Abstract
Introduction
[13N]ammonia PET allows quantification of myocardial perfusion. The similarity between peripheral flow and myocardial perfusion is unclear. We compared perfusion flow in the myocardium with the upper limb during rest and adenosine stress [13N]ammonia PET to establish whether peripheral perfusion reserve (PPR) correlates with MPR.
Methods
[13N]ammonia myocardial perfusion PET-scans of 58 patients were evaluated (27 men, 31 women, age 64 ± 13 years) and were divided in four subgroups: patients with coronary artery disease (CAD, n = 15), cardiac syndrome X (SX, n = 14), idiopathic dilating cardiomyopathy (DCM, n = 16), and normal controls (NC, n = 13). Peripheral limb perfusion was measured in the muscular tissue of the proximal upper limb and quantified through a 2-tissue-compartment model and the PPR was calculated (stress/rest ratio). MPR was also calculated by a 2-tissue-compartment model. The PPR results were compared with the MPR findings.
Results
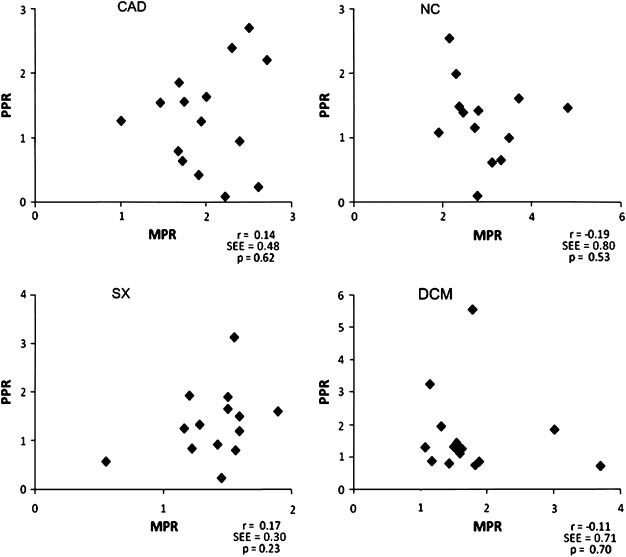
Mean myocardial perfusion increased significantly in all groups as evidenced by the MPR (CAD 1.99 ± 0.47; SX 1.39 ± 0.31; DCM 1.72 ± 0.69; NC 2.91 ± 0.78). Mean peripheral perfusion also increased but not significantly and accompanied with great variations within and between groups (mean PPR: CAD 1.30 ± 0.79; SX 1.36 ± 0.71; DCM 1.60 ± 1.22; NC 1.27 ± 0.63). The mean difference between PPR and MPR for all subpopulations varied widely. No significant correlations in flow reserve were found between peripheral and myocardial microcirculatory beds in any of the groups (Total group: r = −0.07, SEE = 0.70, CAD: r = 0.14, SEE = 0.48, SX: r = 0.17, SEE = 0.30, DCM: r = −0.11, SEE = 0.71, NC: r = −0.19, SEE = 0.80).
Conclusion
No correlations between myocardial and peripheral perfusion (reserve) were found in different patient populations in the same PET session. This suggests a functional difference between peripheral and myocardial flow in the response to intravenously administered adenosine stress.
Keywords: Myocardial perfusion imaging, peripheral perfusion imaging, coronary flow reserve, vasodilator stress, N-13 ammonia
Introduction
Evaluation of systemic arterial dilatory function, mostly by ultrasonography, is a widely available technique. Its findings have been shown to predict coronary artery disease.1 For the microvascular component of myocardial and peripheral perfusion, these correlations are less obvious and probably different.2 Patients with coronary artery disease (CAD) risk factors such as hypercholesterolemia and diabetes mellitus exhibit not only large-vessel dysfunction but also microvascular dysfunction, e.g., reduced responses to vasoactive substances such as adenosine and dipyridamole.3 PET is an accurate technique for the quantification of myocardial perfusion reserve (MPR). An earlier PET study showed no correlation between Doppler ultrasound flow measurement in the forearm and dipyridamole-induced myocardial hyperemia,2 whereas another study comparing acetylcholine-induced myocardial hyperemia to brachial artery dilation did find some weak correlation.4 A possible limitation of these previous studies is the differences in technique between the myocardial and the peripheral measurements. The microvascular component of myocardial and peripheral perfusion may however differ also. To evaluate this possible limitation we studied the peripheral and myocardial perfusion in rest [13N]ammonia PET and during adenosine stress [13N]ammonia PET and calculated the MPR and peripheral perfusion reserve (PPR) in patients with documented coronary artery disease (CAD), microcardiovascular disease (cardiac syndrome X, SX), idiopathic dilating cardiomyopathy (DCM), and normal controls (NC). By including three separate clinical entities we intended also to compare the differences between atherosclerotic macro- and microvascular disease (CAD) and microvascular disease without documented macrovascular disease as found in cardiac syndrome X and idiopathic DCM and ascertain whether peripheral perfusion responses were more strongly linked to any of the underlying pathologies. Perfusion reserve is an important parameter for prognosis in patients with cardiovascular disease5 and will be used in this study.
The aim of this study is to evaluate the correlation between the myocardial and the peripheral perfusion reserve when tested during the same PET perfusion session.
Methods
Study Population
The study population comprised 58 subjects retrospectively, submitted for cardiac [13N]ammonia PET analysis in our centre between 1995 and 2003, whose scans allowed for calculation of perfusion in an appropriate area of the proximal upper limb. These patients were divided into four groups: (I) 15 patients (9 male, 6 female) with documented CAD, defined as wall irregularities on coronary angiogram; (II) 14 patients (4 males, 10 females) with SX, defined as typical chest pain, positive exercise ECG-changes and a normal coronary angiogram; (III) 16 patients (12 males, 4 females) with idiopathic DCM without prior evidence of CAD or myocardial infarction; and (IV) 13 healthy control subjects (2 males, 11 females) without cardiac or pulmonary disease.
Patients’ current medication was continued, except for dipyridamole and diuretics due to interaction with adenosine and to prevent urinary urge during acquisition, respectively. Characteristics of each group are shown in Table 1.
Table 1.
Patients’ characteristics
| CAD | SX | DCM | Control | |
|---|---|---|---|---|
| N | 15 | 14 | 16 | 13 |
| Mean age (range) | 64 (50–81) | 55 (34–76) | 78 (45–91) | 58 (48–73) |
| Use of β-blocker (%) | 100 | 21 | 75 | 54 |
| Use of ACE-inhibitor (%) | 67 | 36 | 81 | 8 |
| Use of vasodilator (%) | 13 | 57 | 50 | 38 |
| Use of statin (%) | 53 | 43 | 0 | 15 |
| Use of diuretics (%) | 27 | 14 | 56 | 14 |
| Known with diabetes (%) | 0 | 7 | 6 | 8 |
CAD, Coronary artery disease; SX, syndrome X, DCM, dilating cardiomyopathy
Acquisition Protocol
PET scans were performed on an ECAT EXACT HR + PET camera (Siemens Medical Systems, Knoxville, TN, USA). Positioning of the subjects was done with the aid of a rectilinear scan. Photon attenuation was measured using an external ring source filled with 68Ge/68Ga and data were automatically corrected for accidental coincidence and dead time. Patients were continuously monitored with 12-lead electrocardiography and blood pressure was obtained every 10 minutes. During adenosine-induced stress, blood pressure was measured every minute. Myocardial perfusion was measured using [13N]ammonia as a perfusion tracer and adenosine as the pharmacological stress agent. After injection of 400 MBq of [13N]ammonia a dynamic rest imaging was performed for 15 minutes. Adenosine stress imaging was performed identically after 140 μg/kg/min of adenosine infusion 3 minutes prior to and 3 minutes following the injection of 400 MBq of [13N]ammonia.
Measurement of Myocardial Perfusion
The quantification of myocardial perfusion has been described previously.6 In summary, [13N]ammonia was injected intravenously over 30 seconds while acquisition of a dynamic sequence of images to obtain time-activity curves from the blood pool and from the myocardial tissue was started. 17 regions of interest (ROIs) were placed within the left ventricular myocardium in the three territories of the major coronary arteries. These ROIs were subsequently copied to the dynamic image sequence. In this way, myocardial tissue time-activity curves for [13N]ammonia were obtained. The arterial input function was obtained from a small ROI in the left ventricular blood pool. Reproducibility has been assessed previously.7 Myocardial perfusion was calculated by fitting the corrected tissue and blood pool time-activity curves to a validated 2-tissue-compartment model for [13N]ammonia using the Hutchins model.8
Measurement of Peripheral Perfusion
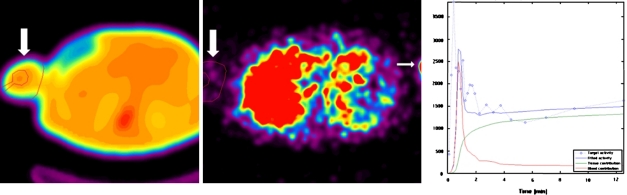
For each study transmission images were used to draw six ROIs around the upper limb (opposite to the injected arm) in six consecutive slices, excluding the areas of highest density correlating with the location of bone. These ROIs were then copied to the dynamic sequences to obtain time-activity curves for [13N]ammonia in the peripheral vascular bed. This is illustrated in Figure 1. A small ROI in the left ventricular blood pool was used for the arterial input function. Peripheral perfusion was calculated by fitting the tissue and blood pool time-activity curves to the aforementioned 2-tissue-compartment model for [13N]ammonia. Since the myocardial perfusion and peripheral perfusion were calculated in the same studies, all circumstances (blood pressure, heart rate) were equal for both measurements.
Figure 1.
Measurement of peripheral perfusion. Regions of interest (ROI) are drawn on the transmission dataset in the upper limb, excluding the area of the humerus. These ROI are copied to the perfusion dataset (large arrows) and the measured time/activity curve is fitted to the compartment model for [13N] ammonia. In the opposite arm the influx of activity from the infusion site is just visible (small arrow)
Perfusion Reserve Calculation
Myocardial and peripheral perfusion flow reserves were calculated by dividing the perfusion results of the adenosine [13N]ammonia stress study by the results of the [13N]ammonia rest study.
Statistical Analysis
The mentioned values are the mean values ± SD. standard error of estimates (SEE) was added in graphs. A paired t test or the nonparametric Wilcoxon signed rank test was used to compare individual values. Correlations were sought by standard linear regression. A P value <.05 was considered significant.
Results
Hemodynamics
The hemodynamic values are listed in Table 2 for the four patient groups. During adenosine infusion, there were no statistically significant changes in blood pressure in any of the groups. The heart rate and accordingly the rate-pressure product increased in all groups, though the increase was not statistically significant in the SX-group. The highest increase was noted in the DCM-group, showing a mean rise in heart rate of 50%, as opposed to the other groups which all showed less than 25% increase in heart rate.
Table 2.
Hemodynamic values
| CAD | SX | DCM | Control | |
|---|---|---|---|---|
| Heart rate at rest per minute | 64 ± 10 | 82 ± 17 | 76 ± 9 | 65 ± 7 |
| Heart rate at stress per minute | 72 ± 11* | 85 ± 23 | 114 ± 10* | 81 ± 14* |
| Systolic blood pressure at rest (mmHg) | 131 ± 16 | 159 ± 3 | 102 ± 5 | 132 ± 9 |
| Systolic blood pressure at stress (mmHg) | 136 ± 15 | 144 ± 8 | 95 ± 6 | 132 ± 13 |
| Diastolic blood pressure at rest (mmHg) | 75 ± 11 | 89 ± 9 | 57 ± 6 | 78 ± 7 |
| Diastolic blood pressure at stress (mmHg) | 79 ± 8 | 85 ± 8 | 66 ± 7 | 76 ± 7 |
| Rate-pressure product at rest | 8340 | 11939 | 7752 | 8526 |
| Rate-pressure product at stress | 9757* | 12166 | 10830* | 10704* |
CAD, Coronary artery disease; SX, syndrome X, DCM, dilating cardiomyopathy
All values mean ± SD
* Statistically significant increase compared to rest (P < 0.05)
Myocardial Perfusion (Reserve)
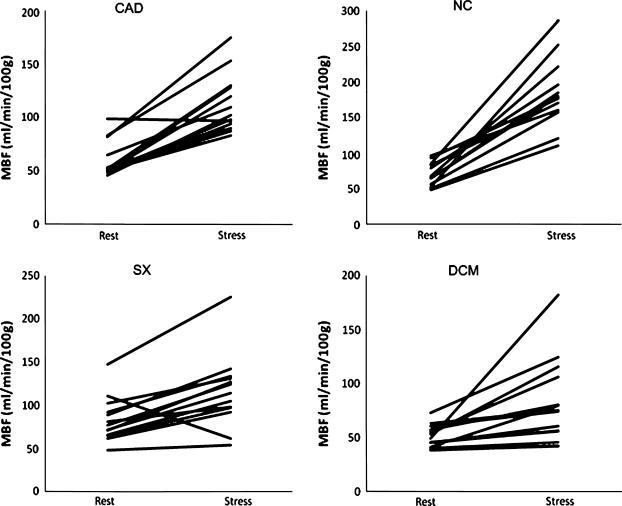
The mean resting flow in the myocardium in mL/min/100 g per group was: CAD 60.3 ± 16.2; SX 83.4 ± 24.6; DCM 50.4 ± 9.8; and NC 70.3 ± 15.8. During adenosine infusion, the measurements were: CAD 112.8 ± 26.4; SX 115.8 ± 40.6; DCM 80.1 ± 36.9; and NC 184.7 ± 47.6. All these changes were highly significant (CAD: P = .007, SX: P = .002, DCM: P = .005, NC: P = .005). The resulting calculated MPR per group was: CAD 1.99 ± 0.47; SX 1.39 ± 0.31; DCM 1.72 ± 0.69; NC 2.91 ± 0.78. The changes in myocardial perfusion between rest and pharmacological stress are shown in Figure 2.
Figure 2.
Myocardial perfusion at rest vs during adenosine stress. CAD, Coronary artery disease; NC, normal controls; SX, syndrome X; DCM, dilating cardiomyopathy; MBF, myocardial blood flow
Peripheral Perfusion (Reserve)
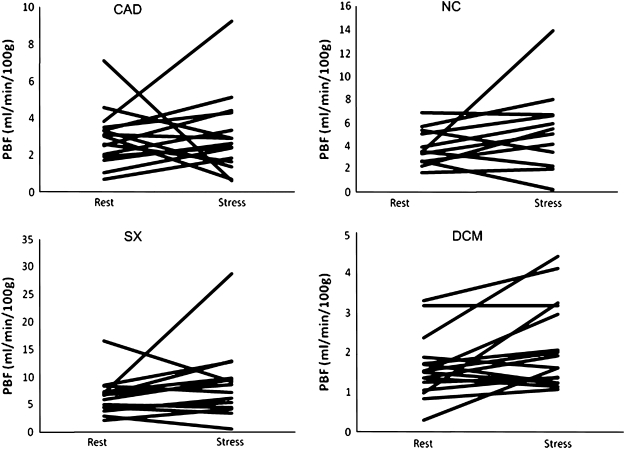
The mean resting flow in the peripheral vasculature in mL/min/100 g per group was: CAD 3.0 ± 1.5; SX 6.5 ± 3.4; DCM 1.6 ± 0.8; and NC 3.9 ± 1.5. During adenosine infusion, the measurements were: CAD 3.1 ± 2.1; SX 8.8 ± 6.5; DCM 2.2 ± 1.0; and NC 5.3 ± 3.4. None of these changes were significant, nor were the differences between the groups. The resulting calculated PPR per group was: CAD 1.30 ± 0.79; SX 1.36 ± 0.71; DCM 1.60 ± 1.22; NC 1.27 ± 0.63. No correlation was found with the rate-pressure product. The changes in peripheral perfusion between rest and pharmacological stress are shown in Figure 3.
Figure 3.
Peripheral perfusion at rest vs during adenosine stress. CAD, Coronary artery disease; NC, normal controls; SX, syndrome X; DCM, dilating cardiomyopathy; PBF, peripheral blood flow
Comparison Between the Different Groups
Myocardial blood flow during adenosine infusion was significantly higher in normal controls compared to all other groups (P < .001 vs all groups), as was the MPR (P ≤ .001 vs all groups).
Myocardial blood flow was significantly higher in SX compared to DCM (P = .03) and in CAD compared to DCM (P = .02). The difference between CAD and SX was not significant.
The MPR was significantly higher in CAD compared to SX (P = .001) whereas all other groups comparisons showed no significant differences.
Peripheral blood flow during adenosine infusion was significantly higher when comparing NC to DCM (P = .015), SX to CAD (P = .014), and SX to DCM (P = .005), other comparisons showed no significant differences.
There were no significant differences in PPR between any of the groups.
Correlation Between Myocardial and Peripheral Blood Flow During Adenosine Infusion
No significant correlations were found between the two circulatory beds during adenosine infusion for the group as a whole (r = 0.13, SEE = 54.32, P = .36) or any of the subgroups (CAD: r = 0.03, SEE = 1.46; SX: r = 0.16, SEE = 43.57; DCM: r = −0.35, SEE = 37.15; NC: r = −0.31, SEE = 49.57).
Correlation Between MPR and PPR
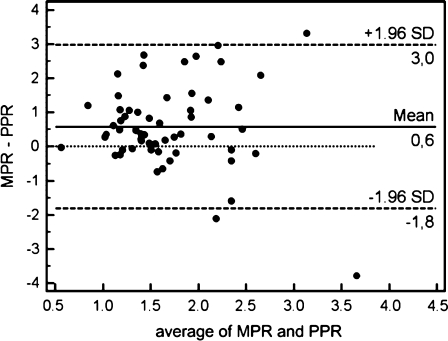
The correlations between the myocardial perfusion reserve and the peripheral perfusion reserve in the separate groups are shown in Figure 4. No correlations were found in the whole group (r = −0.07, SEE = 0.70, P = .47), or for any of the four separate groups (values provided in Figure 4). Figure 5 shows the Bland-Altman plot for the comparison in the whole group.
Figure 4.
Correlations between MPR and PPR. CAD, Coronary artery disease; NC, normal controls; SX, syndrome X; DCM, dilating cardiomyopathy; PPR, peripheral perfusion reserve; MPR, myocardial perfusion reserve
Figure 5.
Bland-Altman plot for MPR/PPR comparison
Discussion
In our study, we evaluated the effects of vasodilator-stress between the two vascular beds in various underlying cardiovascular pathologies.
Our data showed that during the same dose of i.v. adenosine infusion the response in perfusion in the heart and the musculature of the upper limb is not related. Although we noted a minor increase in blood flow in the upper limb, this response was not statistically significant and showed no differences between normal controls and the three patient groups.
There has been an interest in the past in the correlation between abnormalities in the coronary and the peripheral vasculature. Endothelial dysfunction has been linked to atherosclerotic risk factors, paving the way for ultrasound-based assessment of endothelial dysfunction. It has been shown that endothelial dysfunction in the brachial artery has a predictive value for coronary artery disease.1
The resting myocardial perfusion values and the hemodynamic response to adenosine infusion in our patient groups were comparable to other findings,9-11 even though changes in the SX-group were not statistically significant. The myocardial blood flow response to vasodilator-stress in our four patient groups was also comparable to other studies.3,5,12,13 As expected, coronary perfusion increased more than 2-fold in normal controls, whereas in the three patient groups with vascular disease these diseases blocked an adequate increase in blood flow. In the group with CAD, inadequate blood flow response was more localized due to the relatively focal lesion of the vasculopathy when compared to the SX and DCM groups. As the blood flow response is normal in non-atherosclerotic vessels in patients with CAD, in contrast to generally diseased myocardium in SX and DCM, the global MPR in CAD will be less affected. The relatively low MPR found in the SX group is at least partially based on a higher blood flow in rest, presumably due to the inherently higher metabolic rate in these patients. A higher resting flow further decreases the ratio between rest and stress flow measurements, explaining why the MPR in this group was lower than in any of the other groups even though the blood flow during adenosine infusion was comparable to that of the CAD group.
The resting blood flow in the peripheral circulation, in our case mostly in the biceps and triceps muscles, was also comparable to findings in different peripheral muscles in other studies.14,15
A major difference between our study and previous described literature about the relation between myocardial and peripheral blood flow is the used method of a single session adenosine infusion. Most other studies used ischemia-driven blood flow increase in the upper limb after a period of occlusion, usually by means of an inflatable cuff, coupled with hand exercises.
Though the vasodilative effects of adenosine have been reported over 8 decades ago,16 the precise mechanism through which it induces vasodilation is still not completely understood. Biaggioni wrote a commentary17 where he listed a number of mechanisms that may play a role in the vasodilative effects of adenosine, pointing out that the findings of various studies on the subject are contradictory. In an overview article on the role of PET in the understanding of coronary physiology, Schindler et al18 describe the mechanism of dipyridamole and adenosine as being a mixture of predominantly endothelium-independent flow responses, with shear sensitive endothelial components adding to these responses. Adenosine-induced hyperemia is therefore partially endothelium-independent and partially endothelium-dependent.
It is therefore possible that the responses elicited with intravenous adenosine differ significantly from other vasodilative stimuli such as hypoxia (mostly myogenic with supposed added effects from prostaglandins and adenosine), acetylcholine (fully endothelium dependent), or papaverine (smooth muscle relaxation) due to the different pathways that govern each of these responses, and that these responses in turn differ between the coronary and the systemic circulation. Anderson et al4 did find some weak correlation between myocardial vessel response to acetylcholine and flow-mediated brachial artery diameter measurements, though with significant scatter in their findings. In this study, sublingual nitroglycerin, a non-endothelium dependent vasodilator, did not show the same effect as reactive hyperemia, showing the variability in response to differing vasodilators.
Even dipyridamol elicits a different response to purely intravenous adenosine as it increases not only the intravenous but also the interstitial levels of adenosine. This affects the peripheral vasculature in a different way than purely intravenous administration of adenosine does, as adenosine concentrations are raised concomitantly on both sides of the vascular wall.
In conscious subjects, systemic infusion of adenosine has been shown to stimulate sympathetic tone, most probably due to stimulation of arterial chemoreceptors.19 Whether this affects flow in myocardial and peripheral vascular beds differently is not known.
Our data do suggest a possible explanation for the differences between the two perfusion reserves, in that resting flow in the myocardial vasculature is about 20 times higher than in the periphery. As the total delivered dose of adenosine is flow-dependent, the dose delivered to the myocardial vessels will also be about 20 times higher than peripherally. This may account for the minimal effect of adenosine on the peripheral blood flow in all four patient groups, as a threshold dose may not be reached in the periphery due to the significantly lower flow in the resting phase.
Few data exist on peripheral blood flow during intravenous adenosine stress only, especially as measured with PET. Heinonen et al14 in their study using [15O]-H2O as the PET perfusion tracer, reported an increase in peripheral muscle perfusion during adenosine infusion comparable to exercise that was absent in our study population. However, in their study adenosine was infused directly into the femoral artery which differs from our intravenous infusion. They also reported a decline in blood flow in the opposite limb during adenosine infusion, indicating that the hyperemic response to adenosine was localized rather than widespread throughout the body after passing into the systemic circulation.
Pedrinelli et al20 found that in patients with systemic hypertension the peripheral vascular reserve was lower than the myocardial reserve without correlation between the two values. This patient group is an interesting comparison with the patient groups with different included pathologies in our study. Again due to the retrospective nature of our study, we could not specify such a subgroup of patients with hypertension for inclusion. It is difficult to hypothesize about any relation between our current findings and those of Pedrinelli et al, because peripheral vasodilation was based on postischemic hyperemia as opposed to adenosine infusion.
Conclusion
In this study, we confirmed that perfusion reserves of the myocardial and the peripheral microvasculature during the same PET session were different, indicating different levels of response for the two vascular beds during the same intravenous adenosine infusion session.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
See related editorial, doi:10.1007/s12350-011-9357-0
References
- 1.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, Udelson JE, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38:1843–1849. doi: 10.1016/S0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 2.Bottcher M, Madsen MM, Refsgaard J, Buus NH, Dorup I, Nielsen TT, et al. Peripheral flow response to transient arterial forearm occlusion does not reflect myocardial perfusion reserve. Circulation. 2001;103:1109–1114. doi: 10.1161/01.cir.103.8.1109. [DOI] [PubMed] [Google Scholar]
- 3.Pitkanen OP, Raitakari OT, Niinikoski H, Nuutila P, Iida H, Voipio-Pulkki LM, et al. Coronary flow reserve is impaired in young men with familial hypercholesterolemia. J Am Coll Cardiol. 1996;28:1705–1711. doi: 10.1016/S0735-1097(96)00376-2. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 5.Tio RA, Dabeshlim A, Siebelink HM, de SJ, Hillege HL, Zeebregts CJ, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214–219. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 6.Blanksma PK, Willemsen AT, Meeder JG, de Jong RM, Anthonio RL, Pruim J, et al. Quantitative myocardial mapping of perfusion and metabolism using parametric polar map displays in cardiac PET. J Nucl Med. 1995;36:153–158. [PubMed] [Google Scholar]
- 7.Nagamachi S, Czernin J, Kim AS, Sun KT, Bottcher M, Phelps ME, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med. 1996;37:1626–1631. [PubMed] [Google Scholar]
- 8.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-J. [DOI] [PubMed] [Google Scholar]
- 9.Mamede M, Tadamura E, Hosokawa R, Ohba M, Kubo S, Yamamuro M, et al. Comparison of myocardial blood flow induced by adenosine triphosphate and dipyridamole in patients with coronary artery disease. Ann Nucl Med. 2005;19:711–717. doi: 10.1007/BF02985121. [DOI] [PubMed] [Google Scholar]
- 10.Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62–69. doi: 10.1161/01.cir.88.1.62. [DOI] [PubMed] [Google Scholar]
- 11.Bottcher M, Czernin J, Sun K, Phelps ME, Schelbert HR. Effect of beta 1 adrenergic receptor blockade on myocardial blood flow and vasodilatory capacity. J Nucl Med. 1997;38:442–446. [PubMed] [Google Scholar]
- 12.van den Heuvel AF, van Veldhuisen DJ, van der Wall EE, Blanksma PK, Siebelink HM, Vaalburg WM, et al. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;35:19–28. doi: 10.1016/S0735-1097(99)00499-4. [DOI] [PubMed] [Google Scholar]
- 13.Tio RA, Slart RH, de Boer RA, van der Vleuten PA, de Jong RM, van Wijk LM, et al. Reduced regional myocardial perfusion reserve is associated with impaired contractile performance in idiopathic dilated cardiomyopathy. Neth Heart J. 2009;17:470–474. doi: 10.1007/BF03086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, et al. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol. 2010;108:378–386. doi: 10.1152/japplphysiol.00745.2009. [DOI] [PubMed] [Google Scholar]
- 15.Kalliokoski KK, Kemppainen J, Larmola K, Takala TO, Peltoniemi P, Oksanen A, et al. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur J Appl Physiol. 2000;83:395–401. doi: 10.1007/s004210000267. [DOI] [PubMed] [Google Scholar]
- 16.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biaggioni I. Clinical and molecular pharmacologic characteristics of adenosine-induced vasodilation. Clin Pharmacol Ther. 2004;75:137–139. doi: 10.1016/j.clpt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Schindler TH, Zhang XL, Vincenti G, Mhiri L, Lerch R, Schelbert HR. Role of PET in the evaluation and understanding of coronary physiology. J Nucl Cardiol. 2007;14:589–603. doi: 10.1016/j.nuclcard.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biaggioni I, Killian TJ, Mosqueda-Garcia R, Robertson RM, Robertson D. Adenosine increases sympathetic nerve traffic in humans. Circulation. 1991;83:1668–1675. doi: 10.1161/01.cir.83.5.1668. [DOI] [PubMed] [Google Scholar]
- 20.Pedrinelli R, Dell’Omo G, Gimelli A, Di Bello V, Talarico L, Corchia A, et al. Myocardial and forearm blood flow reserve in mild-moderate essential hypertensive patients. J Hypertens. 1997;15:667–673. doi: 10.1097/00004872-199715060-00013. [DOI] [PubMed] [Google Scholar]