Abstract
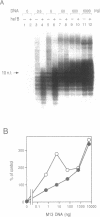
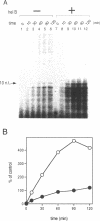
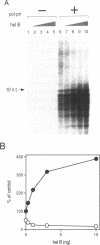
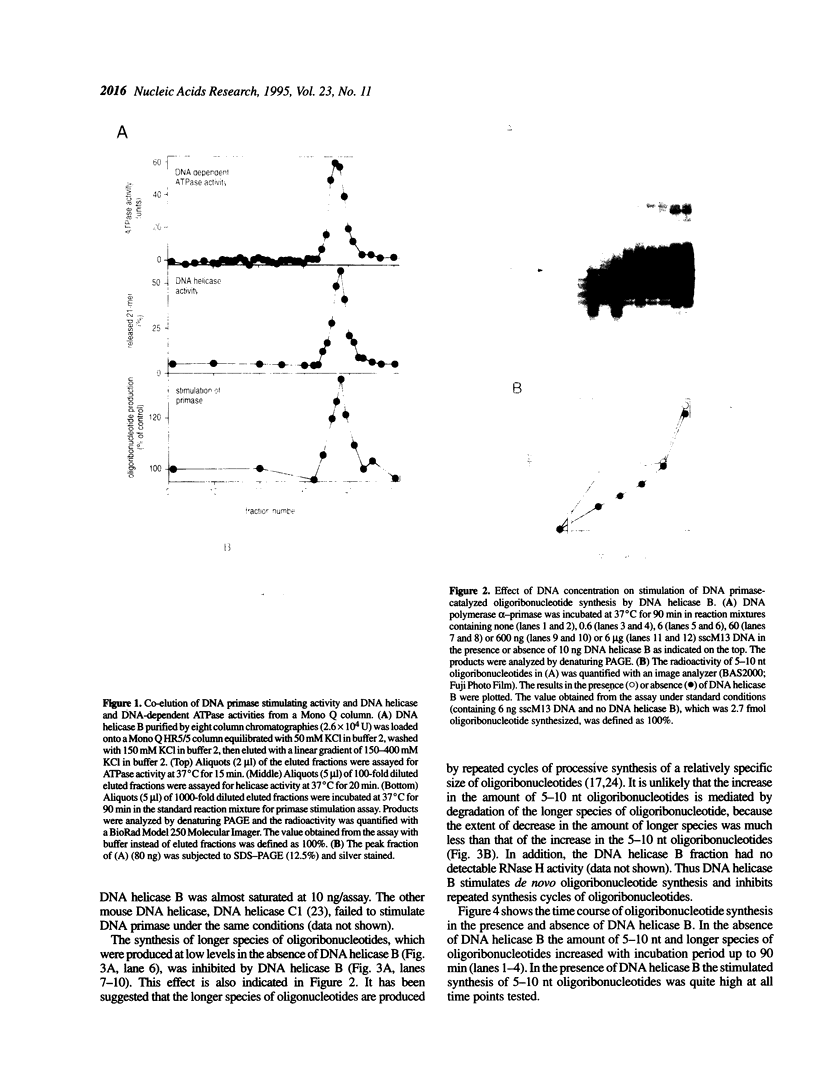
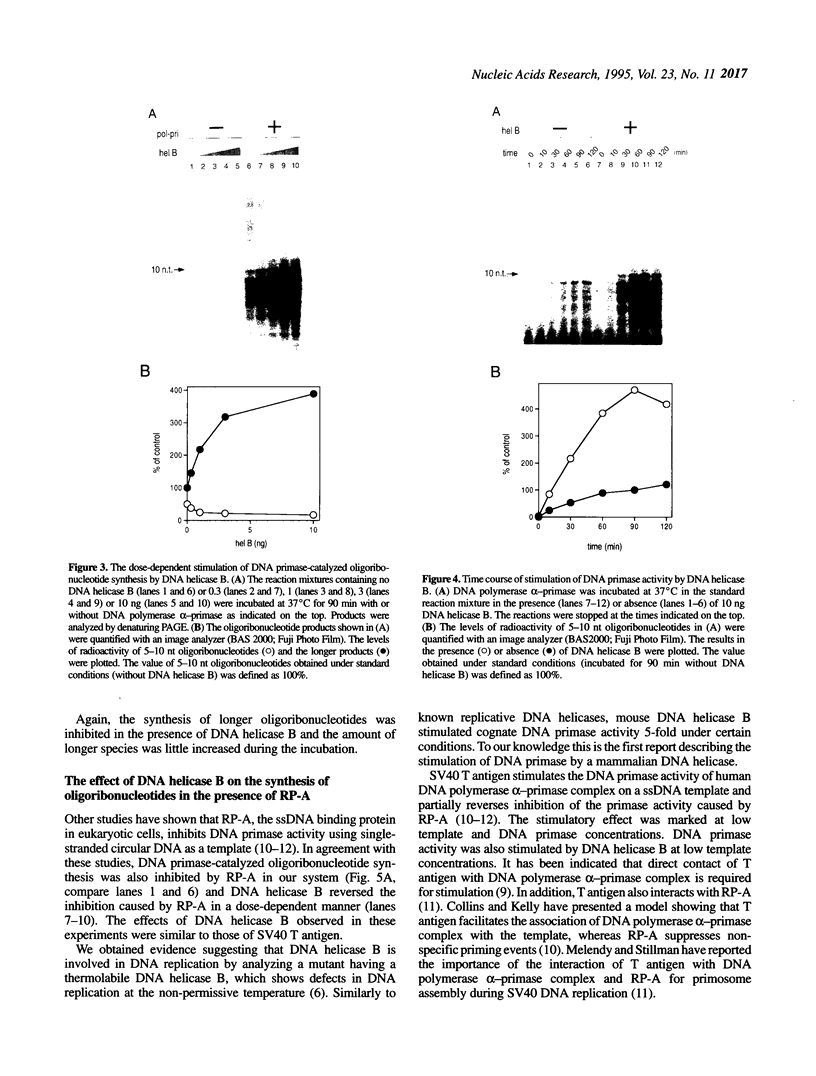
Many prokaryotic and viral DNA helicases involved in DNA replication stimulate their cognate DNA primase activity. To assess the stimulation of DNA primase activity by mammalian DNA helicases, we analyzed the synthesis of oligoribonucleotides by mouse DNA polymerase alpha-primase complex on single-stranded circular M13 DNA in the presence of mouse DNA helicase B. DNA helicase B was purified by sequential chromatography through eight columns. When the purified DNA helicase B was applied to a Mono Q column, the stimulatory activity for DNA primase-catalyzed oligoribonucleotide synthesis and DNA helicase and DNA-dependent ATPase activities of DNA helicase B were co-eluted from the column. The synthesis of oligoribonucleotides 5-10 nt in length was markedly stimulated by DNA helicase B. The synthesis of longer species of oligoribonucleotides, which were synthesized at a low level in the absence of DNA helicase B, was inhibited by DNA helicase B. The stimulatory effect of DNA helicase B was marked at low template concentrations and little or no effect was observed at high concentrations. The mouse single-stranded DNA binding protein, replication protein A (RP-A), inhibited the primase activity of the DNA polymerase alpha-primase complex and DNA helicase B partially reversed the inhibition caused by RP-A.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Collins K. L., Kelly T. J. Effects of T antigen and replication protein A on the initiation of DNA synthesis by DNA polymerase alpha-primase. Mol Cell Biol. 1991 Apr;11(4):2108–2115. doi: 10.1128/mcb.11.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J. J., Tsurumi T., Zhu L. A., Weller S. K., Olivo P. D., Challberg M. D., Mocarski E. S., Lehman I. R. Herpes simplex virus 1 helicase-primase: a complex of three herpes-encoded gene products. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2186–2189. doi: 10.1073/pnas.86.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornreiter I., Höss A., Arthur A. K., Fanning E. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 1990 Oct;9(10):3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Dean F. B., Kwong A. D., Lee S. H. The in vitro replication of DNA containing the SV40 origin. J Biol Chem. 1990 Oct 25;265(30):18043–18046. [PubMed] [Google Scholar]
- Ishimi Y., Matsumoto K. Model system for DNA replication of a plasmid DNA containing the autonomously replicating sequence from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5399–5403. doi: 10.1073/pnas.90.12.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman I. R., Kaguni L. S. DNA polymerase alpha. J Biol Chem. 1989 Mar 15;264(8):4265–4268. [PubMed] [Google Scholar]
- Masutani C., Enomoto T., Suzuki M., Hanaoka F., Ui M. DNA primase stimulatory factor from mouse FM3A cells has an RNase H activity. Purification of the factor and analysis of the stimulation. J Biol Chem. 1990 Jun 25;265(18):10210–10216. [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Melendy T., Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J Biol Chem. 1993 Feb 15;268(5):3389–3395. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Schneider C., Weisshart K., Guarino L. A., Dornreiter I., Fanning E. Species-specific functional interactions of DNA polymerase alpha-primase with simian virus 40 (SV40) T antigen require SV40 origin DNA. Mol Cell Biol. 1994 May;14(5):3176–3185. doi: 10.1128/mcb.14.5.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M., Enomoto T., Watanabe Y., Tawaragi Y., Kawasaki K., Hanaoka F., Yamada M. Purification and characterization of a deoxyribonucleic acid dependent adenosinetriphosphatase from mouse FM3A cells: effects of ribonucleoside triphosphates on the interaction of the enzyme with single-stranded DNA. Biochemistry. 1986 Jun 3;25(11):3239–3245. doi: 10.1021/bi00359a024. [DOI] [PubMed] [Google Scholar]
- Seki M., Enomoto T., Yanagisawa J., Hanaoka F., Ui M. Further characterization of DNA helicase activity of mouse DNA-dependent adenosinetriphosphatase B (DNA helicase B). Biochemistry. 1988 Mar 8;27(5):1766–1771. doi: 10.1021/bi00405a057. [DOI] [PubMed] [Google Scholar]
- Seki M., Kohda T., Yano T., Tada S., Yanagisawa J., Eki T., Ui M., Enomoto T. Characterization of DNA synthesis and DNA-dependent ATPase activity at a restrictive temperature in temperature-sensitive tsFT848 cells with thermolabile DNA helicase B. Mol Cell Biol. 1995 Jan;15(1):165–172. doi: 10.1128/mcb.15.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Enomoto T., Masutani C., Hanaoka F., Yamada M., Ui M. DNA primase-DNA polymerase alpha assembly from mouse FM3A cells. Purification of constituting enzymes, reconstitution, and analysis of RNA priming as coupled to DNA synthesis. J Biol Chem. 1989 Jun 15;264(17):10065–10071. [PubMed] [Google Scholar]
- Takada-Takayama R., Tada S., Hanaoka F., Ui M. Peptide mapping of the four subunits of the mouse DNA polymerase alpha-primase complex. Biochem Biophys Res Commun. 1990 Jul 31;170(2):589–595. doi: 10.1016/0006-291x(90)92132-j. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Nagata K., Tawaragi Y., Enomoto T., Hanaoka F., Yamada M. DNA-dependent ATPase B of FM3A cells. Its separation from DNA polymerase alpha. FEBS Lett. 1982 Nov 22;149(1):44–46. doi: 10.1016/0014-5793(82)81067-3. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA replicase. DNA polymerase associated with a novel RNA polymerase activity to synthesize initiator RNA of strict size. J Biol Chem. 1982 Sep 25;257(18):11121–11127. [PubMed] [Google Scholar]
- Yanagisawa J., Seki M., Kohda T., Enomoto T., Ui M. DNA-dependent adenosinetriphosphatase C1 from mouse FM3A cells has DNA helicase activity. J Biol Chem. 1992 Feb 25;267(6):3644–3649. [PubMed] [Google Scholar]
- Ziegelin G., Scherzinger E., Lurz R., Lanka E. Phage P4 alpha protein is multifunctional with origin recognition, helicase and primase activities. EMBO J. 1993 Sep;12(9):3703–3708. doi: 10.1002/j.1460-2075.1993.tb06045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]