Abstract
BACE1 (β-site β-amyloid precursor protein (APP)-cleaving enzyme 1) mediates the first proteolytic cleavage of APP, leading to amyloid β-peptide (Aβ) production. It has been reported that BACE1 intracellular trafficking, in particular endosome-to-TGN sorting, is mediated by adaptor complexes, such as retromer and Golgi-localized γ-ear-containing ARF-binding proteins (GGAs). Here we investigated whether sortilin, a Vps10p domain-sorting receptor believed to participate in retromer-mediated transport of select membrane cargoes, contributes to the subcellular trafficking and activity of BACE1. Our initial studies revealed increased levels of sortilin in post-mortem brain tissue of AD patients and that overexpression of sortilin leads to increased BACE1-mediated cleavage of APP in cultured cells. In contrast, RNAi suppression of sortilin results in decreased BACE1-mediated cleavage of APP. We also found that sortilin interacts with BACE1 and that a sortilin construct lacking its cytoplasmic domain, which contains putative retromer sorting motifs, remains bound to BACE1. However, expression of this truncated sortilin redistributes BACE1 from the trans-Golgi network to the endosomes and substantially reduces the retrograde trafficking of BACE1. Site-directed mutagenesis and chimera experiments reveal that the cytoplasmic tail of sortilin, but not those from other VPS10p domain receptors (e.g. SorCs1b and SorLA), plays a unique role in BACE1 trafficking. Our studies suggest a new function for sortilin as a modulator of BACE1 retrograde trafficking and subsequent generation of Aβ.
Keywords: Alzheimer Disease, Amyloid, Endosomes, Protein Sorting, Secretases, Trafficking
Introduction
Alzheimer disease (AD)2 is a progressive and fatal neurodegenerative disorder characterized pathologically in part by the extracellular deposit of the 40–42-amino acid-long amyloid β-peptide (Aβ). Aβ is derived from the β-amyloid precursor protein (APP) via the action of two proteolytic enzymes: β- and γ-secretase (1, 2). BACE1 (β-site APP-cleaving enzyme 1) is a transmembrane aspartyl protease that mediates the first β-secretase cleavage, resulting in a soluble ectodomain and a membrane-tethered fragment that subsequently undergoes presenilin-mediated γ-secretase cleavage (3, 4). The γ-secretase cleavage generates Aβ and the APP intracellular domain (5).
Various subcellular sites for APP proteolytic processing have been identified. Aβ production has been localized to the trans-Golgi network (and/or Golgi-associated vesicles), to the endosomal/lysosomal system (following APP endocytosis), and to a small degree (Aβ42 only) to the endoplasmic reticulum/intermediate compartments (6–9). The protease BACE1 has been shown to transit through the secretory pathway and target the endosomal system, cycling between endosomes and the cell surface probably by way of the TGN (10, 11). There is also speculation that BACE1 can traffic from the trans-Golgi network (TGN) directly to endocytic compartments (12). Endosomal trafficking of BACE1 appears to be at least partially governed by an acidic cluster-dileucine motif in its cytoplasmic tail (12–14). This motif has been shown to interact with the VHS domain of GGA1, -2, and -3 (Golgi-localized γ-ear-containing ARF binding proteins), adaptor proteins that mediate sorting between the TGN and endosomes (13, 15). Recently, it has been shown that GGA3 can bind BACE1 via the ubiquitin sorting machinery and regulate BACE1 degradation (16). It is well documented that GGAs mediate anterograde trafficking from the TGN to endosomes, but recent studies have suggested that GGAs might also act in retrograde trafficking of proteins, specifically of BACE1 (15, 17). Furthermore, GGAs have recently been implicated in APP processing (18–20).
A better characterized mechanism for retrograde trafficking involves a protein complex called retromer. Initially identified in yeast, retromer is a pentameric complex consisting of two “subcomplexes.” The first subcomplex consists of Vps35p and Vps29p, which interacts with Vps10p to drive cargo selection. Vps10p is a sorting receptor for carboxypeptidase Y and is functionally equivalent to the cation-independent mannose 6-phosphate receptor (CI-MPR) in mammals. The second subcomplex consists of Vps5p and Vps17p, which drives budding through the self-assembly activity of Vps5p. These two subcomplexes are in turn linked by Vps26p (21). The components of retromer all have mammalian homologues that can act in the endosome-to-Golgi retrieval of the CI-MPR, including Vps35, Vps29, Vps26, and various sorting nexins (SNXs), a family of membrane-associated proteins whose common characteristic property is a Phox homology domain (21, 22). Many retromer components appear to modulate APP cleavage. Evidence suggests that SNX6 associates with BACE1 and regulates APP cleavage (23). Furthermore, RNAi and overexpression studies on the mammalian retromer components, Vps35 and Vps26, have suggested a role for retromer in APP processing (24, 25).
Sortilin is a transmembrane protein that belongs to a family of type I receptors with homology to the yeast receptor Vps10p, the sorting receptor that interacts with retromer (26). It has been demonstrated that the sortilin cytoplasmic domain can convey Golgi localization and Golgi-endosome transport. Such a sorting and trafficking role for sortilin can be attributed to two motifs, a tyrosine-based YSVL motif and an acidic cluster-dileucine motif, both appearing to contribute to the endocytosis and intracellular trafficking of sortilin (27–30). These motifs are likewise found within the CI-MPR (the functional equivalent of Vps10p), which is responsible for transporting lysosomal enzymes from the Golgi to the lysosome and subsequently undergoes retrograde transport via retromer (31–33). Given that the cytoplasmic tail of sortilin binds GGAs and given that sortilin appears to mimic the trafficking patterns of the CI-MPR, it is highly plausible that sortilin participates in retrograde trafficking via GGAs or in association with the retromer complex; SNX1 and Vps26 have been implicated in sortilin retrograde trafficking (33–35). We suspect that BACE1 may interact with sortilin, given that BACE1 endosomal trafficking (like sortilin) is governed by GGAs and given that BACE1 (like sortilin) has been shown to undergo retrograde transport (15, 17, 27, 33).
The intracellular trafficking of BACE1 between endosomes and the TGN appears to be tightly regulated and potentially influential on APP processing and the subsequent generation of Aβ. The convergence of APP and BACE1 in the endosomal pathway is significant and suggests a possible role for various sorting proteins in modulating their proximity. Interestingly, it has been recently found that SorLA/LR11, another member of the Vps10p family of receptors, can regulate the intracellular transport and subsequent processing of APP (36, 37). Furthermore, it was recently reported that inherited variants in SorLA and SorCS1 (a third member of the Vps10p family) are associated with late onset AD (38–40). Given the similar motifs, binding partners, and trafficking characteristics of sortilin and BACE1, as outlined above, we set out to investigate a potential role of sortilin in AD pathogenesis. Our studies indicate that the sortilin cytoplasmic domain, which harbors two identifiable sorting signals, is required for BACE1 subcellular localization and also modulates BACE1-mediated cleavage of APP. The effect of the sortilin cytoplasmic tail on BACE1 trafficking appears to be unique among other Vps10p domain receptors implicated in AD.
EXPERIMENTAL PROCEDURES
Expression Constructs
Full-length human sortilin cDNA was purchased from Origene Technologies (Rockville, MD). For V5- and GFP-tagged fusion proteins, full-length sortilin cDNA was amplified by PCR and cloned in frame to the pEF6/V5-His TOPO vector (Invitrogen) or the pcDNA3.1/CT-GFP-TOPO vector (Invitrogen), respectively. Primers were also designed to PCR-amplify a truncation of sortilin lacking the cytoplasmic tail but maintaining the transmembrane domain. These PCR products were cloned into the above mentioned vectors. To create untagged full-length and C-terminal truncated sortilin constructs, sortilin was PCR-amplified with the appropriate 3′ primers encoding stop codons and subsequently cloned into the pEF6/V5-His TOPO vector. BACE1 expression vectors include pCDNA3.1/Myc-His(−) (Invitrogen), pECFP-N1 (Clontech), and pEGFP-N1 (Clontech). The expression construct encoding BACE1-HA was kindly provided by Robert W. Doms (University of Pennsylvania, Philadelphia, PA). Chimeric constructs containing the luminal and transmembrane domains of sortilin and the cytoplasmic domains of either SorLA or SorCS1b were made by a standard PCR technique using primers generating an XbaI site at the 5′-end and 3′-end. These PCR products were then digested and subcloned into the C-terminal XbaI site of the cDNA3.1/CT-GFP-TOPO vector containing truncated sortilin.
Site-directed mutagenesis was performed using the QuikChange Lightning site-directed mutagenesis kit following the manufacturer's instructions (Stratagene). All mutations were introduced into full-length sortilin that was previously subcloned into the cDNA3.1/CT-GFP-TOPO vector. Missense mutations were introduced with the following primers: Y14A/L17A, 5′-GGTTCCTGGTGCATCGAGCCTCTGTGCTGCAGCAG and 5′-CTGCTGCAGCACAGAGGCTCGATGCACCAGGAACC, followed by 5′-CATCGAGCCTCTGTGGCGCAGCAGCATGCAGAGG and 5′-CCTCTGCATGCTGCTGCGCCACAGAGGCTCGATG; F9A/L10A, 5′-GTCTGTGGGGGAAGGGCCCTGGTGCATCGATAC and 5′-GTATCGATGCACCAGGGCCCTTCCCCCACAGAC, followed by 5′-GTCTGTGGGGGAAGGGCCGCGGTGCATCGATACTCTG and 5′-CAGAGTATCGATGCACCGCGGCCCTTCCCCCACAGAC; and L51A/L52A, 5′-GATGACTCAGATGAGGACGCCGCGGAAAAAGGGCAATTCTGC and 5′-GCAGAATTGCCCTTTTTCCGCGGCGTCCTCATCTGAGTCATC.
Analysis of Post-mortem Brain Tissue
Human brain tissue of the temporal cortex was obtained from the New York Brain Bank at Columbia University. Human brain tissues (diagnosed by histological analysis in the Neuropathology Core) were snap-frozen and stored at −80 °C until required. Tissue samples were soaked with 5 volumes of ice-cold Solution A (0.32 m Sucrose, 0.5 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES, pH 7.4), supplemented with a protease inhibitor mixture (Roche Applied Science) for 30 min, and homogenized using a Teflon pestle homogenizer. Homogenate was centrifuged at 1,500 rpm for 10 min at 4 °C, and 30 μg of the supernatant (S1) was subjected to SDS-PAGE. The blots were sequentially incubated overnight with anti-sortilin (BD Biosciences), anti-SorLA (BD Biosciences), anti-SorSC1b (a gift of Dr. Guido Hermey), anti-β-actin (Sigma), anti-neuronal class III β-tubulin (TUJ1) antibody (Covance, Berkeley, CA), and anti-neuronal nuclei (NeuN) antibody (Millipore, Billerica, MA). Fluorescently labeled secondary antibody was detected and analyzed using the Odyssey infrared imaging system (LI-COR Biotechnology, Lincoln, NE).
Cell Culture
HEK293 and HeLa cells were maintained in DMEM (Invitrogen), supplemented with 10% FBS, 100 units/ml penicillin, 100 units/ml streptomycin, and 2 mm l-glutamine. HEK293 cells stably expressing vector, V5-tagged sortilin (sort-V5), or sortΔC-V5 were additionally maintained with 10 μg/ml blasticidine (Invitrogen). Cells were incubated at 37 °C under 6% CO2.
Immunoprecipitation of Conditioned Media
In triplicate, HEK293 cells that stably express vector, sort-V5, or sortΔC-V5 were incubated for 48 h. Immunoprecipitation of sAPPβ was performed using sβwt antibody and detected using anti-22C11 (Chemicon, Temecula, CA). The antibody sβwt, a sAPPβwt cleavage site-specific antibody, was described previously (23). Immunoprecipitation of total Aβ was performed using anti-4G8 (Covance) and detected using Signet's anti-6E10 antibody (Covance).
Western Blot Analyses of Overexpression Assays
V5-tagged sortilin was resolved via SDS-PAGE using 4–12% Tris/glycine gels, transferred to PVDF, and probed for expression with anti-V5 (Invitrogen). Blots were developed using HRP-conjugated secondary antibodies. Expression of APP and C99 were resolved via SDS-PAGE using 4–12% Tris/glycine gels. APP and C-terminal fragments were probed with APP-CTmax antibody (41) and detected using the Odyssey infrared imaging system (LI-COR Biotechnology).
RNA Interference
shRNA vectors targeting human sortilin (and a non-silencing shRNA vector) were purchased from Open Biosystems (Huntsville, AL). Transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Co-immunoprecipitation
Reciprocal co-immunoprecipitation of sortilin and BACE-HA was performed using monoclonal primary antibodies against sortilin (BD Biosciences) and HA (Covance). HeLa cells were co-transfected with sortilin and/or HA-tagged BACE1, incubated for 48 h, and then lysed with 1% Nonidet P-40 lysis buffer (50 mm Tris-Cl, 150 mm NaCl, 1% Nonidet P-40). Cell lysates were precleared, incubated with anti-sortilin or anti-HA at 4 °C overnight, and subsequently pulled down with protein G Plus/protein A-agarose suspension (Calbiochem). Samples were resolved via SDS-PAGE and Western blotting. Co-immunoprecipitations of full-length sortilin or truncated sortilin and BACE1 were performed using polyclonal antibodies against Myc (Upstate Inc., Charlottesville, VA) or HA (Covance), as well as monoclonal anti-BACE-cat1 antibody (42), a gift of Dr. Robert Vassar. Samples were resolved via Western blotting using anti-V5 (Invitrogen) or anti-sortilin (BD Biosciences). Co-immunoprecipitation of endogenous HEK293 cells was accomplished using 1% Nonidet P-40 lysis buffer and pulling down with anti-BACE-cat1 antibody. An unrelated control antibody, anti-pLCγ2 (Abcam, Cambridge, MA), was included. Samples were resolved via Western blotting using anti-sortilin (BD Biosciences). Co-immunoprecipitation of human post-mortem brain tissue was performed using human brain tissue of the temporal cortex, obtained from the New York Brain Bank at Columbia University. Human brain tissues were snap-frozen and stored at −80 °C until required. Following the preparation of P2 fraction via differential centrifugation, P2 was resuspended in solution B (0.32 m sucrose, 1 mm NaHCO3) and lysed with 2% Nonidet P-40 2× lysis buffer. Lysed P2 was precleared and incubated with anti-BACE1 (Affinity Bioreagents, Golden, CO) at 4 °C overnight and subsequently pulled down with protein G Plus/protein A-agarose suspension (Calbiochem). Samples were resolved via SDS-PAGE and probed with anti-sortilin (BD Biosciences).
Immunocytochemistry
HeLa cells were transiently transfected with GFP-tagged sortilin constructs or untagged SorLA, along with CFP-tagged BACE1. After 24 h, cells were fixed in 4% paraformaldehyde, permeabilized, and blocked for 1 h in 10% normal goat serum or 10% fetal bovine serum. Primary antibodies were diluted in 10% normal goat serum or fetal bovine serum containing 0.1% Triton X-100 and incubated overnight at 4 °C. Organelle localization was detected using anti-syntaxin 6 (BD Biosciences), anti-TGN46 (Novus Biologicals, Littleton, CO), or anti-EEA1 (BD Biosciences). Following incubation of primary antibody, cells were washed and incubated for 1 h at room temperature in Alexa Fluor 568-conjugated secondary antibody (Invitrogen). Immunostained cells were mounted, using Vectashield mounting medium (Vector Laboratories, Burlington, CA), and imaged using the Nikon C1 digital confocal system.
Antibody Uptake Assay
HEK293 cells stably expressing vector, sort-V5, or sortΔC-V5 were transiently transfected with BACE1. After incubation for 24 h, cells were washed and incubated for 15 min on ice with ice-cold PBS. Subsequently, cells were incubated for 30 min on ice with OptiMEM (Gibco) containing an antibody against the ectodomain of BACE1 (Calbiochem). Cells were then incubated at 37 °C in OptiMEM for various time periods and fixed with 4% paraformaldehyde before being processed for immunocytochemistry.
Chloroquine Assay
HEK293 cells stably expressing vector, sort-V5, or sortΔC-V5 were treated overnight with 75 μm chloroquine (Sigma). Immunoprecipitation of conditioned media was performed as described above. Immunoprecipitation of intracellular APPβ was performed on cells lysed with 1% Triton X-100 buffer. Immunoprecipitates were resolved via SDS-PAGE using 4–12% Tris/glycine gels and detected with anti-22C11 (Chemicon). CTFs were detected with APP-CTmax antibody (41).
RESULTS
The Analysis of Vps10p Domain Family Members in AD Post-mortem Brain Tissue
Regions of the temporal cortex in AD undergo major pathological changes involving Aβ deposition, neurofibrillary tangles, and marked neuronal loss (43, 44). All Vps10p domain family members are highly enriched in neurons of the mammalian brain, and sortilin is particularly enriched in neurons of the temporal cortex (45, 46). We began our investigations by analyzing the protein expression of sortilin and other major Vps10p domain family members, including sorCS1 and sorLA, in the temporal cortex of patient-derived post-mortem brain tissue using quantitative Western blot analysis. Given that neuronal loss is a particular neuropathological change in the AD brain, it is necessary to normalize our findings to relevant markers to account for neuron-specific cell loss. In order to account for total cell loss in the post-mortem brain tissue, we normalized our results to β-actin. In order to account for neuron-specific cell loss, we utilized the markers TUJ1 and NeuN.
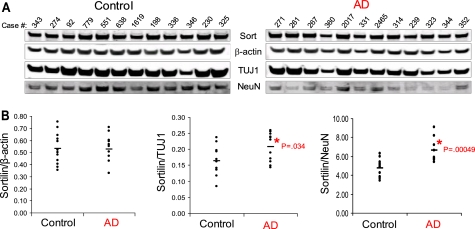
Using infrared-based quantitative Western blotting, we analyzed brain homogenate from the temporal cortex of 12 control subjects and 12 subjects diagnosed with late stage AD. All tissue samples had an average cold post-mortem interval of less than 5 h. Mean age at the time of death was 76.6 years (range, 62–90 years) for control subjects and 79.9 years (range, 71–90 years) for AD subjects (supplemental Fig. 1). Sortilin was detected as a ∼100-kDa protein in human brain tissue as recognized by two independent antibodies, anti-sortCT and anti-sortilin, directed against the C terminus and N terminus of sortilin, respectively (data not shown).
Upon normalization to both TUJ1 and NeuN, we found a significant increase in sortilin (∼25%) as compared with control (Fig. 1, A and B). Additionally, we found an increase in SorLA expression in AD brains, as compared with control (∼15%) but no change in SorCS1b levels (supplemental Fig. 2). Normalization to β-actin levels yielded no significant changes in any of the Vps10p family members. Our results suggest that neuronal expression of sortilin and SorLA, but not that of SorCS1b, is increased in the post-mortem brain tissue of AD.
FIGURE 1.
Normalized expression levels of sortilin in the temporal cortex of patient-derived post-mortem brain tissue. A, human brain tissue from 12 control and 12 AD cases were analyzed via quantitative infrared Western blotting. The immunoblot was probed with sortilin, β-actin, TUJ1, and NeuN. B, plots displaying the immunoreactivity of sortilin antibody as normalized to β-actin, TUJ1, and NeuN for control versus AD brains. The horizontal line represents the normalized mean value in control versus AD subjects (*, p < 0.05).
Sortilin Modulates BACE1-mediated Cleavage of APP
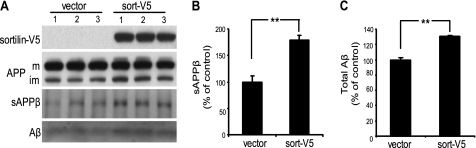
Given the elevated levels of sortilin in the post-mortem AD brain tissue, we next investigated the possible consequences of increased sortilin levels in the proteolytic processing of APP. We were able to detect endogenous proteolytic products in HEK293 cells in which we stably overexpressed either V5-tagged empty vector (vector) or sort-V5 (Fig. 2A). The proteolytic products we assessed include sAPPβ (the N-terminal fragment of APP that is secreted into the medium following BACE1 cleavage) and Aβ (the end product of APP processing, which involves cleavage of APP by both β- and γ-secretases).
FIGURE 2.
Sortilin overexpression increases sAPPβ and secreted Aβ. A, HEK293 cells stably expressing either V5-tagged empty vector (vector) or V5-tagged sortilin (sort-V5) were compared via Western blotting. A, top two panels, representative data showing lysate subjected to SDS-PAGE and immunoblotted with anti-V5 or anti-APP. Full-length APP levels are shown (m, mature APP; im, immature APP). A, bottom two panels, immunoprecipitation of sAPPβ and Aβ from conditioned media. B, infrared quantification of immunoprecipitated sAPPβ. C, chemiluminescent quantification of immunoprecipitated total Aβ. Medium quantifications are normalized to total protein. Each bar represents the average of two experiments assayed in triplicate (lanes 1–3 of A). **, p < 0.01. Error bars, S.D.
Using our HEK293 cells stably overexpressing sortilin, we found, via immunoprecipitation, a 78% increase in sAPPβ upon sortilin overexpression (Fig. 2, A and B). These results suggest that sortilin is affecting the first step in APP proteolysis, mediated by β-secretase. We next analyzed secreted Aβ levels in the media using immunoprecipitation. We found that sortilin overexpression increased total secreted Aβ by ∼30% (Fig. 2, A and C). Our data imply that sortilin mediates BACE1 cleavage of APP and resultant Aβ production.
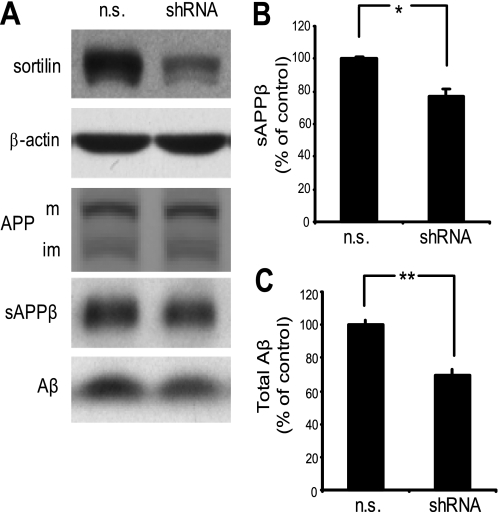
Given that overexpression of sortilin increases BACE1 cleavage of APP, we theorized that a decrease in sortilin expression would harbor the opposite results. To test this, we performed an RNAi experiment in which we transiently transfected HEK293 cells with sortilin-directed shRNA. Sortilin protein levels were substantially reduced compared with cells expressing non-silencing control shRNA (n.s.) (Fig. 3A). To analyze the secreted APP derivatives in sortilin-reduced cells, conditioned medium was subjected to immunoprecipitation using either sAPPβ-specific antibody or antibody against total Aβ. Secretion of sAPPβ was significantly reduced in sortilin knockdown cells compared with control (Fig. 3, A and B). Similarly, total Aβ was reduced by ∼30% (Fig. 3, A and C). Our data indicate that reducing sortilin leads to a reduction in BACE1-mediated cleavage of APP.
FIGURE 3.
Reducing sortilin decreases BACE1-mediated cleavage of APP. A, HEK293 cells were transiently transfected with either non-silencing shRNA vector (n.s.) or shRNA against human sortilin (shRNA). A, top two panels, representative Western blot analysis of sortilin down-regulation. β-Actin serves as a loading control. A, bottom three panels, immunoprecipitation of sAPPβ and Aβ from conditioned media. Full-length APP levels remain unchanged (m, mature APP; im, immature APP). B, chemiluminescent quantification of immunoprecipitated sAPPβ. C, chemiluminescent quantification of immunoprecipitated total Aβ. Medium quantifications are normalized to total protein. Each bar represents the average of two experiments assayed in duplicate. *, p < 0.05; **, p < 0.01. Error bars, S.D.
The Interaction and Subcellular Distribution of Sortilin and BACE1
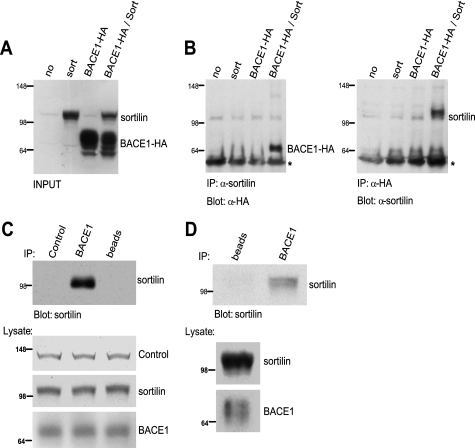
The above data suggest that sortilin modulates BACE1-mediated cleavage of APP. Furthermore, both sortilin and BACE1 follow a similar trafficking pattern in which both proteins are subjected to the retromer pathway (10, 27, 30). We therefore wanted to determine whether sortilin and BACE1 interact and exhibit similar subcellular distribution. Using HeLa cells transiently transfected with HA-tagged BACE1 (BACE1-HA) and sortilin (sort), we performed reciprocal co-immunoprecipitation. Negative controls included mock-transfected cells (no), sortilin expressed alone, and BACE1-HA expressed alone (Fig. 4A). Cell lysates were immunoprecipitated with either anti-sortilin or anti-HA antibodies, and immunoprecipitates were resolved via Western blotting and probed with either anti-HA or anti-sortilin, respectively (Fig. 4B). Co-immunoprecipitation of sortilin and BACE1 was detected in cells co-expressing sortilin and BACE1-HA but not in cells expressing sortilin or BACE1-HA alone. As an additional negative control, we also performed reciprocal co-immunoprecipitation between sortilin and HA-tagged Notch1 and found no evidence of binding (data not shown). We also found similar results showing the interaction of endogenous sortilin and stably expressed BACE1 in neuro2a cells (data not shown). Our data collectively suggest an interaction between sortilin and BACE1.
FIGURE 4.
Co-immunoprecipitation analysis of exogenously and endogenously expressed sortilin and BACE1. A, Western blot analysis of co-immunoprecipitation input. HeLa cells were transiently co-transfected with sortilin (sort) and/or HA-tagged BACE1 (BACE1-HA). Negative controls include a mock transfection (no), sortilin transfection alone, and BACE1-HA transfection alone. B, co-immunoprecipitation (IP) results. Cells were lysed and subjected to co-immunoprecipitation using anti-sortilin or anti-HA. Samples were resolved via Western blotting using anti-HA and anti-sortilin, respectively. *, IgG. C, co-immunoprecipitation of HEK293 lysate by anti-BACE1 antibody. Negative controls include no antibody (beads) and a control antibody (mouse-derived) against an unrelated protein. Samples were resolved via Western blotting using anti-sortilin. D, human post-mortem brain tissue was co-immunoprecipitated with either no antibody (beads) or anti-BACE1. Samples were resolved via Western blotting using anti-sortilin.
We next sought to confirm this interaction between sortilin and BACE1 utilizing endogenously expressed proteins. We accomplished this in two ways: first via co-immunoprecipitation of HEK293 cell lysate and then using homogenized post-mortem brain tissue. Cell lysates were immunoprecipitated with either anti-BACE1 antibody, beads alone, or a negative control mouse-derived antibody against an unrelated protein. Co-immunoprecipitates were then resolved via Western blotting and probed with anti-sortilin (Fig. 4C). Our results indicate that sortilin and BACE1 are co-immunoprecipitated, suggesting a specific interaction. To further confirm our endogenous results, post-mortem brain tissue was homogenized and subjected to co-immunoprecipitation using no antibody (beads) or anti-BACE1. Immunodetection of sortilin shows that anti-BACE1 is able to pull down sortilin in post-mortem human brain tissue (Fig. 4D). This finding further indicates an interaction between BACE1 and sortilin.
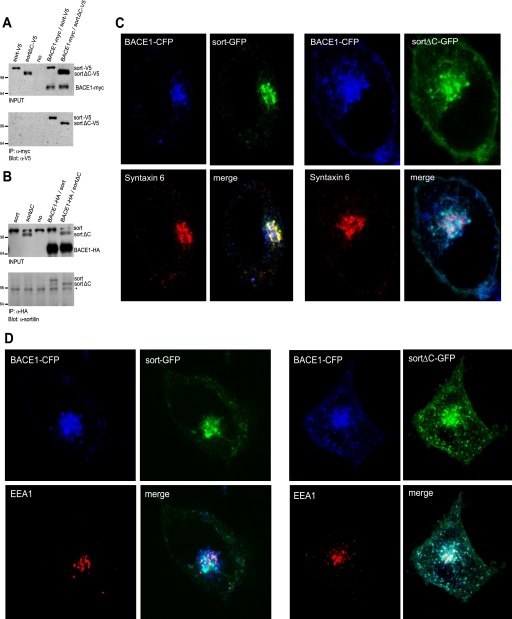
Deletion of the Cytoplasmic Tail of Sortilin Preserves the Interaction but Alters the Subcellular Distribution of BACE1 and Sortilin
The cytoplasmic tail of sortilin harbors various sorting motifs by which sortilin directs its bound cargo. Hypothesizing that BACE1 might act as cargo for sortilin, it is likely that the sortilin cytoplasmic tail modulates BACE1 subcellular distribution. In order to address this question, we generated truncated sortilin constructs in which we deleted the cytoplasmic tail. We first wanted to test whether the truncation of sortilin's cytoplasmic tail would affect its interaction with BACE1. To this end, we performed co-immunoprecipitation assays using two sortilin deletion constructs, one tagged with V5 (sortΔC-V5) and one untagged (sortΔC). For the first co-immunoprecipitation, we transiently transfected HeLa cells with Myc-tagged BACE1 (BACE1-Myc) along with either sort-V5 or sortΔC-V5. Negative controls included mock-transfected cells (no), sort-V5 expressed alone, and sortΔC-V5 expressed alone (Fig. 5A, top). Cell lysates were immunoprecipitated with anti-Myc and subsequently probed with anti-V5 (Fig. 5A, bottom). Both sort-V5 and sortΔC-V5 immunoprecipitated with BACE1-Myc, suggesting that the sortilin deletion construct is still able to interact with BACE1. To confirm these results, we performed an additional co-immunoprecipitation assay in which we transiently transfected HeLa cells with BACE-HA along with either untagged full-length sortilin (sort) or untagged sortΔC. Negative controls included mock-transfected cells (no), sort expressed alone, and sortΔC expressed alone (Fig. 5B, top). Cell lysates were immunoprecipitated with anti-HA and subsequently probed with anti-sortilin (Fig. 5B, bottom). Again we found that both full-length sortilin and truncated sortilin were able to interact with BACE1. These results suggest that deletion of the sortilin cytoplasmic domain retains the ability to form a complex with BACE1.
FIGURE 5.
A and B, truncated sortilin and BACE1 maintain binding affinity. A, co-immunoprecipitation (IP) of tagged full-length and truncated sortilin. HeLa cells were transiently co-transfected with either V5-tagged full-length sortilin (sort-V5), a cytoplasmic truncation of sortilin tagged with V5 (sortΔC-V5), and/or Myc-tagged BACE1 (BACE1-myc). Western blot analysis of input is depicted in the top panel. Negative controls include a mock transfection (no), sort-V5 alone, and sortΔC-V5 alone. The cells were lysed and subjected to immunoprecipitation using anti-Myc antibody. Samples were resolved via Western blotting using anti-V5. B, co-immunoprecipitation of untagged full-length and truncated sortilin. HeLa cells were transiently co-transfected with full-length sortilin (sort), a cytoplasmic truncation of sortilin (sortΔC), and/or HA-tagged BACE1 (BACE1-HA). Western blot analysis of input is depicted in the top panel. Negative controls include a mock transfection (no), sort alone, and sortΔC. The cells were lysed and subjected to immunoprecipitation using anti-HA. Samples were resolved via Western blotting using anti-sortilin. *, nonspecific band. C, truncated sortilin redistributes BACE1 from the TGN to peripheral punctate structures. C, left panels, distribution of full-length sortilin. HeLa cells were transiently transfected with sort-GFP and BACE1-CFP. Following fixation and permeabilization, the cells were stained with anti-syntaxin 6. C, right panels, distribution of truncated sortilin. HeLa cells were transiently transfected with sortΔC-GFP and BACE1-CFP. Following fixation and permeabilization, the cells were stained with anti-syntaxin 6. Magnification was ×100. D, truncated sortilin redistributes BACE1 from the TGN to the early endosomes. D, left panels, distribution of full-length sortilin. HeLa cells were transiently transfected with sort-GFP and BACE1-CFP. Following fixation and permeabilization, the cells were stained with anti-EEA1. D, right panels, distribution of truncated sortilin. HeLa cells were transiently transfected with sortΔC-GFP and BACE1-CFP. Following fixation and permeabilization, the cells were stained with anti-EEA1. Magnification was ×100.
To determine the specific subcellular localization of full-length sortilin with BACE1 as well as truncated sortilin, we performed immunocytochemistry analysis. To accomplish this, we utilized antibodies against the TGN (anti-syntaxin 6) and early endosomes (anti-EEA1). Following co-transfection of HeLa cells with BACE1-CFP and either sortFL-GFP or sortΔC-GFP, we further stained with anti-syntaxin 6 against the TGN (Fig. 5C). We found that full-length sortilin co-localized greatly with BACE1 in the perinuclear region, specifically within the TGN. Truncated sortilin likewise co-localized with BACE1, but the co-localization showed more peripheral and cell surface staining than with full-length sortilin. Importantly, we observed that truncated sortilin and BACE1 co-localization showed little coincidence with the TGN (Fig. 5C). This was in contrast to full-length sortilin and BACE1 co-localization, which almost fully overlapped with the TGN. We next stained with anti-EEA1 against the early endosomes and found that full-length sortilin and BACE1 co-localization only partially overlapped with the early endosomes (Fig. 5D). In contrast, truncated sortilin and BACE1 almost fully overlapped with the early endosomes (Fig. 5D). Furthermore, upon immunocytochemistry analysis of truncated sortilin, the redistribution of sortilin and BACE1 did not appear to coincide with the lysosomes as identified by the anti-lamp2 lysosomal marker (supplemental Fig. 3). Our immunocytochemistry analyses suggest that sortilin might play a role in trafficking BACE1 from the early endosomes to the TGN. Upon truncation of the sortilin cytoplasmic tail, sortilin and BACE1 expression is decreased in the TGN and increased in the early endosomes. This is consistent with the fact that the sortilin cytoplasmic domain is required for retromer- and/or GGA-mediated transport from the endosome to the TGN (27, 30). To substantiate our immunocytochemistry results and verify a biochemical interaction using the same constructs that we used for confocal analyses, we performed a further co-immunoprecipitation experiment utilizing the GFP-tagged sortilin constructs (sortFL-GFP and sortΔC-GFP) and CFP-tagged BACE1 (BACE1-CFP) (supplemental Fig. 4). We found that this system again showed an interaction between sortilin and BACE1. We theorize that our results indicate failed retrograde trafficking of BACE1 by truncated sortilin.
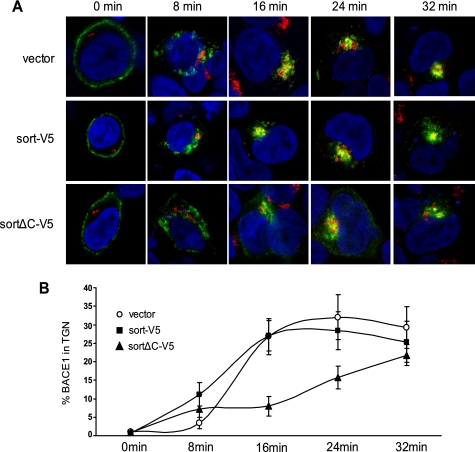
In order to investigate the hypothesis that sortilin participates in retrograde trafficking of BACE1, we performed an antibody uptake experiment, which monitors the endocytic transport of antibody-bound cell surface proteins (23). We compared the kinetics of BACE1-antibody transport from the cell surface to the TGN (as identified by anti-TGN46 antibody) using three HEK293 stable cell lines expressing either vector alone, sort-V5, or sortΔC-V5 and transiently transfected BACE1 (Fig. 6A). Cell surface-located BACE1 was labeled by N-terminal BACE1 antibody at 4 °C and allowed to internalize at 37 °C from 8 to 32 min. Immunocytochemistry revealed that in cells expressing the vector alone, BACE1 internalized to peripheral vesicles, suggestive of early endosomal structures, and showed maximal TGN localization after 24 min. The sort-V5-expressing stable line likewise showed BACE1 internalization to peripheral vesicles but showed maximal TGN localization after only 16 min. Finally, the sortΔC-V5 stable line was internalized, but even after 32 min, it never appeared to reach TGN capacity. These results suggest that by deleting the sortilin cytoplasmic domain, BACE1 retrograde trafficking is severely compromised (Fig. 6B). It appears that sortilin is responsible for endosome to TGN trafficking, dependent upon the sortilin cytoplasmic domain.
FIGURE 6.
Deletion of the cytoplasmic tail of sortilin leads to delayed retrograde trafficking of BACE1. A, following transient transfection of BACE1 in HEK293 cells stably expressing vector, sort-V5, and sortΔC-V5, BACE1 at the cell surface was labeled by N-terminal BACE1 antibody on ice (green). The labeled BACE1 was allowed to internalize at 37 °C and subsequently chased for the indicated time periods. Following fixation and permeabilization, the cells were stained with anti-TGN46 antibody (red). Representative confocal images are shown. Magnification was ×100. B, quantitative analysis using ImageJ image analysis software was performed to measure the co-localization of BACE1 with the TGN, revealing the percentage of BACE1 successfully delivered to the TGN (n = 5).
Mutagenesis Analysis of Sortilin Reveals Two Cytoplasmic Motifs That Regulate the Trafficking of BACE1
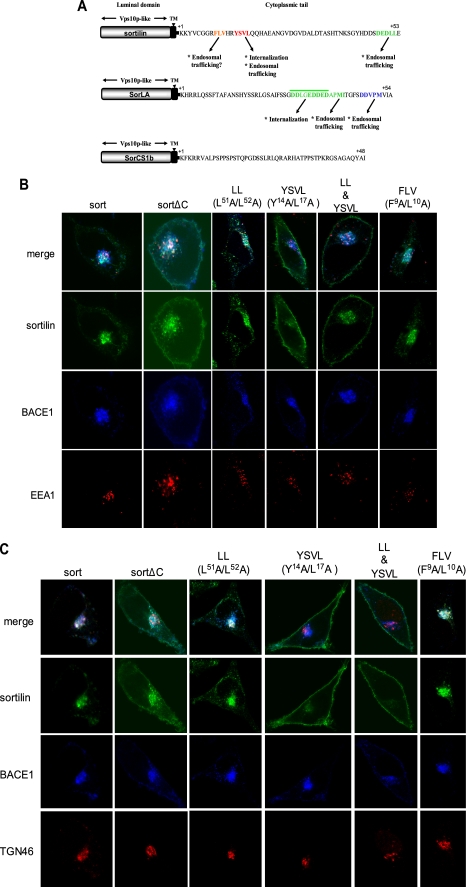
Nielsen et al. (27) identified a 9-residue stretch (HDDSDEDLL) in the cytoplasmic tail of sortilin bearing homology to the extreme C terminus of MPR. This stretch contains the recognizable acidic cluster dileucine (DEDLL) motif that mediates protein internalization and TGN-endosomal sorting. They also reported a second motif in the cytoplasmic tail, YSVL, abiding by the consensus motif YXXΦ, where Φ represents a bulky hydrophobic residue. This consensus motif is likewise found in MPR and other transmembrane proteins and mediates rapid internalization and intracellular transport (27). Recently, another highly conserved motif was found that could potentially mediate the intracellular transport of sortilin and MPR. This tripeptide motif (W/F)L(M/V), FLV in sortilin, was found to be necessary for endosome to Golgi retrieval (49).
In order to investigate the relevance of these motifs in the redistribution of BACE1 by sortilin, we introduced various mutations within these regions to determine the contribution of each motif to BACE1 trafficking, as analyzed by immunocytochemistry (Fig. 7A). The mutations we introduced to each motif have been previously identified as the key residues involved in their respective trafficking roles (27, 49). The mutations we analyzed included a double mutation in the dileucine motif (L51A/L52A), a double mutation in the YSVL motif (Y14A/L17A), both of these alterations together (LL & YSVL), and a double mutation in the FLV motif (F9A/L10A). The constructs were all tagged with GFP and compared with sort-GFP and sortΔC-GFP (supplemental Fig. 5A). The immunocytochemistry involved the transient co-transfection of HeLa cells with each of these constructs along with BACE1-CFP. We then utilized the organelle markers, EEA1 and TGN46, to examine the exact subcellular redistribution of our sortilin double mutants and BACE1 (Fig. 7, B and C). Like sort-GFP (and unlike sortΔC-GFP), the dileucine mutation is able to effectively endocytose BACE1 from the plasma membrane. It is also likely that this mutation also targets BACE1 to the TGN, where it co-localizes with the marker TNG46. However, there is much greater early endosome accumulation as compared with sort-GFP, suggesting an interruption in endosome to TGN retrograde trafficking. The YSVL double mutation, however, fails to undergo endocytosis, although it still shows mild co-localization with BACE1 (mainly intracellularly). This interaction, however, does not occur in either the early endosomes or the TGN. It appears that BACE1 intracellular trafficking by sortilin first necessitates the endocytosis of sortilin via the YSVL motif. The combined mutations of the dileucine and YSVL motifs (LL & YSLV) have a plasma membrane phenotype equivalent to that of the YSVL mutation alone. As such, it appears that the dileucine motif cannot exert its effects on BACE1 localization without sortilin first being internalized by the YSVL motif. In turn, the specific contribution of the YSVL motif cannot be adequately assessed because, without this motif, sortilin fails to internalize and have any further downstream effects on BACE1 trafficking. Interestingly, the FLV mutation appears to have no effect on either sortilin or BACE1 subcellular localization. The FLV mutation distribution phenotype is equivalent to sort-GFP, where sortilin and BACE1 primarily co-localize to the TGN. Supplemental Fig. 5B summarizes these immunocytochemistry results.
FIGURE 7.
A, comparison of the cytoplasmic sorting motifs in Vps10p domain-sorting receptors. The luminal domains all contain at least one Vps10p domain. The sorting motifs in the short cytoplasmic tails are highlighted and differ between receptors. In the case of SorCS1b, no known sorting motifs have been identified. Their functions are indicated below the amino acid sequences. B, distribution of BACE1 with sortilin mutants in the early endosomes. HeLa cells were transiently co-transfected with BACE1-CFP along with either GFP-tagged full-length sortilin (sort), truncated sortilin (sortΔC), or various sortilin cytoplasmic motif double mutations. The mutation constructs harbor double mutations in the dileucine motif (L51A/L52A), a double mutation in the YSVL motif (Y14A/L17A), both of these alterations together (LL & YSVL), and a double mutation in the FLV motif (F9A/L10A). The cells were further stained with anti-EEA1 antibody (red) and analyzed via confocal microscopy. Magnification was ×100. C, distribution of BACE1 with sortilin mutants in the TGN. HeLa cells were transiently co-transfected with BACE1-CFP and either GFP-tagged full-length sortilin (sort), truncated sortilin (sortΔC), or various sortilin cytoplasmic motif double mutations. The mutation constructs harbor double mutations in the dileucine motif (L51A/L52A), a double mutation in the YSVL motif (Y14A/L17A), both of these alterations together (LL & YSVL), and a double mutation in the FLV motif (F9A/L10A). The cells were further stained with anti-TGN46 antibody (red) and analyzed via confocal microscopy. Magnification was ×100. TM, transmembrane domain.
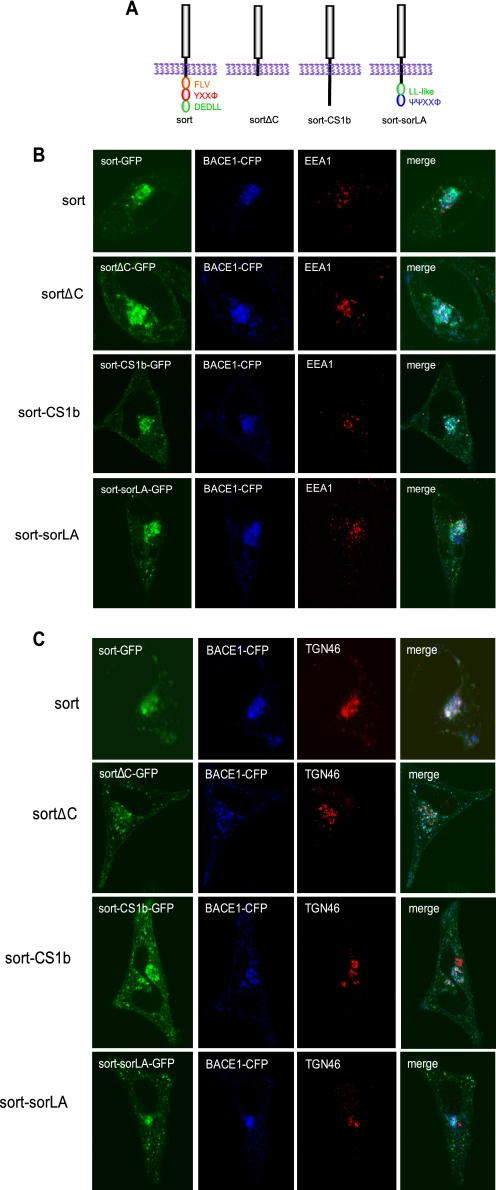
The Cytoplasmic Tails of Vps10p Domain Family Members Are Not Functionally Redundant
Sortilin is one of five known members of the Vps10p domain receptor family, additionally containing SorLA (also known as LR11), SorCS1, SorCS2, and SorCS3 (50). All contain a single Vps10p domain, which can act as a protein interaction motif, at the N terminus of their luminal moiety, but all five members exhibit different combinations of known consensus motifs in their cytoplasmic domains responsible for adaptor protein/trafficking interactions (Figs. 7A and 8A). The known motifs include YXXΦ, DXXLL, XXXL(L/I), ΨΨXXΦ (where Ψ represents Asp, Glu, or phosphoserine). As stated previously, sortilin contains YXXΦ (for rapid internalization and intracellular transport), DXXLL (for TGN-endosomal sorting and minimally for protein internalization), and the newly identified (W/F)L(M/V) (potentially for endosome to Golgi transport). SorLA contains ΨΨXXΦ (a reported GGA binding domain) and an acidic cluster LL-like motif (for endocytosis). SorCS1b, SorCS1e, and SorCS3 display no consensus motifs. Like sortilin, SorCS1a contains YXXΦ and DXXLL. However, it is the DXXLL motif that regulates its internalization (the function of YXXΦ is unknown). Finally, SorCS1c also contains YXXΦ and two LL-like motifs. Their function is undetermined (46, 49, 51–54).
FIGURE 8.
A, schematic illustration of sortilin cytoplasmic domain chimeras. Two chimeric receptors harboring the luminal and transmembrane domains of sortilin and the cytoplasmic domains of either SorLA or SorCS1b. The constructs express GFP. B, analyses of BACE1 redistribution by sortilin chimeras in the early endosomes. Immunocytochemical analyses of sort-CS1b chimera and sort-SorLA chimera as compared with sortFL and sortΔC constructs. HeLa cells were transiently co-transfected with BACE1-CFP and either GFP-tagged sort, sortΔC, sort-CS1b chimera, or sort-SorLA chimera. Following fixation and permeabilization, cells were stained with anti-EEA1 and analyzed via confocal microscopy. Magnification was ×100. C, analyses of BACE1 redistribution by sortilin chimeras in the TGN. Immunocytochemical analyses of sort-CS1b chimera and sort-SorLA chimera as compared with sort and sortΔC constructs. HeLa cells were transiently co-transfected with BACE1-CFP and either GFP-tagged sort, sortΔC, sort-CS1b chimera, or sort-SorLA chimera. Following fixation and permeabilization, cells were stained with anti-TGN46 and analyzed via confocal microscopy. Magnification was ×100.
Functional redundancy between Vps10p domain receptors has been suggested, particularly between sortilin and SorLA. In order to address this question and further characterize the functional motifs involved in BACE1 redistribution by the sortilin cytoplasmic tail, we generated two chimeric receptors harboring the luminal and transmembrane domains of sortilin and the cytoplasmic domains of either SorLA or SorCS1b (sort-CS1b and sort-SorLA, respectively) (Fig. 8A). This was accomplished by ligation of the respective cytoplasmic domains into the sortΔC-GFP construct. Upon confirming the expression of the chimeric receptors (supplemental Fig. 6, A and B), we transiently co-transfected HeLa cells with BACE1-CFP and either sort, sortΔC, sort-CS1b, or sort-SorLA. We then stained with organelle markers, EEA1 or TGN46, to confirm subcellular location (Fig. 8, B and C). We found that the sort-CS1b chimera resembled the sortΔC construct, in that lacking the dileucine and YXXΦ motifs, sort-CS1b chimera redistributed BACE1 from the TGN to the early endosomes and plasma membrane. In contrast, the sort-SorLA chimera redistributed BACE1 to structures not associated with EEA1 or the TGN46. Although BACE1 and the sort-SorLA chimera continue to largely co-localize, their subcellular distribution more closely resembles SorLA distribution; namely wild-type SorLA has been shown to accumulate in a more dispersed vesicular pattern than the perinuclear distribution of wild-type sortilin (51). Thus, our data suggest that the cytoplasmic domains of neither sorLA nor SorCS1b can replicate the function of the sortilin cytoplasmic tail. These data, as summarized in supplemental Fig. 6C, reinforce the distinctive contributions of the Vps10p domain family of receptors to subcellular trafficking and demonstrate that their cytoplasmic tails are not functionally equivalent.
Our chimera results appear to demonstrate that the sortilin cytoplasmic domain serves as a functional determinant for BACE1 localization and that this role is unique to sortilin versus other Vps10p family members. In order to substantiate this claim, we wanted to examine the distribution of wild type SorLA and BACE1, given that SorLA is widely implicated in AD-relevant cell biology.
There are conflicting data as to whether SorLA and BACE1 interact via Co-IP analysis (38, 55), so we performed ICC in which we transiently transfected GFP-tagged BACE1 and full-length SorLA in HeLA cells. As noted in our previous experiments, BACE1 localizes predominately to the perinuclear region of the TGN. Full-length SorLA, however, distributes to cytoplasmic punctate regions and minimally co-localizes with BACE1 (supplemental Fig. 7). It appears that sortilin is indeed unique in its effects on BACE1 subcellular distribution.
Truncated Sortilin Decreases sAPPβ and Has the Independent Function of Increasing C99 and Aβ by Inhibiting CTF Degradation
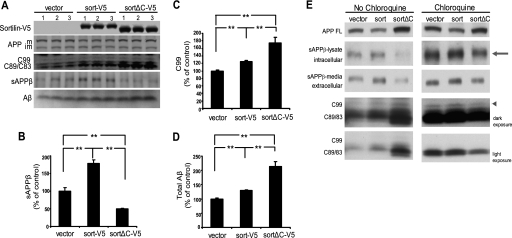
Our results thus far indicate that sortilin and BACE1 interact and that sortilin modulates BACE1-mediated cleavage of APP. Given our immunocytochemistry data, it is likely that sortilin exerts its effect on APP processing by altering BACE1 subcellular distribution. Specifically, the cytoplasmic tail of sortilin appears to be the regulatory domain. As such, we wanted to examine the effects of the cytoplasmic domain on APP processing. To this end, we compared the endogenous levels of APP proteolytic products generated by our three HEK293 stable cell lines expressing either empty-V5, sort-V5, or sortΔC-V5 (Fig. 9A). Immunoprecipitation of sAPPβ revealed that overexpressed sortilin increases sAPPβ by ∼75% as compared with the empty vector cells, whereas truncated sortilin decreases levels of sAPPβ by ∼50%, (Fig. 9, A and B). We saw no significant changes in sAPPα (data not shown). As expected, C99 levels likewise increase in cells overexpressing sortilin, but interestingly, truncated sortilin further increases these levels, by ∼75% over the empty vector cells. Furthermore, Aβ levels (the direct product of C99 cleavage) also increase (Fig. 9, A, C, and D). We likewise see increases in C89 and C83 levels (the CTFs generated by β′- and α-cleavages). The increases in all of the CTFs generated suggest that because BACE1 activity appears to decrease upon transfection of truncated sortilin (as indicated by decreased sAPPβ levels), it might be that CTF degradation is being hindered. Another possibility is that sortilin is affecting the secretion of APPβ, in that the appearance of decreased sAPPβ might actually be a decrease in its secretion, not its generation.
FIGURE 9.
A, truncated sortilin decreases sAPPβ but leads to the accumulation of APP CTFs and increased Aβ. HEK293 cells stably expressing either V5-tagged empty vector (vector), V5-tagged sortilin (sort-V5), or V5-tagged truncated sortilin (sortΔC-V5) were compared. A, top three panels, representative data showing lysate subjected to SDS-PAGE and immunoblotted with anti-V5 or anti-APP. Full-length APP, C99 levels, and C89/C83 levels are shown. A, bottom two panels, immunoprecipitation of sAPPβ and Aβ from conditioned media. B, infrared quantification of immunoprecipitated sAPPβ. C, infrared quantification of immunoprecipitated C99. D, chemiluminescent quantification of immunoprecipitated total Aβ. Medium quantifications are normalized to total protein. Each bar represents the average of two experiments assayed in triplicate (lanes 1–3 in A). **, p < 0.01. E, truncated sortilin decreases sAPPβ and has the independent function of increasing C99 and Aβ by inhibiting CTF degradation. HEK293 cells stably expressing either V5-tagged empty vector (vector), V5-tagged sortilin (sort), or V5-tagged truncated sortilin (sortΔC) were compared for protein degradation. The left panels show untreated cells. The right panel shows cells treated with 75 μm chloroquine. Full-length APP was resolved via chemiluminescent Western blotting. Immunoprecipitated intracellular (arrow) and extracellular APPβ are shown in the second and third rows. Dark and light exposures of CTFs (arrowhead denotes C99) were resolved via quantitative infrared Western blotting.
To explore these issues, we treated our stable cell lines with 75 μm chloroquine to inhibit lysosomal degradation and analyze both secreted and intracellular sAPPβ. Chloroquine is a weak base that, upon cellular uptake, neutralizes acidic organelles, such as the lysosomes. It has been previously reported that chloroquine has no effect on APP maturation or secretion of N-terminal APP fragments but is able to inhibit CTF degradation (56). By blocking the degradation of the CTFs, we sought to inhibit the possible effects of sortilin on CTF degradation and compare our three stable lines with untreated cells. We also immunoprecipitated intracellular, unsecreted APPβ (from the lysate) along with secreted APPβ (from the media) to examine the effects of sortilin on APPβ release. Upon immunoprecipitation of intracellular APPβ, we still found that overexpressed sortilin increased APPβ levels and that truncated sortilin decreased APPβ over the stable line expressing the empty vector (Fig. 9E, top arrow). The pattern of intracellular and extracellular APPβ generation remains the same. These results indicate that sortilin is unlikely to be affecting secretion of APPβ. We then examined the cells treated with chloroquine to see any changes in the pattern of CTF generation. In fact, when CTF degradation is blocked by chloroquine, we see that the levels of CTF generation by truncated sortilin mimic the generation of APPβ, both intracellular and secreted. Truncated sortilin now appears to decrease C99, C89, and C83 (Fig. 9E, arrowhead denotes C99). These results suggest that in untreated cells, sortilin is regulating CTF degradation and, in turn, Aβ production. Overexpressed sortilin results in increased BACE1 cleavage of APP, increased sAPPβ, increased C99, and a resultant increase in Aβ. Upon truncation of the cytoplasmic tail, sortilin decreases BACE1 cleavage of APP. However, this decreased activity of BACE1 does not translate to a decrease in C99 or Aβ because the sortilin cytoplasmic tail appears to have a further function in degrading C99. Therefore, upon deleting the cytoplasmic tail, sortilin is not able to degrade C99, and Aβ increases.
DISCUSSION
Our studies suggest that the sortilin cytoplasmic domain can regulate the subcellular distribution of BACE1 and subsequently affect BACE1-mediated proteolytic processing of APP. It has been demonstrated that sortilin undergoes rapid endocytosis and directed transport to the TGN with little recycling, where it circulates between the endosomes and the TGN (10, 11). This directed TGN transport appears to be governed by two motifs within its cytoplasmic tail: a tyrosine-based YSVL motif and an acidic cluster-dileucine motif (27). Like sortilin, BACE1 also undergoes endocytosis and directed transport to the TGN, from where it can be recycled back to the cell surface (11, 12). Lacking a necessary phosphorylation event, BACE1 appears to get trapped in the early endosomes following endocytosis or re-sorted directly to the plasma membrane (unable to be retrogradely trafficked to the TGN).
Our immunocytochemistry and co-immunoprecipitation studies suggest that sortilin may mediate the retrograde trafficking of BACE1. Sortilin and BACE1 appear to co-localize via confocal microscopy, and our co-immunoprecipitation data suggests that sortilin and BACE1 interact. Furthermore, upon truncation of the sortilin cytoplasmic tail, sortilin and BACE1 together appear to redistribute from the TGN to the early endosomes and other peripheral vesicles. The two proteins continue to interact via co-immunoprecipitation. These data suggest that BACE1, transported by truncated sortilin, is being misdirected from the TGN to the early endosomes, other peripheral endosomal structures (possibly recycling vesicles), and the plasma membrane. BACE1 is accumulating in these endosomal structures because truncated sortilin is unable to retrogradely transport BACE1 from the endosomes to the TGN. Based on these results, we hypothesize that sortilin mediates the retrograde trafficking of BACE1, and the cytoplasmic domain of sortilin is required for the interaction with trafficking cargo adaptors, such as the retromer complex or GGA proteins. Furthermore, the action of the sortilin cytoplasmic domain appears to be unique, in that the cytoplasmic domains of other Vps10p domain family members are not functionally redundant.
We theorize that by overexpressing sortilin, more BACE1 is being retrogradely transported from the endosomes into the TGN, allowing for more BACE1 to be recycled and kept in circulation for prolonged activity upon APP within both the endocytic and TGN recycling pathways. Upon expression of truncated sortilin, however, BACE1 recycling is halted, because sortilin is unable to retrogradely transport it out of the endosomes to the TGN for recycling. As such, BACE1 activity upon APP might likewise be halted, resulting in decreased BACE1 cleavage activity in the TGN. This decreased BACE1 cleavage results in decreased levels of sAPPβ; however, the additional effect of sortilin on CTF degradation leads to increased levels of C99 and Aβ.
In addition to its role in BACE1 trafficking, our data suggest that sortilin is also regulating CTF degradation. Specifically, the deletion of the sortilin cytoplasmic domain appears to enhance C99, leading to an increase in Aβ. Sortilin has been widely implicated in protein delivery to the lysosome. Interestingly, some studies of sortilin lysosomal trafficking have been shown to involve GGA adaptor proteins (27, 28). It is possible that directly, or indirectly, sortilin interacts with the CTFs and promotes their degradation in the lysosome. As such, sortilin might have multiple roles in the production of Aβ.
Interestingly, in our analysis of AD post-mortem brain tissue, we found increased levels of sortilin, which might play a role in the development of AD-related pathological changes. In contrast to our findings, Lah and colleagues (47, 48) have found decreased expression of SorLA in AD brains upon Western blot analysis. Such a discrepancy might be explained by a difference in both experimental and analytical assessment. Whereas our studies focused on protein expression within the temporal cortex, Lah and colleagues (47, 48) examined protein levels within the frontal cortex. Differential expression within various brain regions might account for our dissimilar results. In addition to experimental differences, our analytical assessments also diverge. In order to account for neuronal cell loss in our Western blot analysis, we normalized our Vps10p protein levels to neuron-specific cell markers. In comparison with normalization with β-actin, our results normalized to either TUJ1 or NeuN clearly elucidate Vps10p protein level changes between control versus AD subjects, despite neuronal cell loss incurred by the AD brain.
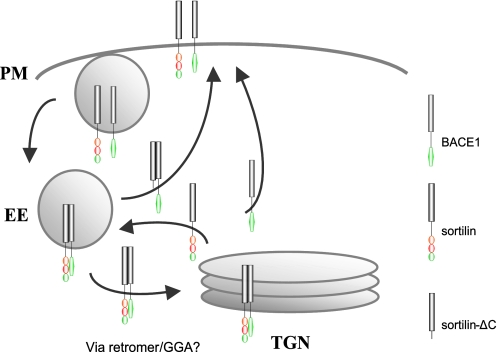
We propose our model for sortilin-mediated trafficking of BACE1 in Fig. 10. Full-length sortilin transports BACE1 from the endosome to the TGN. Endocytosis of BACE1 by sortilin is probably minimal, in which case we see little sortilin and BACE1 co-localization in the early endosomes. Furthermore, sortilin undergoes little recycling to the cell surface, and BACE1 recycling to the cell surface primarily occurs after TGN localization. This could explain why we see little full-length sortilin and BACE1 in the early endosomes and peripheral vesicular structures. In contrast, upon deletion of the sortilin cytoplasmic domain, neither sortilin nor its BACE1 cargo is able to undergo endosome-TGN transport. As a result, both sortilin and BACE1 accumulate within the endocytic pathway and on the plasma membrane. It is possible that this occurs because the proteins simply get “backed up,” unable to traffic through to the TGN. It is likely that truncated sortilin and its BACE1 cargo are being redirected to the cell surface, bypassing the TGN and thereby localizing to peripheral recycling endosomes and the plasma membrane. Sortilin is failing to retrieve BACE1 from the early endosomes to the TGN for recycling to the plasma membrane, and sortilin itself is possibly unable to travel back to the early endosomes from the TGN to retrieve more BACE1.
FIGURE 10.
Model for sortilin-mediated BACE1 trafficking. Trafficking of BACE1 by full-length sortilin. BACE1 and sortilin are endocytosed from the plasma membrane (PM) into the early endosome (EE). BACE1 gets retrogradely trafficked from the early endosome to the TGN by sortilin. We speculate that GGAs or retromer are involved in sortilin retrograde trafficking of BACE1. Sortilin cycles between the early endosome and the TGN. BACE1 is recycled to the plasma membrane via the TGN, forgoing degradation. Also depicted is the trafficking of BACE1 by truncated sortilin. BACE1 and sortilinΔC are endocytosed to the early endosome. Truncated sortilin is unable to retrogradely traffic BACE1 to the TGN, so sortilin and BACE1 trafficking are halted at the early endosome. It is likely that unphosphorylated BACE1 and truncated sortilin enter a direct recycling pathway, back to the plasma membrane, forgoing the TGN.
The specific retrograde transport of BACE1 from endosomes to the TGN probably occurs as a result of sorting motifs contained within the sortilin cytoplasmic tail. Two reported mechanisms for retrograde trafficking include GGA proteins and retromer complex. Both sortilin and BACE1 have been found to bind GGAs, and recent studies have suggested that GGAs might act in retrograde trafficking of proteins, specifically of BACE1 and sortilin (10, 17, 27). Given that sortilin and BACE1 appear to bind, GGA adaptor proteins may be involved in the retrograde trafficking of BACE1 indirectly, as GGAs transport sortilin to the TGN via its cytoplasmic domain. Furthermore, it has recently been found that sortilin interacts with retromer and is responsible for sortilin retrograde trafficking to the TGN (30). Evidence suggests that BACE1 also undergoes retrograde trafficking from the early endosomes to the TGN (10, 30). Given that the sortilin cytoplasmic tail contains similar sorting motifs as the CI-MPR and appears to mimic the trafficking patterns and function of CI-MPR, which itself undergoes retromer transport, it is highly plausible that sortilin also participates in retrograde trafficking of specific cargo, interacting with the Vps35 or Vps26 component of the retromer complex to drive cargo selection (much like the Vps10p and retromer in yeast). Such sortilin-retromer cargo could include BACE1.
Our studies introduce sortilin as an additional Vps10p domain receptor associated with the neuropathology of AD. SorLA has been found to interact directly with APP and co-localize primarily in the Golgi (36, 37). The effect of SorLA on APP appears to be the sequestration of APP to the TGN, inhibiting APP exit to the cell surface, as mediated by the SorLA cytoplasmic domain (36, 57). Whereas SorLA appears to regulate APP distribution and processing through its interaction with APP, sortilin appears to regulate APP processing by its interaction with BACE1 and modulation of BACE1 trafficking. Thus, among several representative members of the VPS10p domain receptors, sortilin plays a unique role in regulating BACE1-mediated cleavage of APP through regulating the subcellular distribution of BACE1.
Acknowledgments
We thank Anna Kane and A. Zenobia Moore for general technical support and members of the Kim laboratory for helpful discussions. We also thank Katarina Mancevska for services at the New York Brain Bank at Columbia University.
This work was supported by grants from the National Institutes of Health Alzheimer's Disease Research Center (AG08702, to T.-W. K.), American Health Assistance Foundation (to T.-W. K.), and the National Institute of Neurological Disorders and Stroke Predoctoral National Research Service Award (NS051957, to G. M. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–7.
- AD
- Alzheimer disease
- Aβ
- amyloid β-peptide
- APP
- β-amyloid precursor protein
- TGN
- trans-Golgi network
- CI-MPR
- cation-independent mannose 6-phosphate receptor
- CTF
- C-terminal fragment
- sort
- sortilin
- CFP
- cyan fluorescent protein.
REFERENCES
- 1. Cummings J. L. (2004) N. Engl. J. Med. 351, 56–67 [DOI] [PubMed] [Google Scholar]
- 2. Van Gassen G., Annaert W. (2003) Neuroscientist 9, 117–126 [DOI] [PubMed] [Google Scholar]
- 3. Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., Doan M., Dovey H. F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S. M., Wang S., Walker D., Zhao J., McConlogue L., John V. (1999) Nature 402, 537–540 [DOI] [PubMed] [Google Scholar]
- 4. Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- 5. Walter J., Kaether C., Steiner H., Haass C. (2001) Curr. Opin. Neurobiol. 11, 585–590 [DOI] [PubMed] [Google Scholar]
- 6. Huse J. T., Doms R. W. (2001) Traffic 2, 75–81 [DOI] [PubMed] [Google Scholar]
- 7. Koo E. H., Squazzo S. L. (1994) J. Biol. Chem. 269, 17386–17389 [PubMed] [Google Scholar]
- 8. Takahashi R. H., Nam E. E., Edgar M., Gouras G. K. (2002) Histol. Histopathol. 17, 239–246 [DOI] [PubMed] [Google Scholar]
- 9. Tienari P. J., Ida N., Ikonen E., Simons M., Weidemann A., Multhaup G., Masters C. L., Dotti C. G., Beyreuther K. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4125–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He X., Li F., Chang W. P., Tang J. (2005) J. Biol. Chem. 280, 11696–11703 [DOI] [PubMed] [Google Scholar]
- 11. Walter J., Fluhrer R., Hartung B., Willem M., Kaether C., Capell A., Lammich S., Multhaup G., Haass C. (2001) J. Biol. Chem. 276, 14634–14641 [DOI] [PubMed] [Google Scholar]
- 12. Huse J. T., Pijak D. S., Leslie G. J., Lee V. M., Doms R. W. (2000) J. Biol. Chem. 275, 33729–33737 [DOI] [PubMed] [Google Scholar]
- 13. He X., Zhu G., Koelsch G., Rodgers K. K., Zhang X. C., Tang J. (2003) Biochemistry 42, 12174–12180 [DOI] [PubMed] [Google Scholar]
- 14. He X., Chang W. P., Koelsch G., Tang J. (2002) FEBS Lett. 524, 183–187 [DOI] [PubMed] [Google Scholar]
- 15. Bonifacino J. S. (2004) Nat. Rev. Mol. Cell Biol. 5, 23–32 [DOI] [PubMed] [Google Scholar]
- 16. Kang E. L., Cameron A. N., Piazza F., Walker K. R., Tesco G. (2010) J. Biol. Chem. 285, 24108–24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wahle T., Prager K., Raffler N., Haass C., Famulok M., Walter J. (2005) Mol. Cell. Neurosci. 29, 453–461 [DOI] [PubMed] [Google Scholar]
- 18. Wahle T., Thal D. R., Sastre M., Rentmeister A., Bogdanovic N., Famulok M., Heneka M. T., Walter J. (2006) J. Neurosci. 26, 12838–12846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Arnim C. A., Spoelgen R., Peltan I. D., Deng M., Courchesne S., Koker M., Matsui T., Kowa H., Lichtenthaler S. F., Irizarry M. C., Hyman B. T. (2006) J. Neurosci. 26, 9913–9922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tesco G., Koh Y. H., Kang E. L., Cameron A. N., Das S., Sena-Esteves M., Hiltunen M., Yang S. H., Zhong Z., Shen Y., Simpkins J. W., Tanzi R. E. (2007) Neuron 54, 721–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seaman M. N. (2005) Trends Cell Biol. 15, 68–75 [DOI] [PubMed] [Google Scholar]
- 22. Cullen P. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 574–582 [DOI] [PubMed] [Google Scholar]
- 23. Okada H., Zhang W., Peterhoff C., Hwang J. C., Nixon R. A., Ryu S. H., Kim T. W. (2010) FASEB J. 24, 2783–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Small S. A., Kent K., Pierce A., Leung C., Kang M. S., Okada H., Honig L., Vonsattel J. P., Kim T. W. (2005) Ann. Neurol. 58, 909–919 [DOI] [PubMed] [Google Scholar]
- 25. Muhammad A., Flores I., Zhang H., Yu R., Staniszewski A., Planel E., Herman M., Ho L., Kreber R., Honig L. S., Ganetzky B., Duff K., Arancio O., Small S. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7327–7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petersen C. M., Nielsen M. S., Nykjaer A., Jacobsen L., Tommerup N., Rasmussen H. H., Roigaard H., Gliemann J., Madsen P., Moestrup S. K. (1997) J. Biol. Chem. 272, 3599–3605 [DOI] [PubMed] [Google Scholar]
- 27. Nielsen M. S., Madsen P., Christensen E. I., Nykjaer A., Gliemann J., Kasper D., Pohlmann R., Petersen C. M. (2001) EMBO J. 20, 2180–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lefrancois S., Zeng J., Hassan A. J., Canuel M., Morales C. R. (2003) EMBO J. 22, 6430–6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takatsu H., Katoh Y., Shiba Y., Nakayama K. (2001) J. Biol. Chem. 276, 28541–28545 [DOI] [PubMed] [Google Scholar]
- 30. Canuel M., Lefrancois S., Zeng J., Morales C. R. (2008) Biochem. Biophys. Res. Commun. 366, 724–730 [DOI] [PubMed] [Google Scholar]
- 31. Johnson K. F., Kornfeld S. (1992) J. Cell Biol. 119, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arighi C. N., Hartnell L. M., Aguilar R. C., Haft C. R., Bonifacino J. S. (2004) J. Cell Biol. 165, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seaman M. N. (2004) J. Cell Biol. 165, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mari M., Bujny M. V., Zeuschner D., Geerts W. J., Griffith J., Petersen C. M., Cullen P. J., Klumperman J., Geuze H. J. (2008) Traffic 9, 380–393 [DOI] [PubMed] [Google Scholar]
- 35. Kim E., Lee Y., Lee H. J., Kim J. S., Song B. S., Huh J. W., Lee S. R., Kim S. U., Kim S. H., Hong Y., Shim I., Chang K. T. (2010) Biochem. Biophys. Res. Commun. 403, 167–171 [DOI] [PubMed] [Google Scholar]
- 36. Andersen O. M., Reiche J., Schmidt V., Gotthardt M., Spoelgen R., Behlke J., von Arnim C. A., Breiderhoff T., Jansen P., Wu X., Bales K. R., Cappai R., Masters C. L., Gliemann J., Mufson E. J., Hyman B. T., Paul S. M., Nykjaer A., Willnow T. E. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Offe K., Dodson S. E., Shoemaker J. T., Fritz J. J., Gearing M., Levey A. I., Lah J. J. (2006) J. Neurosci. 26, 1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogaeva E., Meng Y., Lee J. H., Gu Y., Kawarai T., Zou F., Katayama T., Baldwin C. T., Cheng R., Hasegawa H., Chen F., Shibata N., Lunetta K. L., Pardossi-Piquard R., Bohm C., Wakutani Y., Cupples L. A., Cuenco K. T., Green R. C., Pinessi L., Rainero I., Sorbi S., Bruni A., Duara R., Friedland R. P., Inzelberg R., Hampe W., Bujo H., Song Y. Q., Andersen O. M., Willnow T. E., Graff-Radford N., Petersen R. C., Dickson D., Der S. D., Fraser P. E., Schmitt-Ulms G., Younkin S., Mayeux R., Farrer L. A., St George-Hyslop P. (2007) Nat. Genet. 39, 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bertram L., McQueen M. B., Mullin K., Blacker D., Tanzi R. E. (2007) Nat. Genet. 39, 17–23 [DOI] [PubMed] [Google Scholar]
- 40. Lane R. F., Raines S. M., Steele J. W., Ehrlich M. E., Lah J. A., Small S. A., Tanzi R. E., Attie A. D., Gandy S. (2010) J. Neurosci. 30, 13110–13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landman N., Jeong S. Y., Shin S. Y., Voronov S. V., Serban G., Kang M. S., Park M. K., Di Paolo G., Chung S., Kim T. W. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19524–19529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao J., Fu Y., Yasvoina M., Shao P., Hitt B., O'Connor T., Logan S., Maus E., Citron M., Berry R., Binder L., Vassar R. (2007) J. Neurosci. 27, 3639–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arnold S. E., Hyman B. T., Van Hoesen G. W. (1994) Arch. Neurol. 51, 145–150 [DOI] [PubMed] [Google Scholar]
- 44. Thal D. R., Rüb U., Schultz C., Sassin I., Ghebremedhin E., Del Tredici K., Braak E., Braak H. (2000) J. Neuropathol. Exp. Neurol. 59, 733–748 [DOI] [PubMed] [Google Scholar]
- 45. Sarret P., Krzywkowski P., Segal L., Nielsen M. S., Petersen C. M., Mazella J., Stroh T., Beaudet A. (2003) J. Comp. Neurol. 461, 483–505 [DOI] [PubMed] [Google Scholar]
- 46. Hermey G. (2009) Cell Mol. Life Sci. 66, 2677–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scherzer C. R., Offe K., Gearing M., Rees H. D., Fang G., Heilman C. J., Schaller C., Bujo H., Levey A. I., Lah J. J. (2004) Arch. Neurol. 61, 1200–1205 [DOI] [PubMed] [Google Scholar]
- 48. Dodson S. E., Gearing M., Lippa C. F., Montine T. J., Levey A. I., Lah J. J. (2006) J. Neuropathol. Exp. Neurol. 65, 866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seaman M. N. (2007) J. Cell Sci. 120, 2378–2389 [DOI] [PubMed] [Google Scholar]
- 50. Willnow T. E., Petersen C. M., Nykjaer A. (2008) Nat. Rev. Neurosci. 9, 899–909 [DOI] [PubMed] [Google Scholar]
- 51. Nielsen M. S., Gustafsen C., Madsen P., Nyengaard J. R., Hermey G., Bakke O., Mari M., Schu P., Pohlmann R., Dennes A., Petersen C. M. (2007) Mol. Cell. Biol. 27, 6842–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nielsen M. S., Keat S. J., Hamati J. W., Madsen P., Gutzmann J. J., Engelsberg A., Pedersen K. M., Gustafsen C., Nykjaer A., Gliemann J., Hermans-Borgmeyer I., Kuhl D., Petersen C. M., Hermey G. (2008) Traffic 9, 980–994 [DOI] [PubMed] [Google Scholar]
- 53. Rezgaoui M., Hermey G., Riedel I. B., Hampe W., Schaller H. C., Hermans-Borgmeyer I. (2001) Mech. Dev. 100, 335–338 [DOI] [PubMed] [Google Scholar]
- 54. Westergaard U. B., Kirkegaard K., Sørensen E. S., Jacobsen C., Nielsen M. S., Petersen C. M., Madsen P. (2005) FEBS Lett. 579, 1172–1176 [DOI] [PubMed] [Google Scholar]
- 55. Spoelgen R., von Arnim C. A., Thomas A. V., Peltan I. D., Koker M., Deng A., Irizarry M. C., Andersen O. M., Willnow T. E., Hyman B. T. (2006) J. Neurosci. 26, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caporaso G. L., Gandy S. E., Buxbaum J. D., Greengard P. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2252–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schmidt V., Sporbert A., Rohe M., Reimer T., Rehm A., Andersen O. M., Willnow T. E. (2007) J. Biol. Chem. 282, 32956–32964 [DOI] [PubMed] [Google Scholar]