Abstract
Background
Subcutaneous injections of anti-CD20 antibodies may offer benefits to both patients and the healthcare system for treatment of B-cell malignancies.
Design and Methods
A pilot study was undertaken to evaluate the potential for subcutaneous dosing with 2nd generation anti-CD20 antibody veltuzumab in patients with CD20+ indolent non-Hodgkin’s lymphoma. Patients with previously untreated or relapsed disease received 4 doses of 80, 160, or 320 mg veltuzumab injected subcutaneously every two weeks. Responses were assessed by computed tomography scans, with other evaluations including adverse events, safety laboratories, B-cell blood levels, serum veltuzumab levels, and human anti-veltuzumab antibody (HAHA) titers.
Results
Seventeen patients (14 follicular lymphoma; 13 stage III or IV disease; 5 treatment-naive) completed treatment with only occasional, mild-moderate, transient injection reactions and no other safety issues. Subcutaneous veltuzumab demonstrated a slow release pattern over several days, achieving a mean Cmax of 19, 25 and 63 μg/mL at 80, 160, and 320 mg doses for a total of 4 administrations, respectively. Depletion of circulating B cells occurred after the first injection. The objective response rate (partial responses plus complete responses plus complete responses unconfirmed) was 47% (8/17) with a complete response/complete response unconfirmed rate of 24% (4/17); 4 of 8 objective responses continued for 60 weeks or more. All serum samples evaluated for human anti-veltuzumab antibody were negative.
Conclusions
Subcutaneous injections of low-dose veltuzumab are convenient, well tolerated, and capable of achieving sustained serum levels, B-cell depletion, and durable objective responses in indolent non-Hodgkin’s lymphoma. (Clinicaltrials.gov identifier: NCT00546793)
Keywords: non-Hodgkin’s lymphoma, CD20, antibody, subcutaneous therapy, hA20
Introduction
Following the successful application of the chimeric anti-CD20 monoclonal antibody (MAb) rituximab in the therapy of non-Hodgkin’s lymphoma (NHL)1–10 and autoimmune disorders,11–14 2nd generation anti-CD20 antibodies have been developed to increase efficacy, decrease toxicity (primarily infusion reactions) or immunogenicity, and allow more rapid administration.15–22 Veltuzumab (hA20) is a humanized, anti-CD20 MAb with similarities as well as structural and functional differences from rituximab.23–26 In the complementarity-determining regions, veltuzumab differs from rituximab by only one amino acid, but has completely different framework regions. Veltuzumab showed anti-proliferative, apoptotic, and antibody-dependent cellular cytotoxicity effects in vitro similar to rituximab, but with other qualitative differences, including slower off-rates and increased complement-dependent cytotoxicity in several human lymphoma cell lines. In mice bearing human lymphoma xenografts, low doses of veltuzumab controlled tumor growth, even producing a number of cures.
In the initial non-Hodgkin’s lymphoma clinical study, 82 patients received veltuzumab intravenously, given once weekly for four weeks.27 That study demonstrated the safety of veltuzumab at doses of up to twice the standard rituximab dose of 375 mg/m2. Interestingly, objective responses, including complete responses, occurred at doses as low as 80–120 mg/m2 weekly for a total of 4 administrations. Therefore, veltuzumab was reformulated in a more concentrated form (approximately 80 mg/mL) and this pilot study was undertaken to evaluate the feasibility for delivery of low doses by subcutaneous (SC) injection. Anticipating that a fluid volume of 2 mL or less could be administered by subcutaneous injection, 3 doses were selected for evaluation, with doses of 80 and 160 mg to be delivered by one injection, and doses of 320 mg by two separate injections. All patients received a total of 4 doses of veltuzumab, but with a two week dosing interval to allow for anticipated slow release into the blood. This initial study also focused exclusively on patients with indolent lymphomas for whom the convenience of this route of administration may be of particular benefit.
Design and Methods
Design
In this open-label, multicenter phase I study, patients with indolent non-Hodgkin’s lymphoma received 80, 160 or 320 mg subcutaneous veltuzumab administered every other week for a total of 4 administrations. The primary objectives were to evaluate the safety, tolerance and immunogenicity of veltuzumab with this route of administration and dosing schedule. Secondary objectives were to obtain preliminary evidence of efficacy and to assess pharmacokinetics and pharmacodynamics. Neither steroids nor other pre-medications were routinely required unless clinically indicated. Initially, a standard dose escalation design was used, with escalation continuing as long as none of 3 or one of 6 patients encountered dose limiting toxicity. The study ended after several additional patients were then entered to provide more experience with 160 mg and 320 mg doses.
Patients
Eligible patients were at least 18 years old with CD20-positive follicular lymphoma (FL), small lymphocyctic lymphoma (SLL) or marginal zone lymphoma (MZL), and at least one measurable lesion of 1.5 cm or larger by CT (but none > 10 cm). Previously untreated or relapsed patients were eligible, but patients receiving more than 4 prior treatment regimens, untreated patients with only Stage I or II disease (Ann Arbor classification), or rituximab-resistant patients (progression during or within six months of a rituximab-containing regimen) were excluded. Patients had 0–1 ECOG performance status, hemoglobin 10 g/dL or over, absolute neutrophil count (ANC) 1.0×109/L or over, platelets 50×109/L or over (all without transfusional support), creatinine and bilirubin 1.5 x institutional upper limit of normal (IULN) or under, AST and ALT 2.5 x IULN or under, and be five years beyond any other cancers (except non-melanoma skin cancer or cervical carcinoma in situ), 12 months beyond any prior rituximab treatment, 12 weeks beyond any autologous stem cell transplant, and with no major surgery or chemotherapy, other experimental treatments, or any radiation therapy to the index lesion(s) within four weeks or corticosteroids within two weeks (except ≤ 20 mg/day prednisone or equivalent, if continued unchanged). Patients with central nervous system or pleural effusion involvement by lymphoma, HIV lymphoma, transformed lymphoma (Richter’s lymphoma), known HIV, hepatitis B (must be screened following NCCN guidelines and negative), active hepatitis C or presence of hepatitis C antibody, infection within seven days or requiring antibiotic use, prior therapy with other human or humanized monoclonal antibodies (unless human anti-veltuzumab antibody (HAHA) tested negative), excessive (Grade 3 or 4) toxicity to rituximab, or known human anti-chimeric antibody positivity (testing was not required) were ineligible. At each participating institution, the governing ethics committee approved the study and written informed consent was obtained from all patients.
Assessment
Non-Hodgkin’s lymphoma subtypes were determined for each patient by WHO classification28 and FLIPI prognostic scores were assigned for follicular lymphomas.29 Tumor assessments were based on physical examinations and CT scans (neck, thorax, abdomen, pelvis, other sites of known disease) at baseline, four and 12 weeks after last injection, and then every three months for up to two years or until disease progression with bone marrow biopsy repeated only if positive at baseline to confirm a complete response. Treatment responses were classified at each site by International Working Group (IWG) Criteria30 as either complete response (CR), complete response unconfirmed (CRu), partial response (PR), stable disease, or progressive disease.
Safety was assessed in all patients with adverse events (AEs) classified by the Medical Dictionary for Regulatory Activities (MedRA) system organ class and preferred terms, vital signs and physical examinations, serum chemistries, hematology, urinalysis, serum immunoglobulins, and T-cell levels (CD3+). Laboratory and adverse event toxicity was graded according to National Cancer Institute (NCI) Common Toxicity Criteria (CTC), version 3.0, with dose-limiting toxicity (DLT) defined as any treatment-related Grade 3 or 4 events or Grade 2 events of autoimmune reaction, bronchospasm, or generalized urticaria. Pharmacodynamics were assessed by blood B-cell levels (CD19+). For pharmacokinetics (PK) and immunogenicity, enzyme-linked immunosorbent assay (ELISA) tests, performed by the Sponsor, measured serum levels of veltuzumab and any human anti-veltuzumab antibody reactions, respectively, while pharmacokinetic parameters following last injection were determined by WinNonLin 2.1 (Pharsight Corporation, Mountain View, CA, USA) using a single compartment model.
Statistical analysis
Response rates were summarized using descriptive statistics. Progression-free survival (PFS) was calculated from first injection to disease progression, death, or last contact, whichever occurred earliest. Duration of response (DR) was calculated from the onset of an objective response (OR; i.e., CR, CRu, or PR) to the same events. The Kaplan-Meier method was used to generate progression-free and duration of response survival curves and the corresponding confidence intervals for the estimated medians.
Results
Patient enrollment
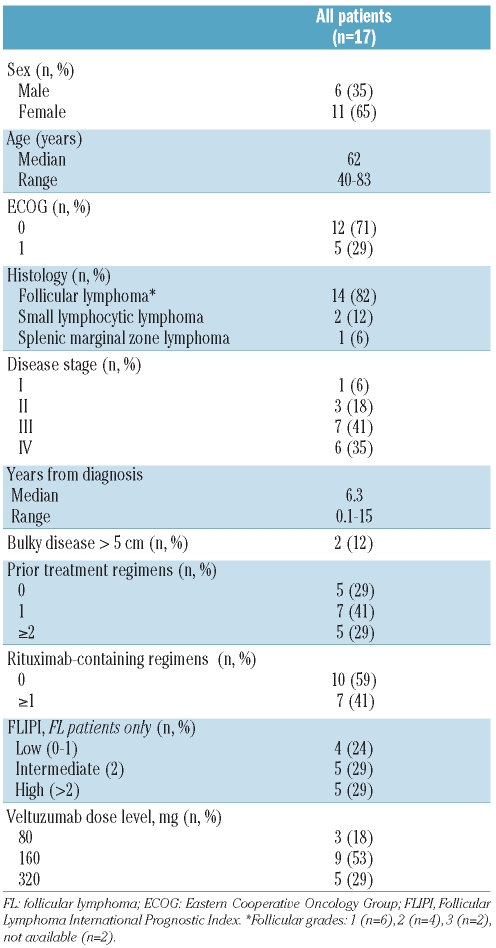
Seventeen patients (14 patients with follicular lymphoma, 3 patients with other indolent histologies) were enrolled between March 2008 and August 2009. All patients met protocol entry criteria except for waivers granted to 2 patients, one admitted despite bulky disease (masses >10 cm) since the risk of tumor lysis following subcutaneous injections was considered low due to slow release and the other with Stage I breast cancer within less than five years, but no evidence of disease. Demographics and patients’ characteristics are summarized in Table 1.
Table 1.
Demographics and baseline characteristics.
Study drug administration
Patients were treated at all 3 dose levels with no occurrences of dose limiting toxicity. All patients completed treatment, receiving 4 subcutaneous doses administered every two weeks at their assigned dose level (Table 1).
Treatment responses
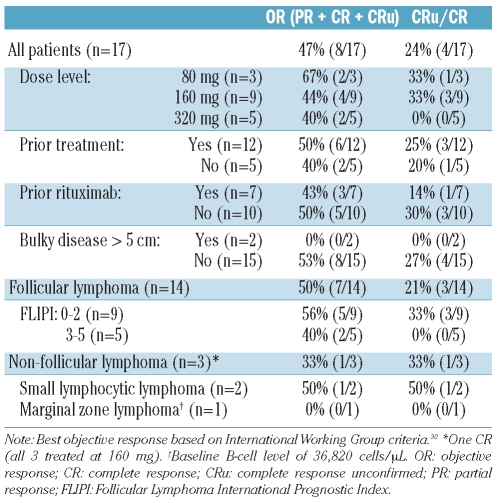
Across all histologies, the objective response (CR + CRu + PR) rate was 47.1% (3 CRs, one CRu, and 4 PRs of 17 patients) with 23.5% (4 of 17) complete response/complete response unconfirmed. The median time to onset of an objective response was 11 weeks from first injection. Of the 8 responders, one with complete response declined follow up after 12 weeks, 3 (one CR, 2 PRs) progressed at 24 weeks, and 4 (one CR, one CRu, 2 PRs) continued in remission for more than 60 weeks, including one patient at 93 weeks. Of the 9 patients who did not achieve an overall response, 2 progressed by the first evaluation four weeks post-treatment. Five of 7 others with stable disease either progressed or withdrew at 12 or 24 weeks, but 2 patients remained progression-free at last evaluations at 48 and 60 weeks. Fourteen of these patients had follicular lymphoma, with 7 (50.0%) achieving an objective response including 3 (21.4%) complete response/complete response unconfirmed, and 2 (14.3%) complete response. Among the 3 patients with other histologies, 2 with high circulating B-cell levels did not achieve an objective response, while the third patient with small lymphocytic lymphoma (SLL) had a complete response continuing 72 weeks after the first subcutaneous injection. Table 2 lists response rates by histology, dose level, and other factors.
Table 2.
Treatment responses.
Adverse events
Thirteen patients had one or more adverse events during the study (Online Supplementary Table S1). Events occurring in more than one patient were injection site reaction (35%), pain in extremity (24%), upper respiratory tract infection (18%), nausea (18%), chills (12%), and dyspnea (12%). One patient had an isolated pulmonary nodule on CT prior to study entry which was confirmed as a squamous cell carcinoma while on study. Otherwise, there were no serious events (SAEs) and all other adverse events were either mild or moderate (i.e. Grade 1 or 2 toxicity). Most events were injection reactions, which occurred in 9 patients: 8 with localized pain or tenderness (n=7) or redness (n=6) at the injection site; 5 had chills or nausea (n=2 each), thoracic pain, general aches, headache, swollen tongue or rash (n=1 each). These were predominantly only mild (Grade 1) transient events that typically resolved either spontaneously or with symptomatic topical or oral medication (no steroids were given). Four patients had infections, but these were all mild (Grade 1) events which involved the upper respiratory tract (n=3) or sinuses (n=1), and were resolved with oral medication.
Safety laboratories
Blood samples for hematology and serum chemistries were obtained before each injection, four and 12 weeks after the last injection, and every three months during follow up. No abnormal pattern of changes occurred (Online Supplementary Tables S2 and S3), and there were no cases of delayed neutropenia (including 10 patients monitored for six months, and 6 patients for one year).
Pharmacokinetics
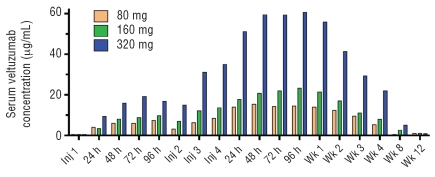
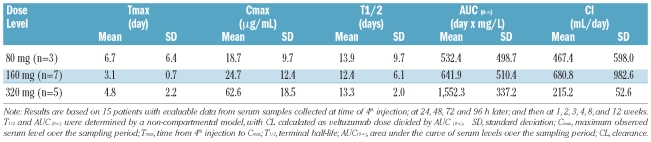
One patient did not undergo the schedule of serum sampling required for pharmacokinetic analysis and another patient with splenic marginal zone lymphoma (MZL) and extremely elevated peripheral blood B-cell levels had unmeasurable serum levels of veltuzumab (< 0.5 μg/mL) at all time points. Pharmacokinetics were analyzed for the other 15 patients, all of whom had measurable veltuzumab levels over the course of treatment. Figure 1 summarizes the mean serum levels for each dose group at scheduled times for serum sampling, whereas Table 3 summarizes pharmacokinetic parameters after the 4th and final injection.
Figure 1.
Mean serum levels of veltuzumab for each dose group. All patients received SC injections once every two weeks for a total of 4 administrations, with cohorts receiving veltuzumab at dose levels of 80 mg, 160 mg, or 320 mg. Serum samples were obtained at time of the 1st injection, 24, 48, 76, and 96 h later; at the 2nd, 3rd and 4th injections, 24, 48, 76 and 96 h after the 14th injection; and then 1, 2, 3, 4, 8, and 12 weeks later. Veltuzumab serum levels were determined by enzyme-linked immunosorbent assay. For plotting purposes, values too low to be measured were assigned the minimum detectable level of the assay (0.5 μg/mL).
Table 3.
Veltuzumab serum levels (pharmacokinetics after 4th injection)
Hematologic changes
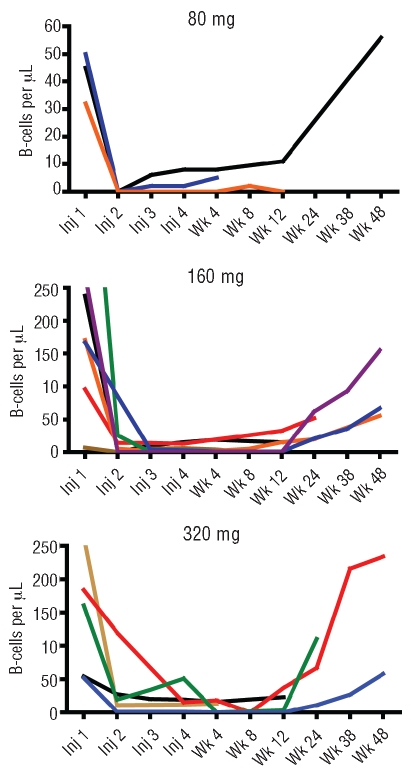
Two patients in this study had leukemic involvement with elevated circulating B-cell levels. The single patient with splenic marginal zone lymphoma had 36,820 cells/μL that decreased by 42% to 21,387 cells/μL over the course of treatment, while one of the 2 patients with small lymphocyctic lymphoma had 2,845 cells/μL that decreased by 98% to 43 cells/μL. Figure 2 graphs the time course of B-cell levels for the other 15 patients all of whom had non-elevated B-cell levels at baseline (7–601 cells/μL). The pattern appears similar for all 3 dose groups, with depletion after first injection and recovery toward baseline by nine to 12 months.
Figure 2.
Circulating B-cell levels for patients having non-elevated levels at study entry. All patients received SC veltuzumab once every two weeks for a total of 4 administrations. Blood samples for B-cell levels were obtained at time of each injection and then 4, 8, 12, 24, 36, and 48 weeks after the 4th administration. Panels show results for cohorts receiving veltuzumab at 320 mg, 160 mg, or 80 mg (note change of scale for the 80 mg dose group).
Quantitative serum immunoglobulins (Ig) and T-cell levels were measured at baseline, at last injection (T cells only), four and 12 weeks later, and in a limited number of patients in subsequent follow up at 24, 36 and 48 weeks. For IgG, IgA, and T cells, median changes from baseline were variable, but small at most time points (typically < 10%). Median IgM levels were consistently decreased, but not clinically significantly, with median decreases at each time point ranging between 16% and 37%.
Immunogenicity (HAHA)
Serum samples were obtained at baseline, prior to the 3rd injection, then four and 12 weeks after the 4th injection, and analyzed for human anti-veltuzumab antibody by ELISA. All samples were negative (<50 ng/mL).
Discussion
By avoiding lengthy intravenous administration and the need for dedicated infusion suites and staff, subcutaneous injections of veltuzumab for treatment of B-cell malignancies may offer benefits to both patients and the healthcare system. Following the demonstration of clinical activity in non-Hodgkin’s lymphoma with intravenous veltuzumab, with complete responses at doses as low as 80–120 mg/m2 weekly for a total of 4 administrations,27 a higher concentration formulation was developed suitable for delivering low doses by subcutaneous injection. In this study, subcutaneous injections of 80–320 mg absolute doses of veltuzumab were found to be convenient and well tolerated. The only treatment related adverse events were local site reactions occasionally accompanied by constitutional symptoms, all transient and mild-moderate events (predominantly Grade 1). Most importantly, there was no evidence of immunogenicity with this route of administration with all serum samples being HAHA-negative.
Subcutaneous injections of low-dose veltuzumab were clinically active with 47% (8/17) achieving an overall response, including 24% (4/17) complete response/complete response unconfirmed. The median time to onset of an objective response of 11 weeks from start of treatment is close to the 3.3-month interval reported with intravenous veltuzumab, indicating that there is no apparent delay of treatment response associated with the subcutaneous route of administration. Objective responses occurred at all dose levels; although only 3 patients were treated with 80 mg for a total of 4 doses, one achieved a complete response. Similarly, responses, including complete response/complete response unconfirmed, occurred in treatment-naïve as well as previously treated patients, including those exposed to rituximab-containing regimens. While almost half of the patients are still in ongoing follow up, 4 of the 8 responders had durable responses of over 60 weeks, and 3 other patients who did not achieve an overall response by International Working Group criteria remained progression-free for at least one year.
The response rates with subcutaneous dosing in follicular non-Hodgkin’s lymphoma are particularly encouraging. While comparisons are limited by the small numbers in this study, the results for the 11 previously treated follicular lymphoma patients (55% OR, 27% CR/CRu) are similar to those obtained in previously treated follicular lymphoma patients who received 4 weekly intravenous doses of veltuzumab at 80–750 mg/m2 (44% OR, 27% CR/Cru),27 and although speculative, these subcutaneous low-dose results (with a CR rate alone of 14%) are also similar to those obtained with 4 doses of once-weekly 375 mg/m2 rituximab in relapsed/refractory indolent non-Hodgkin’s lymphoma, either in rituximab-naïve patients (48% OR, 6% CR)1 or those retreated after previously responding to rituximab (40% OR, 11% CR).31 Two patients with bulky disease (both with follicular lymphoma, one receiving doses of 160 mg and the other of 80 mg) did not achieve an objective response, suggesting that higher doses or more frequent dosing may be required for patients with higher tumor burdens. Only 5 follicular lymphoma patients in the present study had a high risk of poor outcome (3–5 FLIPI scores) and 2 achieved partial responses to therapy; further experience will be needed to evaluate the utility of subcutaneous injections in this important group.
Three patients enrolled in this study had non-follicular lymphoma histologies. One with small lymphocytic lymphoma achieved a complete response, currently ongoing now 72 weeks after treatment. The other 2 patients (one SLL, one MZL) entered with high levels of circulating B cells and failed to achieve an objective response. We have reported that in patients with chronic lymphocytic leukemia (CLL), the same subcutaneous doses and dosing schedule of veltuzumab used here also failed to achieve meaningful clinical benefit, but had evidence of pharmacological activity with transient decreases in the high levels of circulating leukemic B cells.32 For subcutaneous injections, more frequent or extended dosing or combination therapy with other agents will likely be required to overcome the higher antigen burden in these settings, consistent with the experience of other anti-CD20 antibodies which are given intravenously at much higher doses for patients with chronic lymphocytic leukemia.21
Compared to the peak concentrations seen immediately after intravenous infusions, veltuzumab was released slowly into the blood after subcutaneous injections, achieving peak levels only after several days. In spite of the low doses administered here on an every other week dosing schedule, sustained serum levels of veltuzumab were achieved across the treatment period and were still measurable for four to eight weeks afterwards. As expected with this slower release, the mean maximum antibody serum levels of 19, 25 and 63 μg/mL obtained at the 80, 160 and 320 mg for a total of 4 doses, respectively, are generally lower than the maximum values that occurred with bolus delivery of higher intravenous veltuzumab doses.27 Nonetheless, this subcutaneous dosing achieved serum levels that are close to or exceed the 25-μg/mL value associated with maintained efficacy of rituximab,33 thus supporting the clinical activity that was observed at all dose levels in this study. Veltuzumab was pharmacologically active when given by subcutaneous injection. Similar to what was observed with higher intravenous doses of veltuzumab,27 B-cell depletion occurred after the first administration, even at a dose of only 80 mg. Interestingly, the mean terminal half-lives for the three subcutaneous dose levels studied here were similar following the last administration (12.4–13.9 days) and also generally comparable to half-lives previously reported with higher doses of veltuzumab given intravenously (13.3–19.7 days).27 While these pharmacokinetic and pharmacodynamic findings generally support the use of subcutaneous dosing, serum levels of veltuzumab were very low or unmeasurable for 2 patients with leukemic involvement (one splenic MZL, one SLL) whose elevated peripheral B-cell levels decreased, but were not depleted with doses of 160 mg and in an additional patient with follicular lymphoma treated at 80 mg who had extensive adenopathy (including >10 cm masses). These results suggest that while the subcutaneous treatment schedule in this study may be sufficient to cover the antigen sink in most indolent non-Hodgkin’s lymphoma patients, those with greater tumor burden will likely require higher doses or more frequent dosing to overcome leukemic involvement or extensive adenopathy. As mentioned, this is particularly relevant in chronic lymphocytic lymphoma patients.
More than ten years after the initial approval of the first anti-CD20 antibody, rituximab, the relationship between anti-CD20 dose levels and treatment response remains to be clarified.33–35 Most 2nd generation antibodies have pursued doses close to or higher than the 375 mg/m2 dose typically given with rituximab.8–21 However, a proper dose-ranging study has never been reported even though the initial studies of rituximab in non-Hodgkin’s lymphoma had demonstrated that treatment responses could also occur at much lower doses.36,37 In other diseases like immune thrombocytopenia (ITP), with presumably less antigen burden, 100 mg rituximab doses have shown activity,38 and even in the face of the high leukemic burden in chronic lymphocytic leukemia, frequently repeated 20 mg subcutaneous rituximab doses given to 4 patients were shown to be active in one.39 Thus, it is not surprising that low doses of veltuzumab could also be effective in non-Hodgkin’s lymphoma, as shown previously with intravenous administrations27 or here with subcutaneous injections. In addition, subcutaneous injections of veltuzumab at the same low doses used here were also effective in immune thrombocytopenia with doses given only twice, two weeks apart,40 and were capable of transiently decreasing the high levels of circulating leukemic cells in chronic lymphocytic leukemia, when given only 4 times with the same dosing schedule used in the present study.32
In conclusion, this study shows that subcutaneous injections of low-dose veltuzumab given every two weeks for a total of 4 administrations are well tolerated, achieving slow but effective delivery into the blood that is pharmacologically active and capable of achieving durable clinical responses in patients with indolent non-Hodgkin’s lymphoma. The use of anti-CD20 antibodies is likely to continue to grow in both oncology and autoimmune disease indications, including repeated applications for long-term maintenance use. The lengthy time often required for intravenous administration and the need for infusion suites and supervisory staff places great demands on both patients and the healthcare system. Given the convenience and potency of subcutaneous veltuzumab injections, further studies are warranted in diverse CD20+ neoplasms to confirm these initial findings in indolent non-Hodgkin’s lymphoma and to explore other doses and dosing schedules.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 2.Traullé C, Coiffier BB. Evolving role of rituximab in the treatment of patients with non-Hodgkin’s lymphoma. Future Oncol. 2005;1(3):297–306. doi: 10.1517/14796694.1.3.297. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Hagenbeek A. The therapeutic use of rituximab in non-Hodgkin’s lymphoma. Eur J Haematol Suppl. 2007:5–14. doi: 10.1111/j.1600-0609.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheung MC, Haynes AE, Meyer RM, Stevens A, Imrie KR Members of the Hematology, Disease Site Group of the Cancer Care Ontario Program in Evidence-Based Care. Rituximab in lymphoma: a systematic review and consensus practice guideline from Cancer Care Ontario. Cancer Treat Rev. 2007;33(2):161–76. doi: 10.1016/j.ctrv.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-Lopez AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22(23):4711–6. doi: 10.1200/JCO.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346(4):235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 7.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 8.Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–23. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 9.Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27(10):1607–14. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 11.Chambers SA, Isenberg D. Anti-B cell therapy (rituximab) in the treatment of autoimmune diseases. Lupus. 2005;14(3):210–4. doi: 10.1191/0961203305lu2138oa. [DOI] [PubMed] [Google Scholar]
- 12.Silverman GJ. Anti-CD20 therapy and autoimmune disease: therapeutic opportunities and evolving insights. Front Biosci. 2007;12:2194–206. doi: 10.2741/2222. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 14.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 15.Dörner T, Burmester GR. New approaches of B-cell-directed therapy: beyond rituximab. Curr Opin Rheumatol. 2008;20(3):263–8. doi: 10.1097/BOR.0b013e3282f5e08d. [DOI] [PubMed] [Google Scholar]
- 16.Martin P, Furman RR, Ruan J, Elstrom R, Barrientos J, Niesvizky R, et al. Novel and engineered anti–B-cell monoclonal antibodies for non-Hodgkin’s lymphoma. Semin Hematol. 2008;45(2):126–32. doi: 10.1053/j.seminhematol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Czuczman MS, Gregory SA. The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma. 2010;51(6):983–94. doi: 10.3109/10428191003717746. [DOI] [PubMed] [Google Scholar]
- 18.Genovese MC, Kaine JL, Lowenstein MB, Giudice JD, Baldassare A, Schechtman J, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(9):2652–61. doi: 10.1002/art.23732. [DOI] [PubMed] [Google Scholar]
- 19.Morschhauser F, Marlton P, Vitolo U, Lindén O, Seymour JF, Crump M, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol. 2010;21(9):1870–6. doi: 10.1093/annonc/mdq027. [DOI] [PubMed] [Google Scholar]
- 20.Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase I/II trial. Blood. 2008;111(12):5486–95. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 21.Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111(3):1094–100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 22.Robak T. GA-101, a third-generation, humanized and glycol-engineered anti-CD20 mAb for the treatment of B-cell lymphoid malignancies. Curr Opin Investig Drugs. 2009;10(6):588–96. [PubMed] [Google Scholar]
- 23.Stein R, Qu Z, Chen S, Rosario A, Shi V, Hayes M, et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin’s lymphoma. Clin Cancer Res. 2004;10(8):2868–78. doi: 10.1158/1078-0432.ccr-03-0493. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg DM, Rossi EA, Stein R, Cardillo TM, Czuczman MS, Hernandez-Ilizaliturri FJ, et al. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113(5):1062–70. doi: 10.1182/blood-2008-07-168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldenberg DM, Morschhauser F, Wegener WA. Veltuzumab, a novel humanized anti-CD20 monoclonal antibody: Characterization, current clinical results, and future prospects. Leuk Lymphoma. 2010;51(5):747–55. doi: 10.3109/10428191003672123. [DOI] [PubMed] [Google Scholar]
- 26.Milani C, Castillo J. Veltuzumab, an anti-CD20 mAb for the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia and immune thrombocytopenic purpura. Curr Opin Mol Ther. 2009;11(2):200–7. [PubMed] [Google Scholar]
- 27.Morschhauser F, Leonard JP, Fayad L, Coiffier B, Petillon MO, Coleman M, et al. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: Phase I/II results. J Clin Oncol. 2009;27(20):3346–53. doi: 10.1200/JCO.2008.19.9117. [DOI] [PubMed] [Google Scholar]
- 28.Harris NL, Ferry JA. Classification of non-Hodgkin’s lymphomas. In: Knowles DM, editor. Neoplastic Hematopathology. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 691–753. [Google Scholar]
- 29.Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–65. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 31.Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: Safety and efficacy of re-treatment. J Clin Oncol. 2000;18(17):3135–43. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 32.Negrea OG, Allen SL, Rai KR, Elstrom R, Abbasi MR, Farber CM, et al. Subcutaneous injections of low doses of humanized antiCD20 veltuzumab for treatment of indolent B-cell malignancies. Blood. 2009;114(22) [Google Scholar]
- 33.Cartron G, Blasco H, Paintaud G, Watier H, Le Guellec C. Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit Rev in Oncol Hematol. 2007;62(1):43–52. doi: 10.1016/j.critrevonc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Berinstein NL, Grillo-López AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9(9):995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 35.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44(16):3823–37. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 36.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–66. [PubMed] [Google Scholar]
- 37.Maloney DG, Grillo-López AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: Results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma, relapsed low-grade or follicular B-cell lymphoma. J Clin Oncol. 1997;15(10):3266–74. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 38.Provan D, Butler T, Evangelista ML, Amadori S, Newland AC, Stasi R. Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica. 2007;92(12):1695–8. doi: 10.3324/haematol.11709. [DOI] [PubMed] [Google Scholar]
- 39.Aue G, Lindorfer MA, Beum PV, Pawluczkowycz AW, Vire B, Hughes T, et al. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica. 2010;95(2):329–32. doi: 10.3324/haematol.2009.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh MN, Liebman H, Bernstein ZP, Negrea OG, Bussel JB, Onyegbula AC, et al. Subcutaneous injections of low-dose anti-CD20 veltuzumab for treatment of relapsed immune thrombocytopenia (ITP) Blood. 2009;114(22) [Google Scholar]