. 1993 Jul 1;21(13):3089–3092. doi: 10.1093/nar/21.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton G. J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 1993 Jan;6(1):37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- Baxevanis A. D., Bryant S. H., Landsman D. Homology model building of the HMG-1 box structural domain. Nucleic Acids Res. 1995 Mar 25;23(6):1019–1029. doi: 10.1093/nar/23.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D., Lipman D. J., Ostell J. GenBank. Nucleic Acids Res. 1993 Jul 1;21(13):2963–2965. doi: 10.1093/nar/21.13.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M. E., Beltrame M., Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989 Feb 24;243(4894 Pt 1):1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- Boissonneault G., Lau Y. F. A testis-specific gene encoding a nuclear high-mobility-group box protein located in elongating spermatids. Mol Cell Biol. 1993 Jul;13(7):4323–4330. doi: 10.1128/mcb.13.7.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshoy A., McNamara P., Harrington R. E., Trifonov E. N. Curved DNA without A-A: experimental estimation of all 16 DNA wedge angles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2312–2316. doi: 10.1073/pnas.88.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. J., Kellett P. J., Lippard S. J. Ixr1, a yeast protein that binds to platinated DNA and confers sensitivity to cisplatin. Science. 1993 Jul 30;261(5121):603–605. doi: 10.1126/science.8342024. [DOI] [PubMed] [Google Scholar]
- Bruhn S. L., Pil P. M., Essigmann J. M., Housman D. E., Lippard S. J. Isolation and characterization of human cDNA clones encoding a high mobility group box protein that recognizes structural distortions to DNA caused by binding of the anticancer agent cisplatin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2307–2311. doi: 10.1073/pnas.89.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S. H., Lawrence C. E. An empirical energy function for threading protein sequence through the folding motif. Proteins. 1993 May;16(1):92–112. doi: 10.1002/prot.340160110. [DOI] [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Turner C. H., Leung I., Mayes E., Crane-Robinson C. Conformation and domain structure of the non-histone chromosomal proteins HMG 1 and 2. Domain interactions. Eur J Biochem. 1984 Sep 3;143(2):323–330. doi: 10.1111/j.1432-1033.1984.tb08375.x. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Turner C. H., Mayes E., Crane-Robinson C. Conformation and domain structure of the non-histone chromosomal proteins, HMG 1 and 2. Isolation of two folded fragments from HMG 1 and 2. Eur J Biochem. 1983 Mar 15;131(2):367–374. doi: 10.1111/j.1432-1033.1983.tb07272.x. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Imai H., Hamel J. C., Tan E. M. Human autoantibody to RNA polymerase I transcription factor hUBF. Molecular identity of nucleolus organizer region autoantigen NOR-90 and ribosomal RNA transcription upstream binding factor. J Exp Med. 1991 Nov 1;174(5):1239–1244. doi: 10.1084/jem.174.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. Proteins. One thousand families for the molecular biologist. Nature. 1992 Jun 18;357(6379):543–544. doi: 10.1038/357543a0. [DOI] [PubMed] [Google Scholar]
- Connor F., Cary P. D., Read C. M., Preston N. S., Driscoll P. C., Denny P., Crane-Robinson C., Ashworth A. DNA binding and bending properties of the post-meiotically expressed Sry-related protein Sox-5. Nucleic Acids Res. 1994 Aug 25;22(16):3339–3346. doi: 10.1093/nar/22.16.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coriat A. M., Müller U., Harry J. L., Uwanogho D., Sharpe P. T. PCR amplification of SRY-related gene sequences reveals evolutionary conservation of the SRY-box motif. PCR Methods Appl. 1993 Feb;2(3):218–222. doi: 10.1101/gr.2.3.218. [DOI] [PubMed] [Google Scholar]
- Dalrymple B. P., Peters J. M. Characterization of a cDNA clone from the haemoparasite Babesia bovis encoding a protein containing an "HMG-Box". Biochem Biophys Res Commun. 1992 Apr 15;184(1):31–35. doi: 10.1016/0006-291x(92)91153-h. [DOI] [PubMed] [Google Scholar]
- Davis D. L., Burch J. B. Isolation of a chicken HMG2 cDNA clone and evidence for an HMG2-specific 3'-untranslated region. Gene. 1992 Apr 15;113(2):251–256. doi: 10.1016/0378-1119(92)90403-c. [DOI] [PubMed] [Google Scholar]
- Debuchy R., Coppin E. The mating types of Podospora anserina: functional analysis and sequence of the fertilization domains. Mol Gen Genet. 1992 May;233(1-2):113–121. doi: 10.1007/BF00587568. [DOI] [PubMed] [Google Scholar]
- Denny P., Swift S., Brand N., Dabhade N., Barton P., Ashworth A. A conserved family of genes related to the testis determining gene, SRY. Nucleic Acids Res. 1992 Jun 11;20(11):2887–2887. doi: 10.1093/nar/20.11.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooijes D., van de Wetering M., Knippels L., Clevers H. The Schizosaccharomyces pombe mating-type gene mat-Mc encodes a sequence-specific DNA-binding high mobility group box protein. J Biol Chem. 1993 Nov 25;268(33):24813–24817. [PubMed] [Google Scholar]
- Einck L., Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Exp Cell Res. 1985 Feb;156(2):295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- Erondu N. E., Donelson J. E. Differential expression of two mRNAs from a single gene encoding an HMG1-like DNA binding protein of African trypanosomes. Mol Biochem Parasitol. 1992 Mar;51(1):111–118. doi: 10.1016/0166-6851(92)90206-y. [DOI] [PubMed] [Google Scholar]
- Fisher R. P., Topper J. N., Clayton D. A. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell. 1987 Jul 17;50(2):247–258. doi: 10.1016/0092-8674(87)90220-0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Brennan F. E., Hampikian G. K., Goodfellow P. N., Sinclair A. H., Lovell-Badge R., Selwood L., Renfree M. B., Cooper D. W., Graves J. A. Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials. Nature. 1992 Oct 8;359(6395):531–533. doi: 10.1038/359531a0. [DOI] [PubMed] [Google Scholar]
- Funahashi J., Sekido R., Murai K., Kamachi Y., Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993 Oct;119(2):433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- Gastrop J., Hoevenagel R., Young J. R., Clevers H. C. A common ancestor of the mammalian transcription factors TCF-1 and TCF-1 alpha/LEF-1 expressed in chicken T cells. Eur J Immunol. 1992 May;22(5):1327–1330. doi: 10.1002/eji.1830220531. [DOI] [PubMed] [Google Scholar]
- Giese K., Amsterdam A., Grosschedl R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 1991 Dec;5(12B):2567–2578. doi: 10.1101/gad.5.12b.2567. [DOI] [PubMed] [Google Scholar]
- Giese K., Grosschedl R. LEF-1 contains an activation domain that stimulates transcription only in a specific context of factor-binding sites. EMBO J. 1993 Dec;12(12):4667–4676. doi: 10.1002/j.1460-2075.1993.tb06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozé C., Poulat F., Berta P. Partial cloning of SOX-11 and SOX-12, two new human SOX genes. Nucleic Acids Res. 1993 Jun 25;21(12):2943–2943. doi: 10.1093/nar/21.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser K. D., Feix G. Isolation and characterization of maize cDNAs encoding a high mobility group protein displaying a HMG-box. Nucleic Acids Res. 1991 May 25;19(10):2573–2577. doi: 10.1093/nar/19.10.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser K. D., Wohlfarth T., Bäumlein H., Feix G. Comparative analysis of chromosomal HMG proteins from monocotyledons and dicotyledons. Plant Mol Biol. 1993 Nov;23(3):619–625. doi: 10.1007/BF00019309. [DOI] [PubMed] [Google Scholar]
- Griess E. A., Rensing S. A., Grasser K. D., Maier U. G., Feix G. Phylogenetic relationships of HMG box DNA-binding domains. J Mol Evol. 1993 Aug;37(2):204–210. doi: 10.1007/BF02407357. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Haqq C. M., King C. Y., Ukiyama E., Falsafi S., Haqq T. N., Donahoe P. K., Weiss M. A. Molecular basis of mammalian sexual determination: activation of Müllerian inhibiting substance gene expression by SRY. Science. 1994 Dec 2;266(5190):1494–1500. doi: 10.1126/science.7985018. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi H., Iwai K. Tetrahymena HMG nonhistone chromosomal protein. Isolation and amino acid sequence lacking the N- and C-terminal domains of vertebrate HMG 1. J Biochem. 1989 Apr;105(4):577–581. doi: 10.1093/oxfordjournals.jbchem.a122707. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Hahn M. C-C-A-G-G-C-m5C-T-G-G. Helical fine structure, hydration, and comparison with C-C-A-G-G-C-C-T-G-G. J Biol Chem. 1992 Apr 15;267(11):7332–7341. [PubMed] [Google Scholar]
- Hisatake K., Nishimura T., Maeda Y., Hanada K., Song C. Z., Muramatsu M. Cloning and structural analysis of cDNA and the gene for mouse transcription factor UBF. Nucleic Acids Res. 1991 Sep 11;19(17):4631–4637. doi: 10.1093/nar/19.17.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T., King D. L., LaBonne C., Kafatos F. C. A Drosophila single-strand DNA/RNA-binding factor contains a high-mobility-group box and is enriched in the nucleolus. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6488–6492. doi: 10.1073/pnas.90.14.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. N., Engelsberg B. N., Billings P. C. Purification of nuclear proteins that bind to cisplatin-damaged DNA. Identity with high mobility group proteins 1 and 2. J Biol Chem. 1992 Jul 5;267(19):13520–13527. [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Johnson M. S., Srinivasan N., Sowdhamini R., Blundell T. L. Knowledge-based protein modeling. Crit Rev Biochem Mol Biol. 1994;29(1):1–68. doi: 10.3109/10409239409086797. [DOI] [PubMed] [Google Scholar]
- Jones D. N., Searles M. A., Shaw G. L., Churchill M. E., Ner S. S., Keeler J., Travers A. A., Neuhaus D. The solution structure and dynamics of the DNA-binding domain of HMG-D from Drosophila melanogaster. Structure. 1994 Jul 15;2(7):609–627. doi: 10.1016/s0969-2126(00)00063-0. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Kaplan D. J., Duncan C. H. Full length cDNA sequence for bovine high mobility group 1 (HMG1) protein. Nucleic Acids Res. 1988 Nov 11;16(21):10375–10375. doi: 10.1093/nar/16.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. Y., Weiss M. A. The SRY high-mobility-group box recognizes DNA by partial intercalation in the minor groove: a topological mechanism of sequence specificity. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11990–11994. doi: 10.1073/pnas.90.24.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Burgum A. Duplicated NHP6 genes of Saccharomyces cerevisiae encode proteins homologous to bovine high mobility group protein 1. J Biol Chem. 1990 Feb 25;265(6):3234–3239. [PubMed] [Google Scholar]
- Landsman D., Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993 Aug;15(8):539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- Landsman D., Bustin M. Assessment of the transcriptional activation potential of the HMG chromosomal proteins. Mol Cell Biol. 1991 Sep;11(9):4483–4489. doi: 10.1128/mcb.11.9.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Stehelin D., Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993 May 25;21(10):2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T., Goldberg R. B. A plant DNA binding protein shares highly conserved sequence motifs with HMG-box proteins. Nucleic Acids Res. 1991 Sep 11;19(17):4769–4769. doi: 10.1093/nar/19.17.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Pentecost B. T., D'Anna J. A., Tobey R. A., Gurley L. R., Dixon G. H. Characterization of cDNA sequences corresponding to three distinct HMG-1 mRNA species in line CHO Chinese hamster cells and cell cycle expression of the HMG-1 gene. Nucleic Acids Res. 1987 Jul 10;15(13):5051–5068. doi: 10.1093/nar/15.13.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N., Thanos D., Brickman J. M., Ma J., Maniatis T., Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994 Sep 8;371(6493):175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. DNA--protein interactions. HMG has DNA wrapped up. Nature. 1992 May 28;357(6376):282–283. doi: 10.1038/357282a0. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Cerdán M. E., Zitomer R. S. A hypoxic consensus operator and a constitutive activation region regulate the ANB1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Nov;10(11):5921–5926. doi: 10.1128/mcb.10.11.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Brown D., Kerby S., Rudzinski I., Polte T., Randhawa Z., Seidman M. M. Sequence of human HMG2 cDNA. Nucleic Acids Res. 1991 Dec 11;19(23):6643–6643. doi: 10.1093/nar/19.23.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay B., Hu C. H., Pikaard C. S., Reeder R. H. xUBF and Rib 1 are both required for formation of a stable polymerase I promoter complex in X. laevis. EMBO J. 1991 Aug;10(8):2297–2303. doi: 10.1002/j.1460-2075.1991.tb07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenmies J., Pihlaskari R., Laitinen J., Wartiovaara J., Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991 Sep 5;266(25):16722–16729. [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Ner S. S., Churchill M. E., Searles M. A., Travers A. A. dHMG-Z, a second HMG-1-related protein in Drosophila melanogaster. Nucleic Acids Res. 1993 Sep 11;21(18):4369–4371. doi: 10.1093/nar/21.18.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Rothblum L. I. Identification of two forms of the RNA polymerase I transcription factor UBF. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3180–3184. doi: 10.1073/pnas.88.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Endo Y., Ito M., Miyamoto K., Sasakawa T., Suzuki I., Natori Y. Molecular cloning of chick liver HMG 2a cDNA and developmental expression of HMG 2a mRNA. Biochim Biophys Acta. 1992 Mar 24;1130(2):224–226. doi: 10.1016/0167-4781(92)90534-7. [DOI] [PubMed] [Google Scholar]
- Oosterwegel M. A., van de Wetering M. L., Holstege F. C., Prosser H. M., Owen M. J., Clevers H. C. TCF-1, a T cell-specific transcription factor of the HMG box family, interacts with sequence motifs in the TCR beta and TCR delta enhancers. Int Immunol. 1991 Nov;3(11):1189–1192. doi: 10.1093/intimm/3.11.1189. [DOI] [PubMed] [Google Scholar]
- Oosterwegel M., van de Wetering M., Dooijes D., Klomp L., Winoto A., Georgopoulos K., Meijlink F., Clevers H. Cloning of murine TCF-1, a T cell-specific transcription factor interacting with functional motifs in the CD3-epsilon and T cell receptor alpha enhancers. J Exp Med. 1991 May 1;173(5):1133–1142. doi: 10.1084/jem.173.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paonessa G., Frank R., Cortese R. Nucleotide sequence of rat liver HMG1 cDNA. Nucleic Acids Res. 1987 Nov 11;15(21):9077–9077. doi: 10.1093/nar/15.21.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M. A., Clayton D. A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991 May 17;252(5008):965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- Pauken C. M., Nagle D. L., Bucan M., Lo C. W. Molecular cloning, expression analysis, and chromosomal localization of mouse Hmg1-containing sequences. Mamm Genome. 1994 Feb;5(2):91–99. doi: 10.1007/BF00292334. [DOI] [PubMed] [Google Scholar]
- Pentecost B. T., Wright J. M., Dixon G. H. Isolation and sequence of cDNA clones coding for a member of the family of high mobility group proteins (HMG-T) in trout and analysis of HMG-T-mRNA's in trout tissues. Nucleic Acids Res. 1985 Jul 11;13(13):4871–4888. doi: 10.1093/nar/13.13.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost B., Dixon G. H. Isolation and partial sequence of bovine cDNA clones for the high-mobility-group protein (HMG-1). Biosci Rep. 1984 Jan;4(1):49–57. doi: 10.1007/BF01120823. [DOI] [PubMed] [Google Scholar]
- Philley M. L., Staben C. Functional analyses of the Neurospora crassa MT a-1 mating type polypeptide. Genetics. 1994 Jul;137(3):715–722. doi: 10.1093/genetics/137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pil P. M., Lippard S. J. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992 Apr 10;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- Read C. M., Cary P. D., Crane-Robinson C., Driscoll P. C., Norman D. G. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res. 1993 Jul 25;21(15):3427–3436. doi: 10.1093/nar/21.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read C. M., Cary P. D., Preston N. S., Lnenicek-Allen M., Crane-Robinson C. The DNA sequence specificity of HMG boxes lies in the minor wing of the structure. EMBO J. 1994 Dec 1;13(23):5639–5646. doi: 10.1002/j.1460-2075.1994.tb06902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck G. R., Isackson P. J., Teller D. C. Domain structure in high molecular weight high mobility group nonhistone chromatin proteins. Nature. 1982 Nov 4;300(5887):76–78. doi: 10.1038/300076a0. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Fuchs R., Higgins D. G., Stoehr P. J., Cameron G. N. The EMBL data library. Nucleic Acids Res. 1993 Jul 1;21(13):2967–2971. doi: 10.1093/nar/21.13.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler P., Hug M., Handermann M., Janssen W., Koonin E. V., Delius H., Darai C. Identification of genes encoding zinc finger proteins, non-histone chromosomal HMG protein homologue, and a putative GTP phosphohydrolase in the genome of Chilo iridescent virus. Nucleic Acids Res. 1994 Jan 25;22(2):158–166. doi: 10.1093/nar/22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman I. G., Wang T., Wu M., Bowen J., Cook R. G., Gorovsky M. A., Allis C. D. Macronuclei and micronuclei in Tetrahymena thermophila contain high-mobility-group-like chromosomal proteins containing a highly conserved eleven-amino-acid putative DNA-binding sequence. Mol Cell Biol. 1991 Jan;11(1):166–174. doi: 10.1128/mcb.11.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata M., Hüppi K., Usuda S., Okazaki K., Yoshida K., Sakano H. HMG1-related DNA-binding protein isolated with V-(D)-J recombination signal probes. Mol Cell Biol. 1991 Sep;11(9):4528–4536. doi: 10.1128/mcb.11.9.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H., Tsuda K., Yoshida M. Primary structure of non-histone chromosomal protein HMG2 revealed by the nucleotide sequence. Biochemistry. 1990 May 8;29(18):4419–4423. doi: 10.1021/bi00470a022. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Staben C., Yanofsky C. Neurospora crassa a mating-type region. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4917–4921. doi: 10.1073/pnas.87.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenburg F., Dinkl E., Grummt F. Nucleotide sequence of a mouse cDNA encoding the non-histone chromosomal high mobility group protein-2 (HMG-2) Nucleic Acids Res. 1992 Sep 25;20(18):4927–4927. doi: 10.1093/nar/20.18.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M., Dixon G. H. A retropseudogene for non-histone chromosomal protein HMG-1. Biochim Biophys Acta. 1993 Feb 20;1172(1-2):231–235. doi: 10.1016/0167-4781(93)90303-u. [DOI] [PubMed] [Google Scholar]
- Sugimoto A., Iino Y., Maeda T., Watanabe Y., Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991 Nov;5(11):1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Tremethick D. J., Molloy P. L. Effects of high mobility group proteins 1 and 2 on initiation and elongation of specific transcription by RNA polymerase II in vitro. Nucleic Acids Res. 1988 Dec 9;16(23):11107–11123. doi: 10.1093/nar/16.23.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Kikuchi M., Mori K., Waga S., Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988 Aug 9;27(16):6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- Turgeon B. G., Bohlmann H., Ciuffetti L. M., Christiansen S. K., Yang G., Schäfer W., Yoder O. C. Cloning and analysis of the mating type genes from Cochliobolus heterostrophus. Mol Gen Genet. 1993 Apr;238(1-2):270–284. doi: 10.1007/BF00279556. [DOI] [PubMed] [Google Scholar]
- Waga S., Mizuno S., Yoshida M. Chromosomal protein HMG1 removes the transcriptional block caused by the cruciform in supercoiled DNA. J Biol Chem. 1990 Nov 15;265(32):19424–19428. [PubMed] [Google Scholar]
- Wagner C. R., Hamana K., Elgin S. C. A high-mobility-group protein and its cDNAs from Drosophila melanogaster. Mol Cell Biol. 1992 May;12(5):1915–1923. doi: 10.1128/mcb.12.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Precht P., Balakir R., Horton W. E., Jr Rat and chick cDNA clones encoding HMG-like proteins. Nucleic Acids Res. 1993 Mar 25;21(6):1493–1493. doi: 10.1093/nar/21.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir H. M., Kraulis P. J., Hill C. S., Raine A. R., Laue E. D., Thomas J. O. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993 Apr;12(4):1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Johnson B. H., Reeck G. R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989 Feb 11;17(3):1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski J. R., Schulze E. Insect proteins homologous to mammalian high mobility group protein 1. Characterization and DNA-binding properties. J Biol Chem. 1992 Aug 25;267(24):17170–17177. [PubMed] [Google Scholar]
- Wu M., Allis C. D., Sweet M. T., Cook R. G., Thatcher T. H., Gorovsky M. A. Four distinct and unusual linker proteins in a mitotically dividing nucleus are derived from a 71-kilodalton polyprotein, lack p34cdc2 sites, and contain protein kinase A sites. Mol Cell Biol. 1994 Jan;14(1):10–20. doi: 10.1128/mcb.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. A novel Arabidopsis DNA binding protein contains the conserved motif of HMG-box proteins. Nucleic Acids Res. 1992 Dec 25;20(24):6737–6737. doi: 10.1093/nar/20.24.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi K., Privé G. G., Dickerson R. E. Analysis of local helix geometry in three B-DNA decamers and eight dodecamers. J Mol Biol. 1991 Jan 5;217(1):201–214. doi: 10.1016/0022-2836(91)90620-l. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Clevers H. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. EMBO J. 1992 Aug;11(8):3039–3044. doi: 10.1002/j.1460-2075.1992.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]