Abstract
Neuronal circuitry is often considered a clean slate that can be dynamically and arbitrarily molded by experience. However, when we investigated synaptic connectivity in groups of pyramidal neurons in the neocortex, we found that both connectivity and synaptic weights were surprisingly predictable. Synaptic weights follow very closely the number of connections in a group of neurons, saturating after only 20% of possible connections are formed between neurons in a group. When we examined the network topology of connectivity between neurons, we found that the neurons cluster into small world networks that are not scale-free, with less than 2 degrees of separation. We found a simple clustering rule where connectivity is directly proportional to the number of common neighbors, which accounts for these small world networks and accurately predicts the connection probability between any two neurons. This pyramidal neuron network clusters into multiple groups of a few dozen neurons each. The neurons composing each group are surprisingly distributed, typically more than 100 μm apart, allowing for multiple groups to be interlaced in the same space. In summary, we discovered a synaptic organizing principle that groups neurons in a manner that is common across animals and hence, independent of individual experiences. We speculate that these elementary neuronal groups are prescribed Lego-like building blocks of perception and that acquired memory relies more on combining these elementary assemblies into higher-order constructs.
Keywords: cell assemblies, Edelman, Hebb, brain development, learning
Hebb's (1) contributions to the theory of learning and memory have shaped psychological, philosophical, and neuroscientific theories for over 60 y. Three of the concepts that he put forward were particularly important. The first defines a correlation-based learning rule, namely that when one neuron persistently drives another, then the connection between them will be strengthened. The second states that this leads to the formation of clustered synaptic coupling of neurons into cell assemblies whose network topologies are molded by experience; the third suggests that such elementary cell assemblies are synaptically linked by the same learning rule to form trains of percepts (a phase sequence), constituting thoughts (1–6). There is a vast body of evidence for all three concepts (5, 7–10).
Despite this evidence, theorists have pointed out that the first postulate would cause synapses within cell assemblies to saturate, restricting their dynamic range and limiting memory storage capacity (11–20). Experimental studies have confirmed that saturated long-term potentiation (LTP) is unfavorable to learning and memory (21, 22). Although Hebb (1) had suggested a mechanism for passive weakening of unused synaptic connections, these objections suggested the need for an active depressive mechanism (16, 17, 23), inspiring the discoveries of long-term depression (LTD) (24) and bidirectional and spike timing-dependent plasticity (STDP) (25). Bidirectional plasticity provided a plausible mechanism for synaptic weights to assume arbitrary values depending on correlated activity among neurons. In artificial neural networks incorporating these modified Hebbian mechanisms, experience can mold the network topology of cell assemblies, endowing them with remarkable information processing and memory capabilities (11–20). However, these models are based on two key assumptions. The first is that memory is stored in the configuration of the connectivity of neurons in an assembly and in the set of synaptic weights of the connections; the second is that experience can freely mold the network connectivity and synaptic weights.
What is known about the network topology of cortical microcircuits? Intracellular recordings from multiple neurons have shown that different types of neurons have different connection probabilities and different numbers and distributions of synapses (26–30). Connection probabilities between neocortical neurons also vary according to the layer in which they reside (31, 32), the brain regions to which they project, and the brain regions from which they receive input (33, 34). Although these findings provide evidence for synaptic clustering, it is likely that these forms of clustering also involve genetically determined, topographical, and neuron-specific connectivity principles (35–38).
Evidence for synaptic clustering between neurons of the same type in a microcircuit began with the finding that layer 5 pyramidal neurons are reciprocally connected more often than expected from their unidirectional connection probabilities (39). A systematic study of triplets of these same pyramidal neurons by Song et al. (40) led to the discovery of three-cell motifs of connectivity.
There is considerable evidence for circuit plasticity between neocortical pyramidal neurons. When neurons lie within about 50 μm of each other (i.e., within the dimensions of a minicolumn), the axon of every pyramidal neuron is structurally connected to the dendrites of virtually every other pyramidal neuron in the immediate vicinity (41). This structural tabula rasa offers enormous opportunities for functional rewiring of neuron connectivities without major growth and reorganization of their neural arbors (42–44). Indeed, the synaptic circuitry of these layer 5 pyramidal neurons dynamically rewires after stimulation (45), indicating that activity can influence synaptic connectivity. Transmission across these established synaptic connections also undergoes lasting changes (46) driven by the relative timing of spikes emitted by pre- and postsynaptic neurons (47), providing a mechanism for stimuli to specifically mold the synaptic weights. Indeed, numerous models have shown that timing-dependent bidirectional plasticity rules have the potential to uniquely configure cell assemblies in ways that represent specific stimuli, allowing assemblies to learn from experience (48–52). What is not known, however, is whether such plasticity mechanisms have free range to arbitrarily mold neuron assemblies starting from a clean slate, as suggested by the structural tabula rasa, or whether they must operate under constraints of some prescribed synaptic organization. It is, therefore, important to know whether there exists any preferred synaptic connectivity between neurons at early stages of brain development.
In this paper, we present a study in which we searched for synaptically clustered neurons in the neocortex of neonatal animals (postnatal day 14) in the same neocortical region (somatosensory) and on the same layer 5 pyramidal (thick-tufted subcortically projecting) neurons on which a vast number of structural, functional, and plasticity studies have already generated extensive evidence for experience-dependent plasticity. We studied the topology and weights of the synaptic networks of groups of up to 12 simultaneously recorded neurons using a newly designed multineuron patch-clamp set-up.
Results
Distance-Dependent Connectivity Profiles.
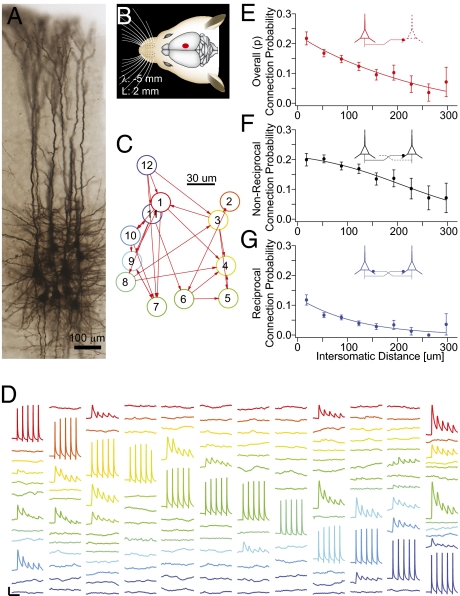
We recorded simultaneously from up to 12 thick-tufted layer 5 pyramidal neurons in somatosensory cortical slices (300 μm thick) from Wistar rats (postnatal days 14–16) (Fig. 1 A and B)—a model system extensively used in previous studies of cortical neurons and plasticity (39, 40, 45–47, 53–58). Connectivity was determined by applying trains (5–15 spikes at 20–70 Hz) of pulses of current (∼2 nA for 2.5–4 ms) to each recorded neuron and measuring the response of the other neurons, in the form of excitatory postsynaptic potentials (EPSPs), in current–clamp mode (Fig. 1 C and D). In 270 experiments, we took measurements from 1,345 neurons and 3,446 pairs of neurons. To ensure statistical robustness, each measurement was repeated at least 20 times. This procedure readily revealed synaptic connections and provided reliable measurements of their strength (Materials and Methods).
Fig. 1.
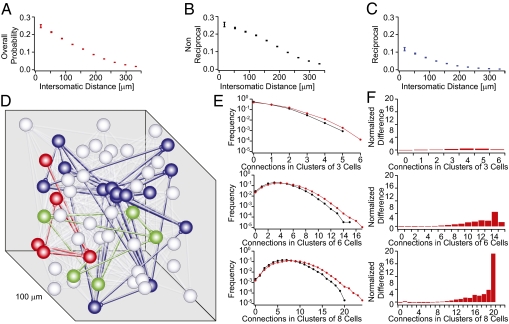
Pair-wise connectivity. (A) Morphological staining of a cluster of 12 cells recorded simultaneously. (B) Region of the somatosensory cortex where recordings were carried out. (C) Connectivity diagram of neurons in D. (D) Example of recorded traces in an experimental session. A different neuron is stimulated and the responses of the remaining neurons were recorded (displayed in columns). [Scale bars: horizontal, 100 ms; vertical, 1 mV (15 mV for action potentials)]. (E–G) Connection probability profiles as a function of distance. Error bars represent SEM.
The arbors of neurons that are farther apart in space naturally intersect more rarely than those of close neighbors. It follows that connectivity among neurons decreases with intersomatic distance (42). This implies that even randomly connected neurons show distance-dependent local clustering. To account for this phenomenon, we quantified distance-dependent connectivity using infrared differential interference contrast microscopy, capturing the precise x, y, and z coordinates of all recorded neurons and computing connection probability as a function of intersomatic distance for unidirectional, bidirectional, and combined connectivity configurations (Fig. 1 E–G). As expected, P decreased with distance in all configurations (P < 0.02; Kruskal–Wallis test), with a less abrupt decrease for unidirectional than bidirectional connections. These data also confirm previous reports that bidirectional connections are more than two times as frequent than predicted by chance (P < 0.001; t test) (39, 40).
Distributed Cell Assemblies.
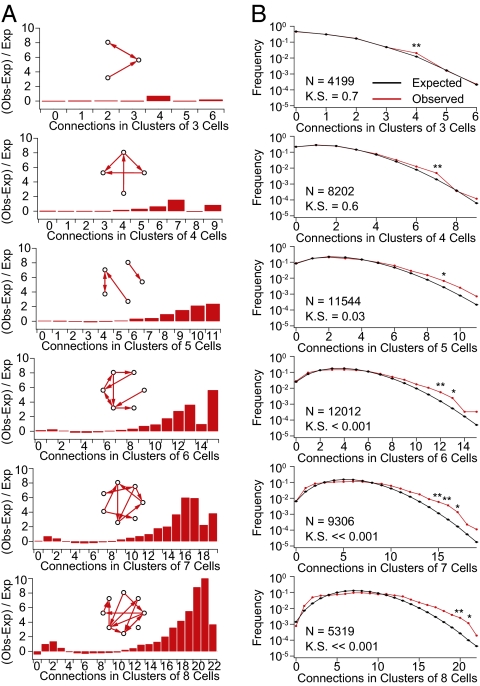
To detect possible patterns of nonrandom connectivity, we measured the number of connections between neurons in all possible groupings containing between three and eight neurons (n = 4,199, 8,202, 11,544, 12,012, 9,306, and 5,319, respectively). To compute expected connection probabilities, we constructed 1,000 simulated sets of networks in which the positions of neurons reflected the measured positions of the neurons obtained from our recordings and connection probabilities matched unidirectional or bidirectional connection probability profiles found earlier (Fig. S1). Finally, we compared the expected and observed distributions. In groups of three and four neurons, we found no significant differences (P = 0.7 and 0.6, respectively; two-sample Kolmogorov—Smirnov test). However, a more detailed analysis of specific connectivity patterns confirmed results from Song et al. (40) showing that certain three- and four-neuron motifs (Fig. S2) were significantly overrepresented (P < 0.01; z test and Bonferroni correction for multiple comparisons). Significant differences in the overall distribution of the number of expected connections first appeared in groups containing six neurons (P < 0.001; two-sample Kolmogorov–Smirnov test) (Fig. 2). This result could be expected if the smaller motifs previously described are not elementary units in their own right but parts of larger assemblies.
Fig. 2.
Higher order connectivity. (A) Difference between the observed and expected values in A divided by the expected values [(observed − expected)/expected]. (Insets) Schematic examples of possible patterns. Notice the increasingly larger disparity for clusters with many connections. (B) Comparison of observed (red) and expected (black) prevalence of given numbers of connections in clusters of three to eight cells. Error bars represent SEM.
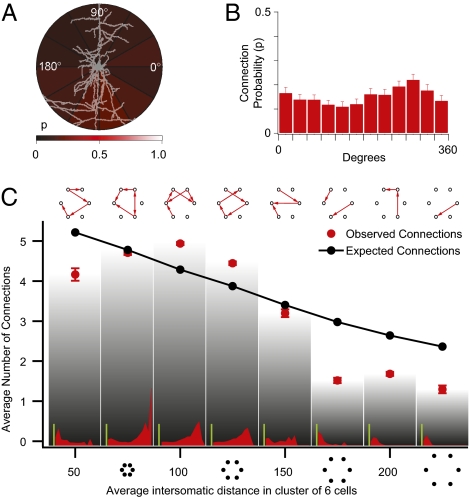
We then proceeded to analyze the principles governing clustering within these cell assemblies. There was no significant correlation between the intersomatic orientation of the neurons and their connection probability (P = 0.23; Kruskal–Wallis test) (Fig. 3 A and B). This is evidence against a lattice-like arrangement of synaptically connected neurons. Testing for neurons with an excessive number of incoming or outgoing connections showed no evidence of hubs (P = 0.6; two-sample Kolmogorov–Smirnov test) (Fig. S3), which characterize scale-free networks (59).
Fig. 3.
Clustering. (A) Intensity plot of outgoing connection probability at different intersomatic orientations; brighter regions have higher connection probability. (B) Bar plot of connection probabilities at different orientations. (C) Average number of connections in clusters of six cells grouped according to cluster dimension. (Insets) Histograms of the difference over expected profiles as in Fig. 2B for different cluster dimensions. (Green scale bars value: 5.) Error bars represent SEM.
Multineuron patch-clamp recordings normally focus on neurons that are within about 50 μm of each other. We therefore searched for synaptic clustering over greater distances (up to 200 μm). Contrary to expectations, we found that the average number of connections in groups of six neurons initially increased rather than decreasing monotonically with mean intersomatic distance (Fig. 3C) (P < 0.01; two-sample Kolmogorov–Smirnov test). The highest numbers of connections were thus not in the most compact groups but in groups of neurons separated by a mean distance of 100–125 μm. This phenomenon was clearly apparent, despite the fact that longer-range connections are more likely lost during the in vitro slicing procedure than the shorter-range connections. This suggests that the peak in the number of connections in this range may be even higher in vivo. Synaptic clusters of neurons are, thus, not confined within individual minicolumns, but extend across distances equivalent to the diameter of a functional neocortical column (300–500 μm).
Constraints on Network Topology.
There are many possible network topologies ranging from Erdös–Rényi random networks to Watts–Strogatz networks, that additionally exhibit various degrees of clustering, to highly organized, scale-free networks that contain hubs; each type implies different degrees and kinds of clustering with particular functional capabilities (60).
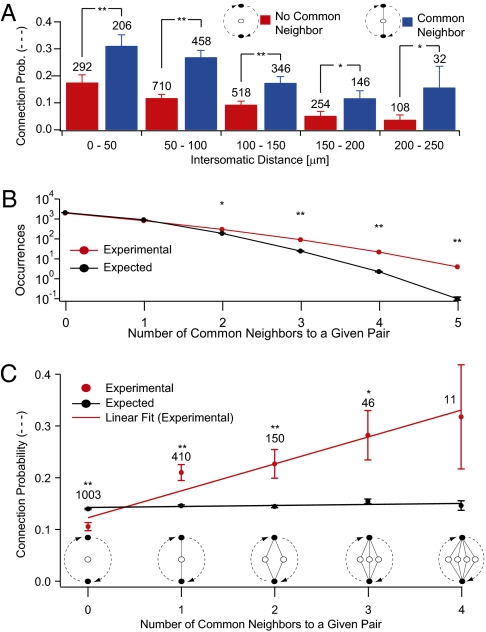
Our experimental data showed that, at any given distance, the connection probability for a pair of neurons with at least one common neighbor was significantly higher than the expected value (Fig. 4A) (P < 0.001, P < 0.001, P < 0.001, P = 0.017, P = 0.0156; paired t test) and that pairs of neurons sharing more than one common neighbor occurred significantly more often than expected (Fig. 4B). The connection probabilities rose linearly with the number of common neighbors (Fig. 4C) (R = 0.969). Connection probabilities, therefore, follow a strict rule and can be accurately predicted. This common neighbor rule is reminiscent of the mutual acquaintance rule described for social networks (61) but without the typical hub-like arrangement. Interestingly, two neurons were more likely to be connected when they both received input from the same common neighbor rather than projecting to a common neighbor, alluding to an even more refined rule relating connection probabilities and types of common neighbors in these directed networks (Fig. S4).
Fig. 4.
Common neighbor effect. (A) Connection probability as a function of distance comparing pairs that share a common neighbor against those that do not. (B) Occurrences of pairs of neurons sharing more than one common neighbor were significantly more numerous than expected by chance. (C) Connection probability between neurons as a function of the number of common neighbors experimentally observed. Error bars represent SEM.
To test whether the common neighbor rule could explain the experimental data, we implemented it in a network model. We first constructed a network of 2,000 point neurons in which neurons were connected according to the average distance-dependent connection probabilities found earlier (Fig. 5 A–C). We then recomputed the connection probabilities using the measured relationship between numbers of common neighbors and average connection probabilities and iterated this procedure until the clustering coefficient converged (Fig. 5D and SI Materials and Methods). Simulated patch-clamp recordings mimicking experiments were performed on the model. No evidence for hubs or a lattice-like organization of connections was found. As in the experimental data, evidence of clustering was stronger in larger than smaller groups of neurons (Fig. 5 E and F). The spatial distribution of clusters was similar to the distribution found in experiments. Taken together, these findings indicate that the common neighbor rule is sufficient to explain the synaptic clustering of neurons we observed in the experiments. This constraint suggests that experience cannot arbitrarily mold the network topology of cell assemblies but must abide by the common neighbor rule.
Fig. 5.
Simulation of clustered connectivity. (A–C) Connection probabilities in reorganized network that reflect the function of distance as found in the experimental data. (D) Three clusters (red, green, and blue) identified with affinity propagation in a network of 2,028 neurons. (E and F) Properties of simulated networks that reflect experimental measurements shown in Fig. 2 when comparing the initial network (black) and its reorganized version (red). Error bars represent SEM.
Constraints on Synaptic Weights.
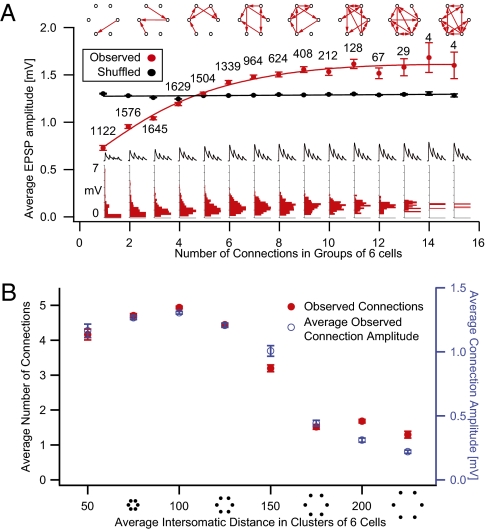
We next measured the synaptic weights in cell assemblies as they became more clustered. We identified all possible groups of six neurons and calculated the average amplitude of excitatory postsynaptic synaptic potentials (EPSPs) for groups with different numbers of connections. The analysis showed that, as groups became more interconnected, average EPSPs increased progressively in amplitude (Fig. 6A). In groups with six or more connections, average EPSPs were already close to saturation. The variance of the average amplitude of EPSPs tended to decrease with increased clustering, indicating a reduction in the dynamic range of the mean synaptic weights across different assemblies (i.e., all assemblies, even in different animals, with the same number of connections tend to the same mean synaptic weight) (Fig. S5A).
Fig. 6.
Synaptic strengths Amplitudes. (A) Average EPSP amplitude as a function of number of connections in groups of six neurons (red) and results after random shuffling of connection amplitudes (black). (Insets) Distributions of average connection amplitude for groups with different numbers of connections. (B) Average number of connections in clusters of six cells grouped according to cluster spatial spread (red) and corresponding average connection amplitude (blue). Error bars represent SEM.
To test the significance of the tight correlation between synaptic weights and numbers of connections, we shuffled the EPSP data, assigning each connection a randomly selected value from our pool of measured amplitudes and removing the value from the pool. For 100 repetitions of this procedure, we consistently measured a significant difference between observed and shuffled data (two-sample Kolmogorov–Smirnov, Bonferroni-corrected test). Plots of average synaptic strengths for groups of six neurons with different mean intersomatic distances showed a relationship with distance that was almost identical to that between distance and connection probabilities (Fig. 6B). This suggests that the distribution of synaptic weights can also be predicted from the number of common neighbors. This finding is supported by the observation that the distribution of synaptic weights shifts to the right with higher numbers of common neighbors (Fig. S5B).
Interlaced Cell Assemblies.
We used our model network with 2,000 neurons to extrapolate beyond the experimental results and explore the likely characteristics of separable cell assemblies in neural microcircuits. We first identified clusters using an affinity propagation algorithm (62) in which affinity was defined by the number of common neighbors. The results showed that each instance of the model contained an average of 38.2 ± 1.9 clusters, with 53.1 ± 35.3 neurons per cluster; all clusters interlaced in the same space. The mean connection probability for pairs of neurons in the same cluster (Pin = 0.14 ± 0.02) was more than two times the probability for pairs belonging to different clusters (Pout = 0.07 ± 0.005). Although these estimates are naturally sensitive to assumptions about neuron density, they were remarkably stable over a wide range of strictness values in the clustering algorithm. These results suggest that the average cluster contains a few dozen neurons and that typical microcircuits contain a few dozen such clusters (Fig. S6). To the upper limits for cluster strictness, the number of clusters increased, indicating that clusters contain clusters within clusters. Some of these subclusters may correspond to the three-cell motifs reported in previous work and to the three- and four-cell motifts reported here.
We proceeded to explore the network topology of the cell assemblies generated in our model network. The degree of clustering was significantly higher than expected for a random network in which clustering depends only on distance (Fig. S7A). The average shortest path between neurons within clusters was 1.80 ± 0.04 hops (1.9 ± 0.3 for the overall network). The distribution of the number of connections per neuron (degree) was binomial-like, with a right skew, and it showed no resemblance to the power-law distributions typical of scale-free networks that have so far characterized virtually all biological and social networks (Fig. S7 B and C) (63). The degree of separation is much smaller than, for example, protein interaction networks with 3° separation, social acquaintances within 6°, and Internet web pages with even higher degrees of separation. The network topology that we found is significantly different from the Erdös–Rényi random models and scale-free models, bearing a closer resemblance to the Watts–Strogatz model (64).
Discussion
This study reports a form of synaptic clustering in neocortical microcircuits, where cell assemblies are not arranged randomly or in a lattice but as small world networks without hubs. These assemblies extend beyond the diameter of neocortical minicolumns, probably contain only a few dozen neurons, and are interlaced with other assemblies within the same space. We found that connection probability between any two neurons increases linearly with the number of their common neighbors, that the mean synaptic weight depends on the number of connections in a group of neurons, and that this mean value increases to saturate after only a small fraction of the maximal clustering is reached. The grouping of neurons into elementary cell assemblies across animals is, therefore, governed by a powerful synaptic organizing principle.
The finding that connection probability between neurons and the mean synaptic weight within groups of neurons is predictable and tightly related to each other indicates that experiences cannot freely mold network topology and synaptic weights. The predictability of network topologies and synaptic weights is particularly surprising if we consider that our study was conducted with early postnatal animals. In such young animals, one might have expected to find a configuration of neural circuitry closer to a clean slate on which any new configuration could be molded—a notion that has profoundly influenced philosophical and psychological theory since the 17th century (65).
We speculate that this synaptic organizing principle is genetically prescribed and developmentally expressed, because it applies across different animals. However, STDP does mold synaptic weights (47), and long-term microcircuit plasticity (LTMP) does mold the connectivity between these pyramidal neurons (45, 66) in this same preparation. The specific neurons involved in a group may, therefore, be selected by experience, but because of the powerful connectivity constraints that we found, these forms of plasticity is likely to add only minor variations to functioning of neuronal groups.
The synaptic clustering we found provides experimental evidence for the primary repertoires proposed earlier by the theory of neuronal group selection by Edelman (67). Unlike Hebb's proposal (1), this theory suggests that functional neural circuitry arises by selection among neuronal groups that already emerged during embryonic development independent of experience. In Edelman's theory, subsequent experience selects neuronal groups to form secondary repertoires that have survival value (67, 68). In Hebb's view, experience carves out and reinforces chains of such elementary assemblies to form phase sequences supporting specific trains of thought (1). A key difference between Edelman's (67, 68) theory and Hebb's (1) proposals is reflected by Edelman's emphasis on selection, as apposed to instruction, of repertoires of neuronal groups iteratively during perception in a process called reentry (67, 68).
The elementary assemblies that we found are interconnected by fewer and weaker strands of connections than within assemblies, which are more amenable to experience-dependent modification (69). This suggests that experience could uniquely mold overall neuronal circuitry by differently combining elementary assemblies into unique superassemblies or secondary repertoires. The various possible combinations of elementary assemblies plus the numerous ways that one assembly could be connected to another (through different neurons, different weights between each neuron, different synaptic dynamics, different directions, or different degrees of reciprocity) may not only provide for vast combinatorics and hence memory storage capacity, but also ensure stability of memories in the face of ongoing activity.
Materials and Methods
Electrophysiological Recordings.
Whole-cell recordings were made from pyramidal neurons at 34 ± 1 °C in rat somatosensory cortex (λ-5, medio-lateral 2) slices from animals at postnatal days 14 to 16. Neuronal connectivity was established with trains of current pulses (5–15 pulses at 20–70 Hz).
Data Analysis.
Data were analyzed using IGOR (WaveMetrics), MATLAB (MathWorks), and C++. The cortical layer 5 neuron models were assembled with 2,000 cells in a cube layout using MATLAB. Data are presented as mean ± SEM.
Computer-Assisted Patch Clamp.
Recordings were achieved with custom C++ software that controlled manipulators, amplifiers, oscilloscopes, pipette pressure, and video display.
Additional details are in SI Material and Methods.
Supplementary Material
Acknowledgments
We thank Michele Pignatelli, Idan Segev, Sean Hill, and Felix Schürmann for discussions and comments. We especially thank the late Philip Goodman for his comments and help on the statistical and clustering analyses. We thank Richard Walker for his editing help on this paper. R.P. and T.L.B. were supported by an EU Synapse grant. This study was also carried out as part of the Blue Brain Project and European Union's Fast Analog Computing on Emergent Transient States (FACETS) program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016051108/-/DCSupplemental.
References
- 1.Hebb DO. The Organization of Behavior—A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 2.Milner PM. The cell assembly: Mark II. Psychol Rev. 1957;64:242–252. doi: 10.1037/h0042287. [DOI] [PubMed] [Google Scholar]
- 3.Sejnowski TJ. The book of Hebb. Neuron. 1999;24:773–776. doi: 10.1016/s0896-6273(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 4.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 5.Morris RG. D.O. Hebb: The organization of behavior, Wiley: New York; 1949. Brain Res Bull. 1999;50:437–438. doi: 10.1016/s0361-9230(99)00182-3. [DOI] [PubMed] [Google Scholar]
- 6.Sejnowski TJ. The once and future Hebb synapse. Can Psychol. 2003;44:17–20. [Google Scholar]
- 7.Kandel ER. In Search of Memory: The Emergence of a New Science of Mind. 1st Ed. W. W. Norton & Company, New York; 2006. [Google Scholar]
- 8.Dudai Y. The Neurobiology of Memory: Concept, Findings, Trends. London: Oxford University Press; 1989. [Google Scholar]
- 9.Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- 10.Byrne JH, Menzel R, Roediger HL. Learning and Memory: A Comprehensive Reference. 1st Ed. Amsterdam: Elsevier; 2008. [Google Scholar]
- 11.Sejnowski TJ. Statistical constraints on synaptic plasticity. J Theor Biol. 1977;69:385–389. doi: 10.1016/0022-5193(77)90146-1. [DOI] [PubMed] [Google Scholar]
- 12.Rochester N, Holland J, Haibt L, Duda W. Tests on a Cell Assembly Theory of the Action of the Brain, Using a Large Digital Computer. 1956. (IRE Transactions on Information Theory), 80–93. [Google Scholar]
- 13.Rosenblatt F. Principles of Neurodynamics; Perceptions and the Theory of Brain Mechanisms. Washington, DC: Spartan Books; 1962. [Google Scholar]
- 14.Miller KD, Mackay DJC. The role of constraints in Hebbian learning. Neural Comput. 1994;6:100–126. [Google Scholar]
- 15.Dayan P, Abbott LF. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 16.Stent GS. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a disMbuted memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 19.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amit DJ, Gutfreund H, Sompolinsky H. Information storage in neural networks with low levels of activity. Phys Rev A. 1987;35:2293–2303. doi: 10.1103/physreva.35.2293. [DOI] [PubMed] [Google Scholar]
- 21.Barnes CA, et al. LTP saturation and spatial learning disruption: Effects of task variables and saturation levels. J Neurosci. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNaughton N, Wickens J. Hebb, pandemonium and catastrophic hypermnesia: The hippocampus as a suppressor of inappropriate associations. Cortex. 2003;39:1139–1163. doi: 10.1016/s0010-9452(08)70882-7. [DOI] [PubMed] [Google Scholar]
- 23.Sejnowski TJ. Storing covariance with nonlinearly interacting neurons. J Math Biol. 1977;4:303–321. doi: 10.1007/BF00275079. [DOI] [PubMed] [Google Scholar]
- 24.Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: A postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- 25.Abbott LF, Nelson SB. Synaptic plasticity: Taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 26.Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2-5 of adult rat and cat neocortex: Triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AM, Morris OT. Selectivity in the inter-laminar connections made by neocortical neurones. J Neurocytol. 2002;31:239–246. doi: 10.1023/a:1024117908539. [DOI] [PubMed] [Google Scholar]
- 28.Thomson AM, Bannister AP, Mercer A, Morris OT. Target and temporal pattern selection at neocortical synapses. Philos Trans R Soc Lond B Biol Sci. 2002;357:1781–1791. doi: 10.1098/rstb.2002.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watts J, Thomson AM. Excitatory and inhibitory connections show selectivity in the neocortex. J Physiol. 2005;562:89–97. doi: 10.1113/jphysiol.2004.076984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West DC, Mercer A, Kirchhecker S, Morris OT, Thomson AM. Layer 6 cortico-thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex. 2006;16:200–211. doi: 10.1093/cercor/bhi098. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- 32.Kampa BM, Letzkus JJ, Stuart GJ. Cortical feed-forward networks for binding different streams of sensory information. Nat Neurosci. 2006;9:1472–1473. doi: 10.1038/nn1798. [DOI] [PubMed] [Google Scholar]
- 33.Le Be JV, Silberberg G, Wang Y, Markram H. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex. 2007;17:2204–2213. doi: 10.1093/cercor/bhl127. [DOI] [PubMed] [Google Scholar]
- 34.Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci USA. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chedotal A, Richards LJ. Wiring the brain: The biology of neuronal guidance. Cold Spring Harb Perspect Biol. 2010;2:a001917. doi: 10.1101/cshperspect.a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepanyants A, Tamas G, Chklovskii DB. Class-specific features of neuronal wiring. Neuron. 2004;43:251–259. doi: 10.1016/j.neuron.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Markram H. A network of tufted layer 5 pyramidal neurons. Cereb Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- 40.Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalisman N, Silberberg G, Markram H. The neocortical microcircuit as a tabula rasa. Proc Natl Acad Sci USA. 2005;102:880–885. doi: 10.1073/pnas.0407088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stepanyants A, Chklovskii DB. Neurogeometry and potential synaptic connectivity. Trends Neurosci. 2005;28:387–394. doi: 10.1016/j.tins.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 44.Chklovskii DB. Synaptic connectivity and neuronal morphology: Two sides of the same coin. Neuron. 2004;43:609–617. doi: 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Le Be JV, Markram H. Spontaneous and evoked synaptic rewiring in the neonatal neocortex. Proc Natl Acad Sci USA. 2006;103:13214–13219. doi: 10.1073/pnas.0604691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 47.Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 48.Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383:76–81. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- 49.Blum KI, Abbott LF. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 1996;8:85–93. doi: 10.1162/neco.1996.8.1.85. [DOI] [PubMed] [Google Scholar]
- 50.Caporale N, Dan Y. Spike timing-dependent plasticity: A Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 51.Rao RP, Sejnowski TJ. Spike-timing-dependent Hebbian plasticity as temporal difference learning. Neural Comput. 2001;13:2221–2237. doi: 10.1162/089976601750541787. [DOI] [PubMed] [Google Scholar]
- 52.Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci. 2000;3:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- 53.Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- 54.Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frick A, Feldmeyer D, Sakmann B. Postnatal development of synaptic transmission in local networks of L5A pyramidal neurons in rat somatosensory cortex. J Physiol. 2007;585:103–116. doi: 10.1113/jphysiol.2007.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krieger P, Kuner T, Sakmann B. Synaptic connections between layer 5B pyramidal neurons in mouse somatosensory cortex are independent of apical dendrite bundling. J Neurosci. 2007;27:11473–11482. doi: 10.1523/JNEUROSCI.1182-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deuchars J, West DC, Thomson AM. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J Physiol. 1994;478:423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- 59.Barabasi AL, Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- 60.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 61.Jin EM, Girvan M, Newman ME. Structure of growing social networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:046132. doi: 10.1103/PhysRevE.64.046132. 10.1103/PhysRevE.64.046132. [DOI] [PubMed] [Google Scholar]
- 62.Frey BJ, Dueck D. Clustering by passing messages between data points. Science. 2007;315:972–976. doi: 10.1126/science.1136800. [DOI] [PubMed] [Google Scholar]
- 63.Barabasi AL. Scale-free networks: A decade and beyond. Science. 2009;325:412–413. doi: 10.1126/science.1173299. [DOI] [PubMed] [Google Scholar]
- 64.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 65.Locke J. 1689. An Essay Concerning Human Understanding (William Tegg, London), 38th Ed. [Google Scholar]
- 66.Silva GT, Le Bé J-V, Riachi I, Rinaldi T, Markram K, Markram H. Enhanced long-term microcircuit plasticity in the valproic acid animal model of autism. Front Syna Neurosci. 2009 doi: 10.3389/neuro.19.001.2009. 10.3389/neuro.19.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edelman GM. Neural Darwinism: The Theory of Neuronal Group Selection. Basic Books, New York; 1987. [DOI] [PubMed] [Google Scholar]
- 68.Edelman GM. Neural Darwinism: selection and reentrant signaling in higher brain function. Neuron. 1993;10:115–125. doi: 10.1016/0896-6273(93)90304-a. [DOI] [PubMed] [Google Scholar]
- 69.van Rossum MC, Bi GQ, Turrigiano GG. Stable Hebbian learning from spike timing-dependent plasticity. J Neurosci. 2000;20:8812–8821. doi: 10.1523/JNEUROSCI.20-23-08812.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.