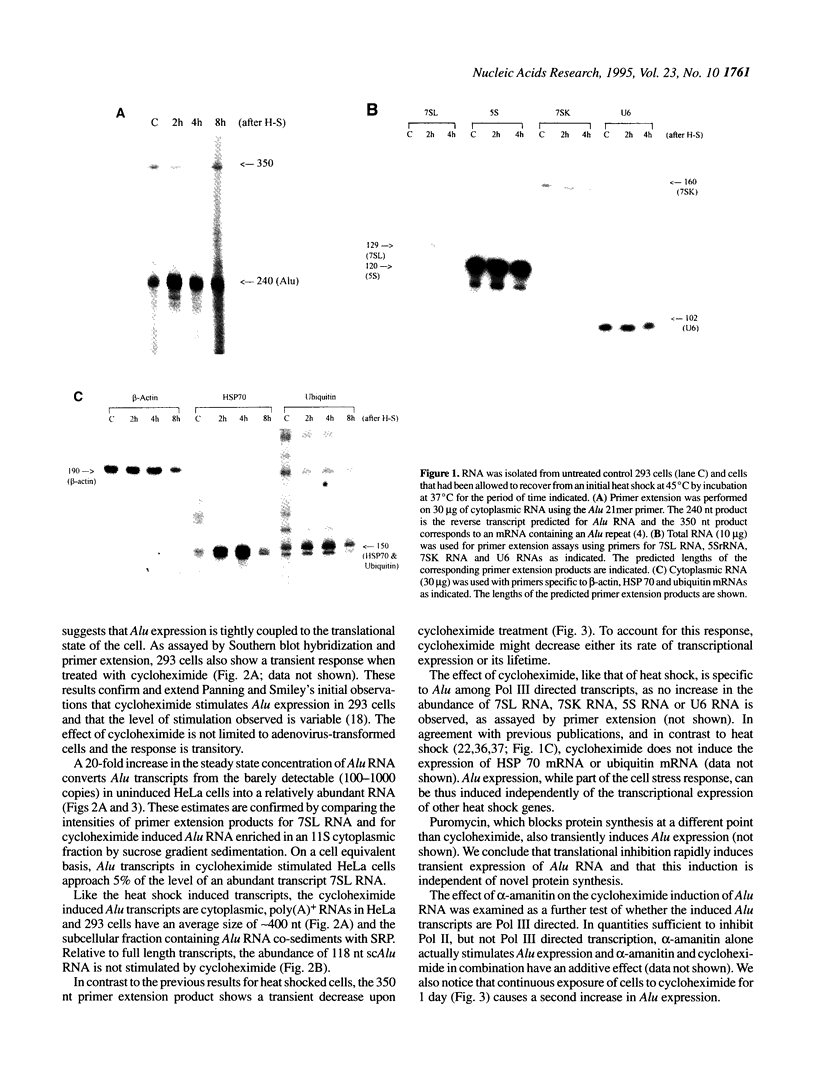
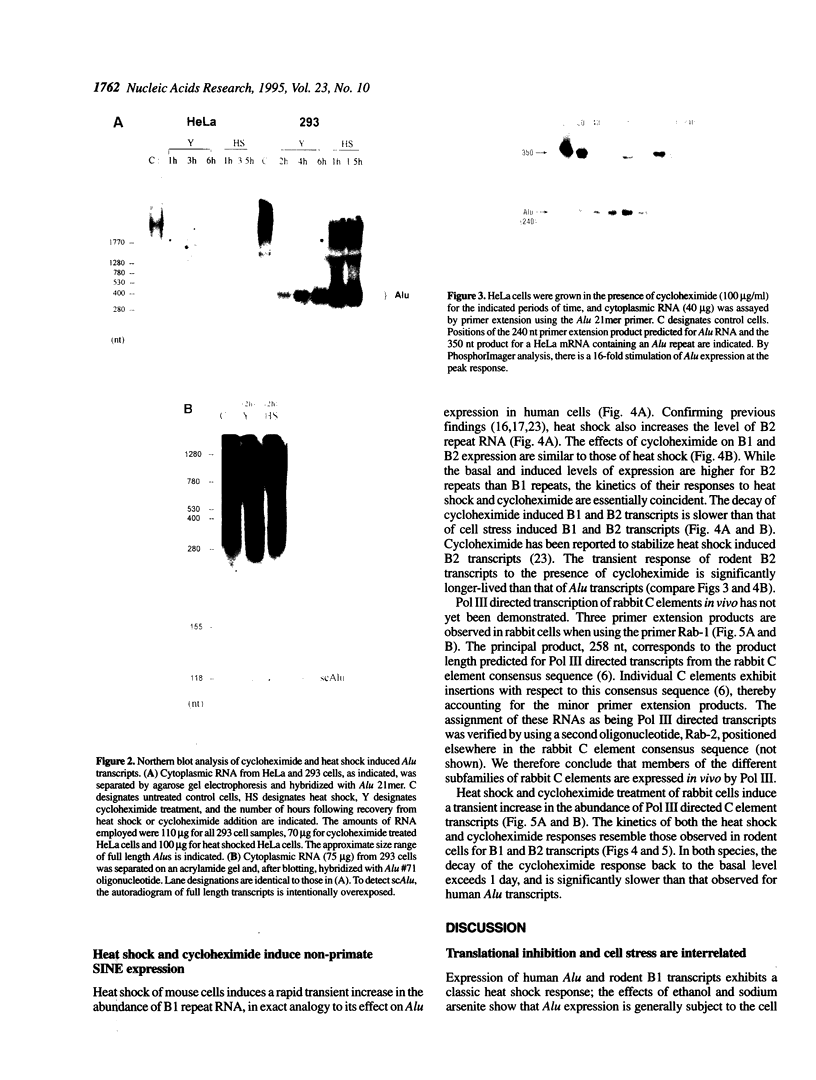
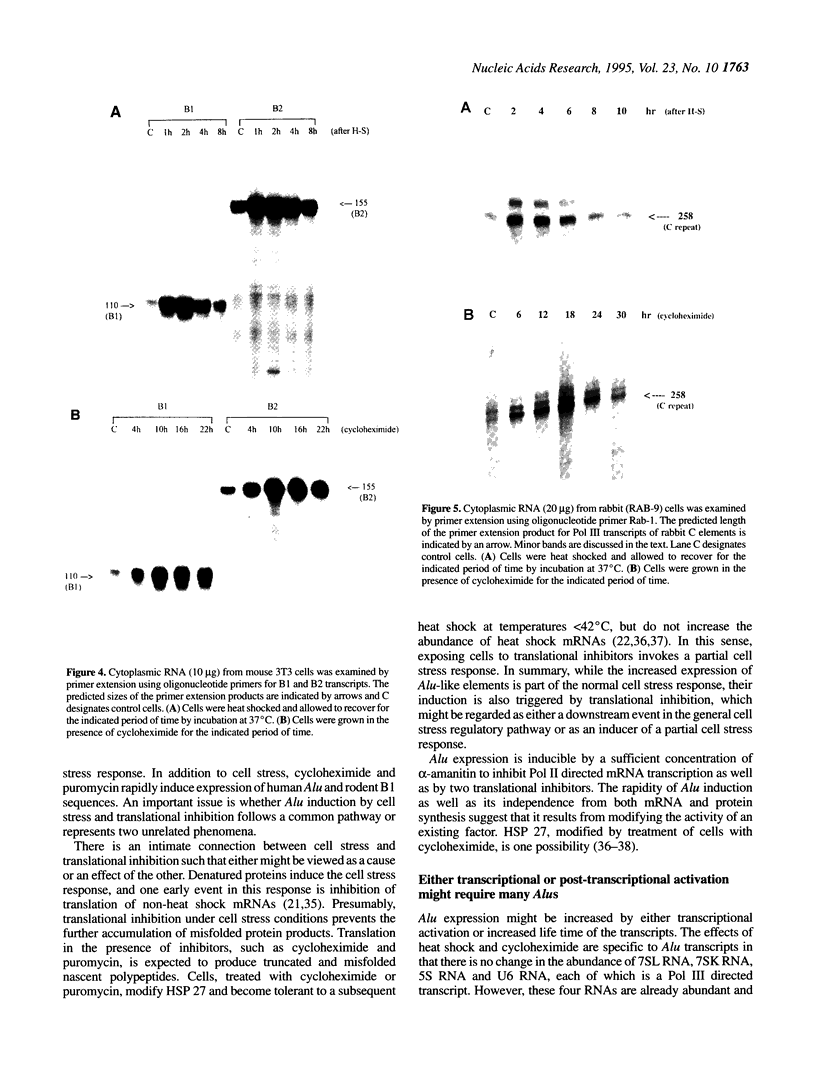
Abstract
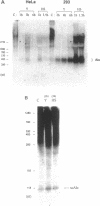
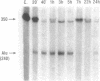
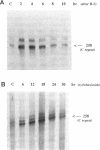
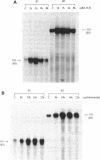
The abundance of Alu RNA is transiently increased by heat shock in human cell lines. This effect is specific to Alu repeats among Pol III transcribed genes, since the abundance of 7SL, 7SK, 5S and U6 RNAs is essentially unaffected by heat shock. The rapid induction of Alu expression precedes the heat shock induction of mRNAs for the ubiquitin and HSP 70 heat shock genes. Heat shock mimetics also transiently induce Alu expression indicating that increased Alu expression is a general cell-stress response. Cycloheximide treatment rapidly and transiently increases the abundance of Alu RNA. Again, compared with other genes transcribed by Pol III, this increase is specific to Alu. However, as distinguished from the cell stress response, cycloheximide does not induce expression of HSP 70 and ubiquitin mRNAs. Puromycin also increases Alu expression, suggesting that this response is generally caused by translational inhibition. The response of mammalian SINEs to cell stress and translational inhibition is not limited to SINEs which are Alu homologues. Heat shock and cycloheximide each transiently induce Pol III directed expression of B1 and B2 RNAs in mouse cells and C-element RNA in rabbit cells. Together, these three species exemplify the known SINE composition of placental mammals, suggesting that mammalian SINEs are similarly regulated and may serve a common function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baler R., Welch W. J., Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J Cell Biol. 1992 Jun;117(6):1151–1159. doi: 10.1083/jcb.117.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredow S., Sürig D., Müller J., Kleinert H., Benecke B. J. Activating-transcription-factor (ATF) regulates human 7S L RNA transcription by RNA polymerase III in vivo and in vitro. Nucleic Acids Res. 1990 Dec 11;18(23):6779–6784. doi: 10.1093/nar/18.23.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. Y., Nelson B., Bilyeu T., Hsu K., Darlington G. J., Maraia R. J. A human Alu RNA-binding protein whose expression is associated with accumulation of small cytoplasmic Alu RNA. Mol Cell Biol. 1994 Jun;14(6):3949–3959. doi: 10.1128/mcb.14.6.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crête P., Landry J. Induction of HSP27 phosphorylation and thermoresistance in Chinese hamster cells by arsenite, cycloheximide, A23187, and EGTA. Radiat Res. 1990 Mar;121(3):320–327. [PubMed] [Google Scholar]
- Doran J. L., Bingle W. H., Roy K. L. The nucleotide sequences of two human 5S rRNA pseudogenes. Nucleic Acids Res. 1987 Aug 11;15(15):6297–6297. doi: 10.1093/nar/15.15.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander E. W., Wolffe A. P., Howard B. H. Nucleosome interactions with a human Alu element. Transcriptional repression and effects of template methylation. J Biol Chem. 1993 Sep 15;268(26):19565–19573. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Induction of heat shock protein transcripts and B2 transcripts by various stresses in Chinese hamster cells. Exp Cell Res. 1989 May;182(1):61–74. doi: 10.1016/0014-4827(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Ubiquitin mRNA is a major stress-induced transcript in mammalian cells. Nucleic Acids Res. 1989 Feb 11;17(3):1215–1230. doi: 10.1093/nar/17.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Mitchell J. B. Induction of B2 RNA polymerase III transcription by heat shock: enrichment for heat shock induced sequences in rodent cells by hybridization subtraction. Nucleic Acids Res. 1986 Jul 25;14(14):5793–5811. doi: 10.1093/nar/14.14.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Hayashi K. Organization of sequences related to U6 RNA in the human genome. Nucleic Acids Res. 1981 Jul 24;9(14):3379–3388. doi: 10.1093/nar/9.14.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann-Blumberg U., Hintz M. F., Gatewood J. M., Schmid C. W. Developmental differences in methylation of human Alu repeats. Mol Cell Biol. 1993 Aug;13(8):4523–4530. doi: 10.1128/mcb.13.8.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K. L., Latchman D. S. HSV infection induces increased transcription of Alu repeated sequences by RNA polymerase III. FEBS Lett. 1989 Dec 4;258(2):255–258. doi: 10.1016/0014-5793(89)81667-9. [DOI] [PubMed] [Google Scholar]
- Kochanek S., Renz D., Doerfler W. DNA methylation in the Alu sequences of diploid and haploid primary human cells. EMBO J. 1993 Mar;12(3):1141–1151. doi: 10.1002/j.1460-2075.1993.tb05755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane D. E., Clark A. G., Cheng J. F., Hardison R. C. Subfamily relationships and clustering of rabbit C repeats. Mol Biol Evol. 1991 Jan;8(1):1–30. doi: 10.1093/oxfordjournals.molbev.a040631. [DOI] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Dewey W. C. Effect of cycloheximide or puromycin on induction of thermotolerance by sodium arsenite in Chinese hamster ovary cells: involvement of heat shock proteins. J Cell Physiol. 1987 Jul;132(1):41–48. doi: 10.1002/jcp.1041320106. [DOI] [PubMed] [Google Scholar]
- Liu W. M., Maraia R. J., Rubin C. M., Schmid C. W. Alu transcripts: cytoplasmic localisation and regulation by DNA methylation. Nucleic Acids Res. 1994 Mar 25;22(6):1087–1095. doi: 10.1093/nar/22.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. M., Schmid C. W. Proposed roles for DNA methylation in Alu transcriptional repression and mutational inactivation. Nucleic Acids Res. 1993 Mar 25;21(6):1351–1359. doi: 10.1093/nar/21.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraia R. J., Driscoll C. T., Bilyeu T., Hsu K., Darlington G. J. Multiple dispersed loci produce small cytoplasmic Alu RNA. Mol Cell Biol. 1993 Jul;13(7):4233–4241. doi: 10.1128/mcb.13.7.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraia R. J. The subset of mouse B1 (Alu-equivalent) sequences expressed as small processed cytoplasmic transcripts. Nucleic Acids Res. 1991 Oct 25;19(20):5695–5702. doi: 10.1093/nar/19.20.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S., Tripodi M., Melli M. A sequence upstream from the coding region is required for the transcription of the 7SK RNA genes. Nucleic Acids Res. 1986 Dec 9;14(23):9243–9260. doi: 10.1093/nar/14.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N. SINEs. Curr Opin Genet Dev. 1991 Dec;1(4):498–504. doi: 10.1016/s0959-437x(05)80198-4. [DOI] [PubMed] [Google Scholar]
- Panning B., Smiley J. R. Activation of RNA polymerase III transcription of human Alu repetitive elements by adenovirus type 5: requirement for the E1b 58-kilodalton protein and the products of E4 open reading frames 3 and 6. Mol Cell Biol. 1993 Jun;13(6):3231–3244. doi: 10.1128/mcb.13.6.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price B. D., Calderwood S. K. Heat-induced transcription from RNA polymerases II and III and HSF binding activity are co-ordinately regulated by the products of the heat shock genes. J Cell Physiol. 1992 Nov;153(2):392–401. doi: 10.1002/jcp.1041530219. [DOI] [PubMed] [Google Scholar]
- Quentin Y. Successive waves of fixation of B1 variants in rodent lineage history. J Mol Evol. 1989 Apr;28(4):299–305. doi: 10.1007/BF02103425. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Ivanov P. L., Kramerov D. A., Georgiev G. P. Mouse ubiquitous B2 repeat in polysomal and cytoplasmic poly(A)+RNAs: uniderectional orientation and 3'-end localization. Nucleic Acids Res. 1983 Sep 24;11(18):6541–6558. doi: 10.1093/nar/11.18.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M., Ohshima K., Mukoyama H., Yasue H., Okada N. A novel tRNA species as an origin of short interspersed repetitive elements (SINEs). Equine SINEs may have originated from tRNA(Ser). J Mol Biol. 1994 Jun 24;239(5):731–735. doi: 10.1006/jmbi.1994.1410. [DOI] [PubMed] [Google Scholar]
- Schmid C., Maraia R. Transcriptional regulation and transpositional selection of active SINE sequences. Curr Opin Genet Dev. 1992 Dec;2(6):874–882. doi: 10.1016/s0959-437x(05)80110-8. [DOI] [PubMed] [Google Scholar]
- Sinnett D., Richer C., Deragon J. M., Labuda D. Alu RNA transcripts in human embryonal carcinoma cells. Model of post-transcriptional selection of master sequences. J Mol Biol. 1992 Aug 5;226(3):689–706. doi: 10.1016/0022-2836(92)90626-u. [DOI] [PubMed] [Google Scholar]
- Tomilin N. V., Bozhkov V. M., Bradbury E. M., Schmid C. W. Differential binding of human nuclear proteins to Alu subfamilies. Nucleic Acids Res. 1992 Jun 25;20(12):2941–2945. doi: 10.1093/nar/20.12.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullu E., Weiner A. M. Human genes and pseudogenes for the 7SL RNA component of signal recognition particle. EMBO J. 1984 Dec 20;3(13):3303–3310. doi: 10.1002/j.1460-2075.1984.tb02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullu E., Weiner A. M. Upstream sequences modulate the internal promoter of the human 7SL RNA gene. 1985 Nov 28-Dec 4Nature. 318(6044):371–374. doi: 10.1038/318371a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Matsumoto S., Kojima S., Ohshima K., Okada N., Machida Y. Molecular characterization of a short interspersed repetitive element from tobacco that exhibits sequence homology to specific tRNAs. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6562–6566. doi: 10.1073/pnas.90.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]