Abstract
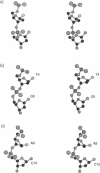
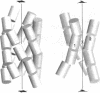
A sequence that is represented frequently in functionally important sites involving protein-DNA interactions is GTG/CAC, suggesting that the trimer may play a role in regulatory processes. The 2.5 A resolution structure of d(CGGTGG)/d(CCACCG), a part of the interior operator (OI, nucleotides +44 to +49) of the gal operon, co-crystallized with spermine, is described herein. The crystal packing arrangement in this structure is unprecedented in a crystal of B-DNA, revealing a close packing of columns of stacked DNA resembling a 5-stranded twisted wire cable. The final structure contains one hexamer duplex, 17 water molecules and 1.5 spermine molecules per crystallographic asymmetric unit. The hexamer exhibits base-pair opening and shearing at T.A resulting in a novel non-Watson-Crick hydrogen-bonding scheme between adenine and thymine in the GTG region. The ability of this sequence to adopt unusual conformations in its GTG region may be a critical factor conferring sequence selectivity on the binding of Gal repressor. In addition, this is the first conclusive example of a crystal structure of spermine with native B-DNA, providing insight into the mechanics of polyamine-DNA binding, as well as possible explanations for the biological action of spermine.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anin M. F., Leng M. Distortions induced in double-stranded oligonucleotides by the binding of cis- or trans-diammine-dichloroplatinum(II) to the d(GTG) sequence. Nucleic Acids Res. 1990 Aug 11;18(15):4395–4400. doi: 10.1093/nar/18.15.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Braunlin W. H., Strick T. J., Record M. T., Jr Equilibrium dialysis studies of polyamine binding to DNA. Biopolymers. 1982 Jul;21(7):1301–1314. doi: 10.1002/bip.360210704. [DOI] [PubMed] [Google Scholar]
- Brennan R. G., Roderick S. L., Takeda Y., Matthews B. W. Protein-DNA conformational changes in the crystal structure of a lambda Cro-operator complex. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8165–8169. doi: 10.1073/pnas.87.20.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briki F., Genest D. Molecular dynamics study of the base pair opening process in the self-complementary octanucleotide d(CTGATCAG). J Biomol Struct Dyn. 1993 Aug;11(1):43–56. doi: 10.1080/07391102.1993.10508708. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. DNA structure from A to Z. Methods Enzymol. 1992;211:67–111. doi: 10.1016/0076-6879(92)11007-6. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. Definitions and nomenclature of nucleic acid structure components. Nucleic Acids Res. 1989 Mar 11;17(5):1797–1803. doi: 10.1093/nar/17.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan M. E., Lu P. Transcriptional enhancer related DNA sequences: anomalous 1H NMR NOE crosspeaks. Nucleic Acids Res. 1992 Feb 11;20(3):525–532. doi: 10.1093/nar/20.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Fawthrop S. A., Yang J. C., Fisher J. Structural and dynamic studies of a non-self-complementary dodecamer DNA duplex. Nucleic Acids Res. 1993 Oct 25;21(21):4860–4866. doi: 10.1093/nar/21.21.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Spermine-DNA interactions: a theoretical study. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5948–5952. doi: 10.1073/pnas.83.16.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Williams L. D., Basu H. S., Marton L. J. Implications and concepts of polyamine-nucleic acid interactions. J Cell Biochem. 1991 May;46(1):37–47. doi: 10.1002/jcb.240460107. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Guéron M., Kochoyan M., Leroy J. L. A single mode of DNA base-pair opening drives imino proton exchange. Nature. 1987 Jul 2;328(6125):89–92. doi: 10.1038/328089a0. [DOI] [PubMed] [Google Scholar]
- Hartmann B., Leng M., Ramstein J. Poly(dA-dT).poly(dA-dT) two-pathway proton exchange mechanism. Effect of general and specific base catalysis on deuteration rates. Biochemistry. 1986 Jun 3;25(11):3073–3077. doi: 10.1021/bi00359a001. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Sakamoto I., Goto N., Kashiwagi K., Honma R., Hirose S. Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys. 1982 Dec;219(2):438–443. doi: 10.1016/0003-9861(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Jordan S. R., Pabo C. O. Structure of the lambda complex at 2.5 A resolution: details of the repressor-operator interactions. Science. 1988 Nov 11;242(4880):893–899. doi: 10.1126/science.3187530. [DOI] [PubMed] [Google Scholar]
- Kahn J. D., Yun E., Crothers D. M. Detection of localized DNA flexibility. Nature. 1994 Mar 10;368(6467):163–166. doi: 10.1038/368163a0. [DOI] [PubMed] [Google Scholar]
- Larsen T. A., Kopka M. L., Dickerson R. E. Crystal structure analysis of the B-DNA dodecamer CGTGAATTCACG. Biochemistry. 1991 May 7;30(18):4443–4449. doi: 10.1021/bi00232a010. [DOI] [PubMed] [Google Scholar]
- Lavery R., Sklenar H. Defining the structure of irregular nucleic acids: conventions and principles. J Biomol Struct Dyn. 1989 Feb;6(4):655–667. doi: 10.1080/07391102.1989.10507728. [DOI] [PubMed] [Google Scholar]
- Leijon M., Gräslund A. Effects of sequence and length on imino proton exchange and base pair opening kinetics in DNA oligonucleotide duplexes. Nucleic Acids Res. 1992 Oct 25;20(20):5339–5343. doi: 10.1093/nar/20.20.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J. L., Gao X. L., Guéron M., Patel D. J. Proton exchange and internal motions in two chromomycin dimer-DNA oligomer complexes. Biochemistry. 1991 Jun 11;30(23):5653–5661. doi: 10.1021/bi00237a003. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Kochoyan M., Huynh-Dinh T., Guéron M. Characterization of base-pair opening in deoxynucleotide duplexes using catalyzed exchange of the imino proton. J Mol Biol. 1988 Mar 20;200(2):223–238. doi: 10.1016/0022-2836(88)90236-7. [DOI] [PubMed] [Google Scholar]
- Lu P., Cheung S., Arndt K. Possible molecular detent in the DNA structure at regulatory sequences. J Biomol Struct Dyn. 1983 Oct;1(2):509–521. doi: 10.1080/07391102.1983.10507458. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Effect of ethylation of operator-phosphates on Gal repressor binding. DNA contortion by repressor. J Mol Biol. 1989 Jul 20;208(2):217–223. doi: 10.1016/0022-2836(89)90383-5. [DOI] [PubMed] [Google Scholar]
- Maltseva T. V., Agback P., Chattopadhyaya J. How much hydration is necessary for the stabilisation of DNA-duplex? Nucleic Acids Res. 1993 Sep 11;21(18):4246–4252. doi: 10.1093/nar/21.18.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Protein-DNA interaction. No code for recognition. Nature. 1988 Sep 22;335(6188):294–295. doi: 10.1038/335294a0. [DOI] [PubMed] [Google Scholar]
- Meers P., Hong K., Bentz J., Papahadjopoulos D. Spermine as a modulator of membrane fusion: interactions with acidic phospholipids. Biochemistry. 1986 Jun 3;25(11):3109–3118. doi: 10.1021/bi00359a007. [DOI] [PubMed] [Google Scholar]
- Narayana N., Ginell S. L., Russu I. M., Berman H. M. Crystal and molecular structure of a DNA fragment: d(CGTGAATTCACG). Biochemistry. 1991 May 7;30(18):4449–4455. doi: 10.1021/bi00232a011. [DOI] [PubMed] [Google Scholar]
- Olson W. K. Computational studies of polynucleotide flexibility. Nucleic Acids Res. 1982 Feb 11;10(3):777–787. doi: 10.1093/nar/10.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Pingoud A. Spermidine increases the accuracy of type II restriction endonucleases. Suppression of cleavage at degenerate, non-symmetrical sites. Eur J Biochem. 1985 Feb 15;147(1):105–109. doi: 10.1111/j.1432-1033.1985.tb08725.x. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Alves J., Ehbrecht H. J., Zabeau M., Gualerzi C. Effect of polyamines and basic proteins on cleavage of DNA by restriction endonucleases. Biochemistry. 1984 Nov 20;23(24):5697–5703. doi: 10.1021/bi00319a006. [DOI] [PubMed] [Google Scholar]
- Plaxco K. W., Goddard W. A., 3rd Contributions of the thymine methyl group to the specific recognition of poly- and mononucleotides: an analysis of the relative free energies of solvation of thymine and uracil. Biochemistry. 1994 Mar 15;33(10):3050–3054. doi: 10.1021/bi00176a038. [DOI] [PubMed] [Google Scholar]
- Pullman B. Electrostatics of Polymorphic DNA. J Biomol Struct Dyn. 1983 Dec;1(3):773–794. doi: 10.1080/07391102.1983.10507481. [DOI] [PubMed] [Google Scholar]
- Quintana J. R., Grzeskowiak K., Yanagi K., Dickerson R. E. Structure of a B-DNA decamer with a central T-A step: C-G-A-T-T-A-A-T-C-G. J Mol Biol. 1992 May 20;225(2):379–395. doi: 10.1016/0022-2836(92)90928-d. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Artz P., Lu P. Origin of the asymmetrical contact between lac repressor and lac operator DNA. J Mol Biol. 1993 Oct 5;233(3):389–399. doi: 10.1006/jmbi.1993.1519. [DOI] [PubMed] [Google Scholar]
- Sarai A., Takeda Y. Lambda repressor recognizes the approximately 2-fold symmetric half-operator sequences asymmetrically. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6513–6517. doi: 10.1073/pnas.86.17.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Ionic and structural effects on the thermal helix-coil transition of DNA complexed with natural and synthetic polyamines. Biopolymers. 1984 Jul;23(7):1295–1306. doi: 10.1002/bip.360230713. [DOI] [PubMed] [Google Scholar]
- Thomas T., Kiang D. T. A twenty-two-fold increase in the relative affinity of estrogen receptor to poly (dA-dC).poly (dG-dT) in the presence of polyamines. Nucleic Acids Res. 1988 May 25;16(10):4705–4720. doi: 10.1093/nar/16.10.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit Y., Westhof E., Fuchs R. P., Moras D. Unusual helical packing in crystals of DNA bearing a mutation hot spot. Nature. 1989 Oct 5;341(6241):459–462. doi: 10.1038/341459a0. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Srivenugopal K. S., Reid B. R., Morris D. R. Nuclear magnetic resonance studies of polyamine binding to a defined DNA sequence. J Mol Biol. 1985 Sep 20;185(2):457–459. doi: 10.1016/0022-2836(85)90418-8. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Frederick C. A., Ughetto G., Rich A. Ternary interactions of spermine with DNA: 4'-epiadriamycin and other DNA: anthracycline complexes. Nucleic Acids Res. 1990 Sep 25;18(18):5533–5541. doi: 10.1093/nar/18.18.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Swank R. A., Matthews H. R. Photoaffinity polyamines: sequence-specific interactions with DNA. Nucleic Acids Res. 1991 Jul 11;19(13):3701–3708. doi: 10.1093/nar/19.13.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska K., Pullman B. Spermine-nucleic acid interactions: a theoretical study. Biopolymers. 1986 Mar;25(3):375–392. doi: 10.1002/bip.360250302. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Kim J., Adhya S. DNA bending by negative regulatory proteins: Gal and Lac repressors. Genes Dev. 1989 May;3(5):606–611. doi: 10.1101/gad.3.5.606. [DOI] [PubMed] [Google Scholar]