Abstract
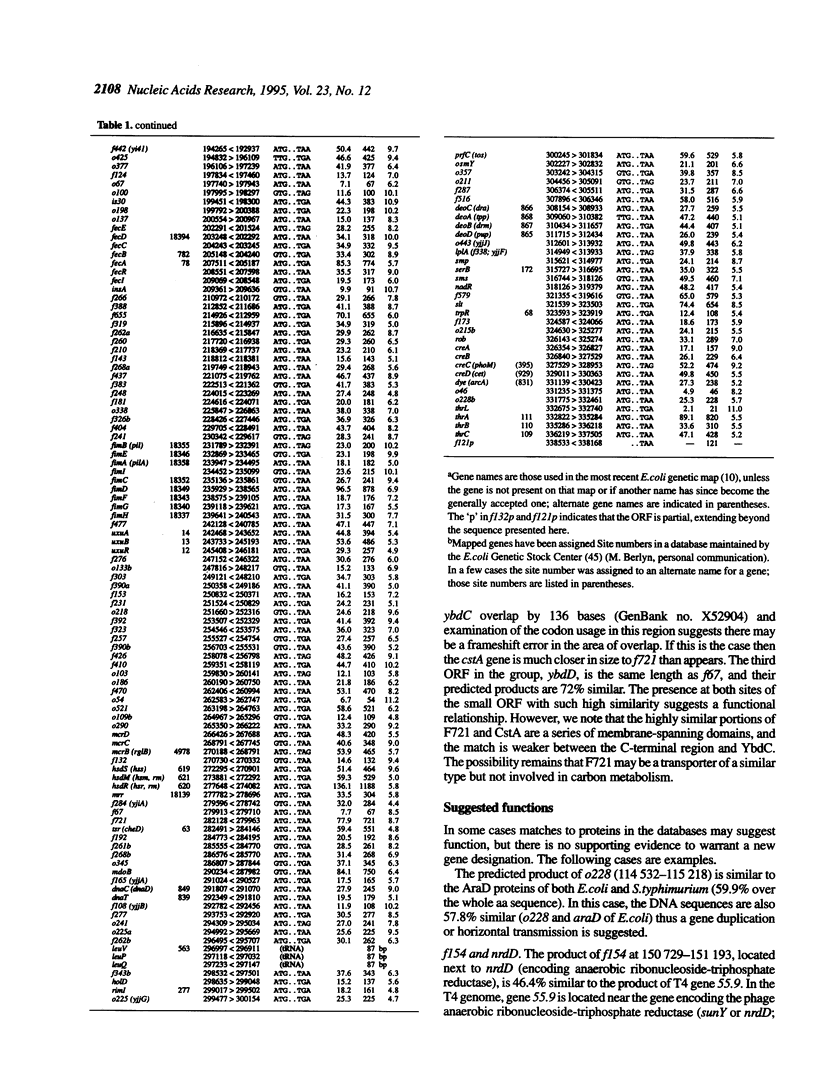
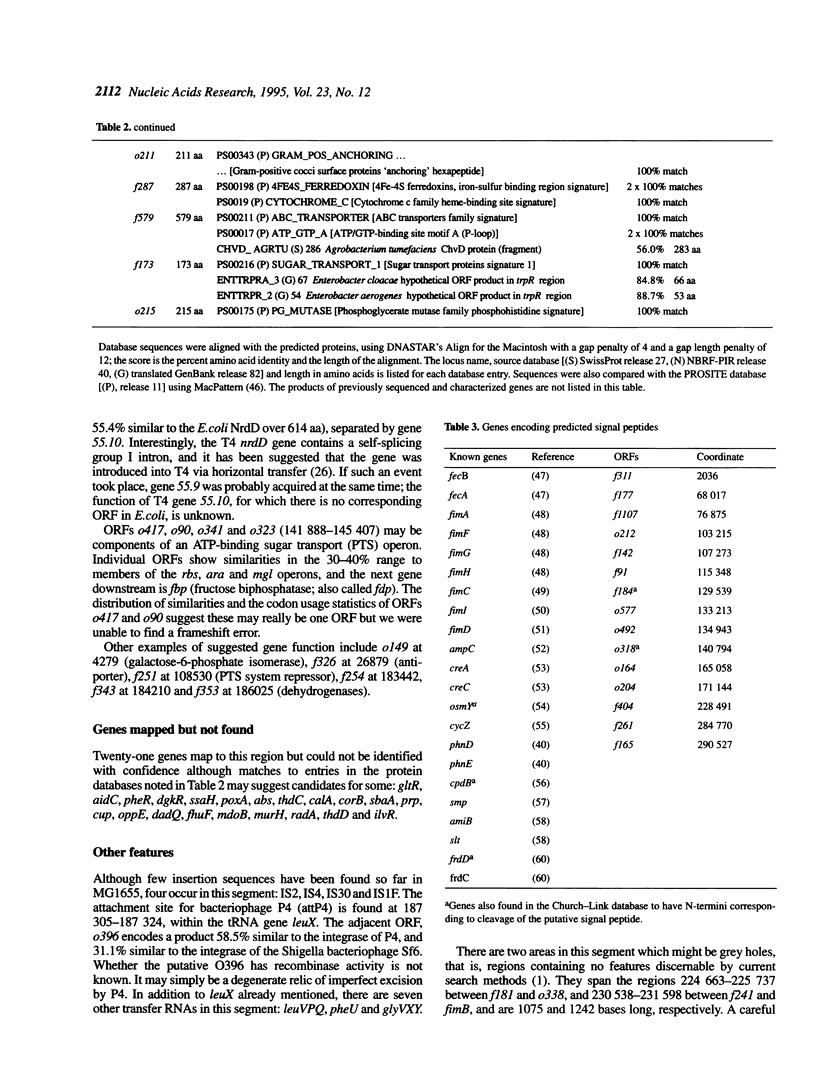
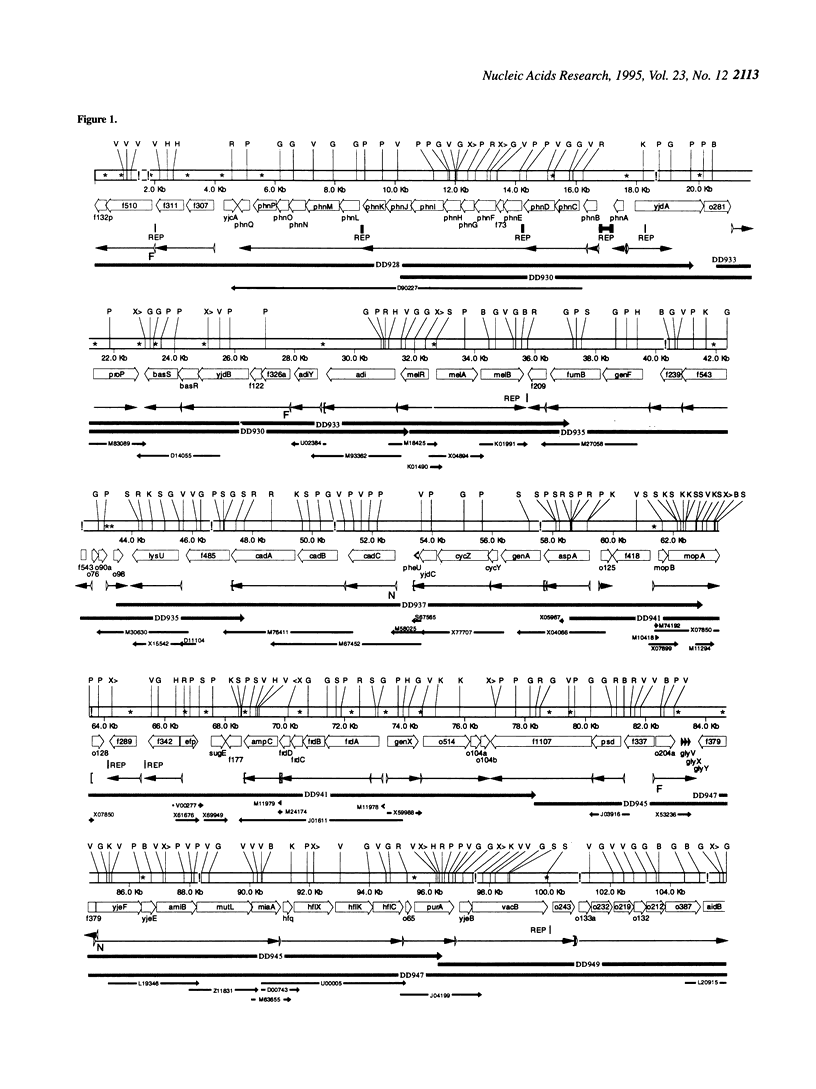
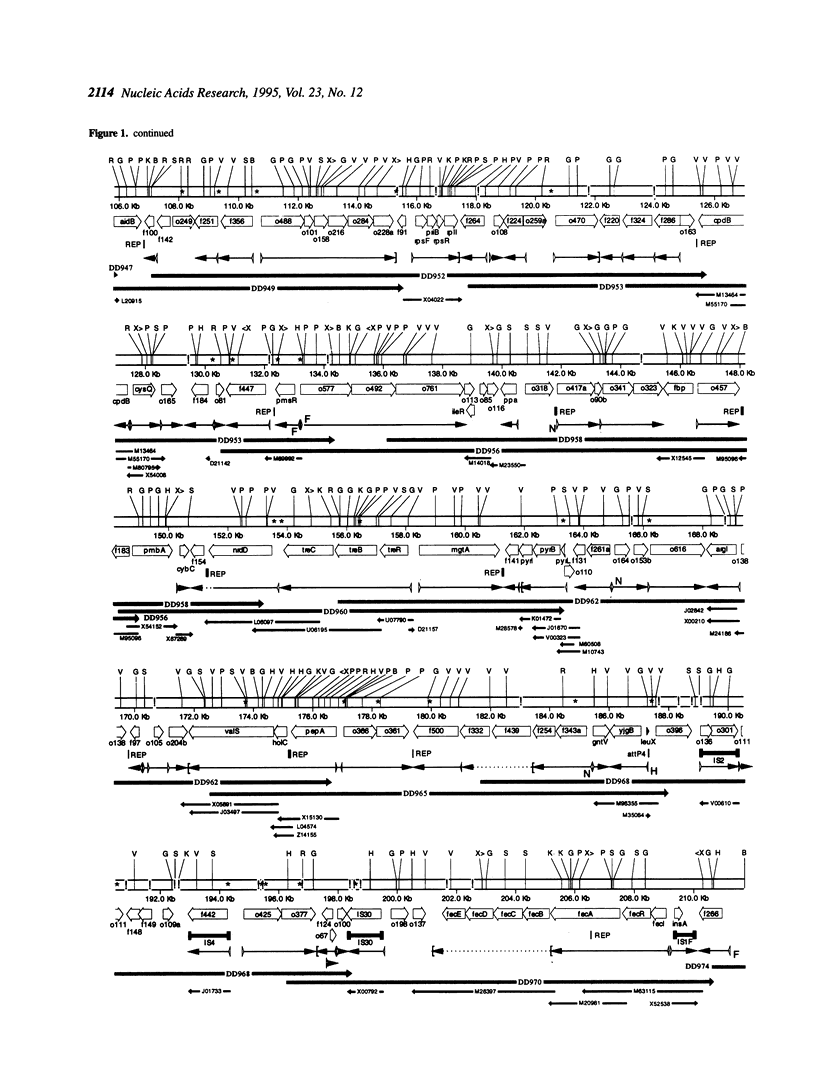
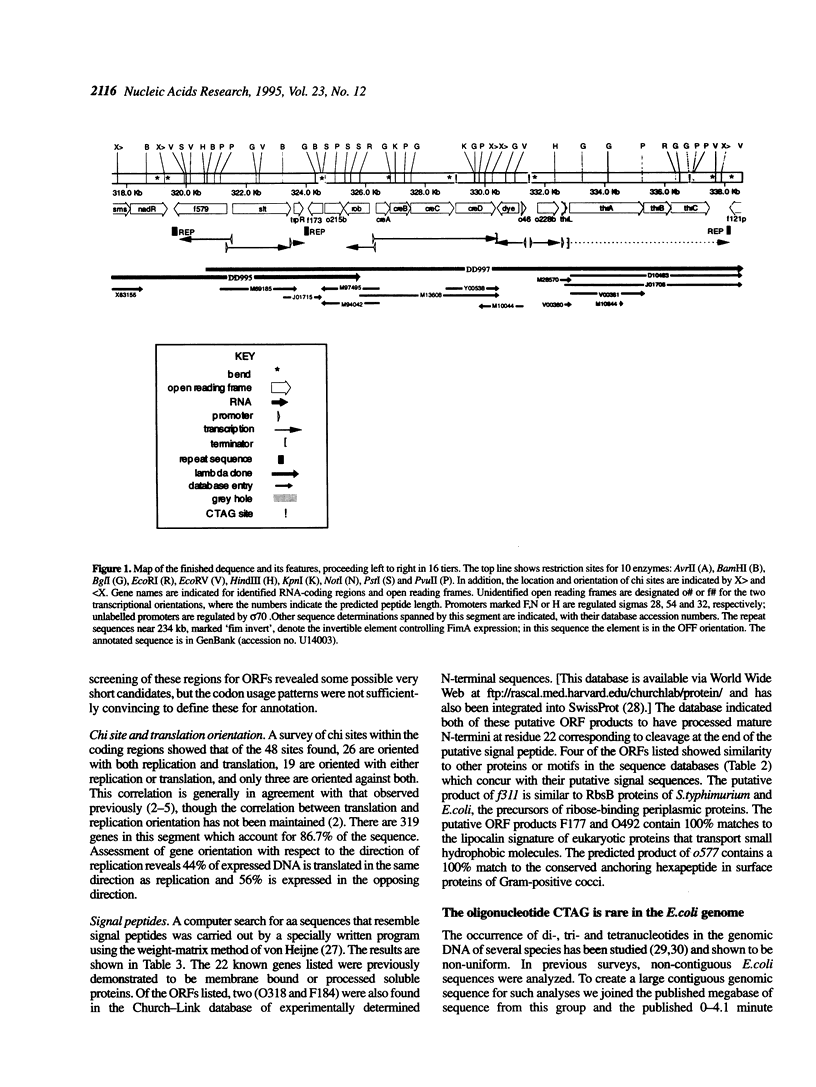
The 338.5 kb of the Escherichia coli genome described here together with previously described segments bring the total of contiguous finished sequence of this genome to > 1 Mb. Of 319 open reading frames (ORFs) found in this 338.5 kb segment, 147 (46%) are potential new genes. The positions of several genes which had been previously located here by mapping or partial sequencing have been confirmed. Several ORFs have functions suggested by similarities to other characterised genes but cannot be assigned with certainty. Fifteen of the ORFs of unknown function had been previously sequenced. Eight transfer RNAs are encoded in the region and there are two grey holes in which no features were found. The attachment site for phage P4 and three insertion sequences were located. The region was also analysed for chi sites, bend sites, REP elements and other repeats. A computer search identified potential promoters and tentative transcription units were assigned. The occurrence of the rare tetramer CTAG was analysed in 1.6 Mb of contiguous E.coli sequence. Hypotheses addressing the rarity and distribution of CTAG are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura M., Makino K., Shinagawa H., Nakata A. Nucleotide sequence of the phoM region of Escherichia coli: four open reading frames may constitute an operon. J Bacteriol. 1986 Oct;168(1):294–302. doi: 10.1128/jb.168.1.294-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B., Letovsky S. Genome-related datasets within the E. coli Genetic Stock Center database. Nucleic Acids Res. 1992 Dec 11;20(23):6143–6151. doi: 10.1093/nar/20.23.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat A. S., McClelland M. DNA mismatch correction by Very Short Patch repair may have altered the abundance of oligonucleotides in the E. coli genome. Nucleic Acids Res. 1992 Apr 11;20(7):1663–1668. doi: 10.1093/nar/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Ritzenthaler P., Kolb A. The regulatory region of the uxuAB operon in Escherichia coli K12. Mol Gen Genet. 1986 Jan;202(1):112–119. doi: 10.1007/BF00330526. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Burland V., Plunkett G., 3rd, Sofia H. J., Daniels D. L. Analysis of the Escherichia coli genome. IV. DNA sequence of the region from 89.2 to 92.8 minutes. Nucleic Acids Res. 1993 Nov 25;21(23):5408–5417. doi: 10.1093/nar/21.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum P. H., Jovanovich S. B., McCann M. P., Schultz J. E., Lesley S. A., Burgess R. R., Matin A. Cloning and in vivo and in vitro regulation of cyclic AMP-dependent carbon starvation genes from Escherichia coli. J Bacteriol. 1990 Jul;172(7):3813–3820. doi: 10.1128/jb.172.7.3813-3820.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge C., Campbell A. M., Karlin S. Over- and under-representation of short oligonucleotides in DNA sequences. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1358–1362. doi: 10.1073/pnas.89.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland V., Daniels D. L., Plunkett G., 3rd, Blattner F. R. Genome sequencing on both strands: the Janus strategy. Nucleic Acids Res. 1993 Jul 25;21(15):3385–3390. doi: 10.1093/nar/21.15.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland V., Plunkett G., 3rd, Daniels D. L., Blattner F. R. DNA sequence and analysis of 136 kilobases of the Escherichia coli genome: organizational symmetry around the origin of replication. Genomics. 1993 Jun;16(3):551–561. doi: 10.1006/geno.1993.1230. [DOI] [PubMed] [Google Scholar]
- Bächi B., Kornberg H. L. Genes involved in the uptake and catabolism of gluconate by Escherichia coli. J Gen Microbiol. 1975 Oct;90(2):321–335. doi: 10.1099/00221287-90-2-321. [DOI] [PubMed] [Google Scholar]
- Chen G. T., Inouye M. Role of the AGA/AGG codons, the rarest codons in global gene expression in Escherichia coli. Genes Dev. 1994 Nov 1;8(21):2641–2652. doi: 10.1101/gad.8.21.2641. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., Plunkett G., 3rd, Burland V., Blattner F. R. Analysis of the Escherichia coli genome: DNA sequence of the region from 84.5 to 86.5 minutes. Science. 1992 Aug 7;257(5071):771–778. doi: 10.1126/science.1379743. [DOI] [PubMed] [Google Scholar]
- Engel H., Kazemier B., Keck W. Murein-metabolizing enzymes from Escherichia coli: sequence analysis and controlled overexpression of the slt gene, which encodes the soluble lytic transglycosylase. J Bacteriol. 1991 Nov;173(21):6773–6782. doi: 10.1128/jb.173.21.6773-6782.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Park Y. K., Penfound T., Fenger T., Spector M. P. Regulation of NAD metabolism in Salmonella typhimurium: molecular sequence analysis of the bifunctional nadR regulator and the nadA-pnuC operon. J Bacteriol. 1990 Aug;172(8):4187–4196. doi: 10.1128/jb.172.8.4187-4196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Penfound T. The bifunctional NadR regulator of Salmonella typhimurium: location of regions involved with DNA binding, nucleotide transport and intramolecular communication. FEMS Microbiol Lett. 1993 Sep 1;112(2):179–183. doi: 10.1111/j.1574-6968.1993.tb06445.x. [DOI] [PubMed] [Google Scholar]
- Fuchs R. MacPattern: protein pattern searching on the Apple Macintosh. Comput Appl Biosci. 1991 Jan;7(1):105–106. doi: 10.1093/bioinformatics/7.1.105. [DOI] [PubMed] [Google Scholar]
- Fujita N., Mori H., Yura T., Ishihama A. Systematic sequencing of the Escherichia coli genome: analysis of the 2.4-4.1 min (110,917-193,643 bp) region. Nucleic Acids Res. 1994 May 11;22(9):1637–1639. doi: 10.1093/nar/22.9.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T., Miwa Y., Nihashi J., Aratani Y. Organization and transcription of the gluconate operon, gnt, of Bacillus subtilis. J Biol Chem. 1986 Oct 15;261(29):13744–13753. [PubMed] [Google Scholar]
- Greener T., Govezensky D., Zamir A. A novel multicopy suppressor of a groEL mutation includes two nested open reading frames transcribed from different promoters. EMBO J. 1993 Mar;12(3):889–896. doi: 10.1002/j.1460-2075.1993.tb05729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istúriz T., Palmero E., Vitelli-Flores J. Mutations affecting gluconate catabolism in Escherichia coli. Genetic mapping of the locus for the thermosensitive gluconokinase. J Gen Microbiol. 1986 Nov;132(11):3209–3219. doi: 10.1099/00221287-132-11-3209. [DOI] [PubMed] [Google Scholar]
- Jones C. H., Pinkner J. S., Nicholes A. V., Slonim L. N., Abraham S. N., Hultgren S. J. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8397–8401. doi: 10.1073/pnas.90.18.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Cardon L. R. Computational DNA sequence analysis. Annu Rev Microbiol. 1994;48:619–654. doi: 10.1146/annurev.mi.48.100194.003155. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. The fimD gene required for cell surface localization of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1990 Jan;220(2):334–338. doi: 10.1007/BF00260505. [DOI] [PubMed] [Google Scholar]
- Klemm P., Krogfelt K. A., Hedegaard L., Christiansen G. The major subunit of Escherichia coli type 1 fimbriae is not required for D-mannose-specific adhesion. Mol Microbiol. 1990 Apr;4(4):553–559. doi: 10.1111/j.1365-2958.1990.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Lanfroy E., Bohin J. P. Physical map location of the Escherichia coli gene encoding phosphoglycerol transferase I. J Bacteriol. 1993 Sep;175(17):5736–5737. doi: 10.1128/jb.175.17.5736-5737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Burns D. M., Beacham I. R. Isolation and sequence analysis of the gene (cpdB) encoding periplasmic 2',3'-cyclic phosphodiesterase. J Bacteriol. 1986 Mar;165(3):1002–1010. doi: 10.1128/jb.165.3.1002-1010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Kim S. K., Shinagawa H., Amemura M., Nakata A. Molecular analysis of the cryptic and functional phn operons for phosphonate use in Escherichia coli K-12. J Bacteriol. 1991 Apr;173(8):2665–2672. doi: 10.1128/jb.173.8.2665-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Bhagwat A. S. Biased DNA repair. Nature. 1992 Feb 13;355(6361):595–596. doi: 10.1038/355595b0. [DOI] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. Selection against dam methylation sites in the genomes of DNA of enterobacteriophages. J Mol Evol. 1984;21(4):317–322. doi: 10.1007/BF02115649. [DOI] [PubMed] [Google Scholar]
- Merkl R., Kröger M., Rice P., Fritz H. J. Statistical evaluation and biological interpretation of non-random abundance in the E. coli K-12 genome of tetra- and pentanucleotide sequences related to VSP DNA mismatch repair. Nucleic Acids Res. 1992 Apr 11;20(7):1657–1662. doi: 10.1093/nar/20.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf W. W., Wanner B. L. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene. 1993 Jul 15;129(1):27–32. doi: 10.1016/0378-1119(93)90692-v. [DOI] [PubMed] [Google Scholar]
- Metcalf W. W., Wanner B. L. Mutational analysis of an Escherichia coli fourteen-gene operon for phosphonate degradation, using TnphoA' elements. J Bacteriol. 1993 Jun;175(11):3430–3442. doi: 10.1128/jb.175.11.3430-3442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Daniel A. S., Cowan G. M., Sharp P. M. Conservation of motifs within the unusually variable polypeptide sequences of type I restriction and modification enzymes. Mol Microbiol. 1993 Jul;9(1):133–143. doi: 10.1111/j.1365-2958.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Médigue C., Rouxel T., Vigier P., Hénaut A., Danchin A. Evidence for horizontal gene transfer in Escherichia coli speciation. J Mol Biol. 1991 Dec 20;222(4):851–856. doi: 10.1016/0022-2836(91)90575-q. [DOI] [PubMed] [Google Scholar]
- Neuwald A. F., Stauffer G. V. An Escherichia coli membrane protein with a unique signal sequence. Gene. 1989 Oct 30;82(2):219–228. doi: 10.1016/0378-1119(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Plunkett G., 3rd, Burland V., Daniels D. L., Blattner F. R. Analysis of the Escherichia coli genome. III. DNA sequence of the region from 87.2 to 89.2 minutes. Nucleic Acids Res. 1993 Jul 25;21(15):3391–3398. doi: 10.1093/nar/21.15.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler P., Mata-Gilsinger M., Stoeber F. Construction and expression of hybrid plasmids containing Escherichia coli K-12 uxu genes. J Bacteriol. 1980 Sep;143(3):1116–1126. doi: 10.1128/jb.143.3.1116-1126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler P., Mata-Gilsinger M. Use of in vitro gene fusions to study the uxuR regulatory gene in Escherichia coli K-12: direction of transcription and regulation of its expression. J Bacteriol. 1982 Jun;150(3):1040–1047. doi: 10.1128/jb.150.3.1040-1047.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C. Mutations affectant le catabolisme du glucuronate chez Escherichia coli K12. Mol Gen Genet. 1974;131(1):31–46. doi: 10.1007/BF00269385. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J., Portalier R., Stoeber F. Regulation of hexuronate system genes in Escherichia coli K-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J Bacteriol. 1981 Jan;145(1):211–220. doi: 10.1128/jb.145.1.211-220.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossolini G. M., Muscas P., Chiesurin A., Satta G. Analysis of the Salmonella fim gene cluster: identification of a new gene (fimI) encoding a fimbrin-like protein and located downstream from the fimA gene. FEMS Microbiol Lett. 1993 Dec 15;114(3):259–265. doi: 10.1111/j.1574-6968.1993.tb06583.x. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Matin A. Molecular and functional characterization of a carbon starvation gene of Escherichia coli. J Mol Biol. 1991 Mar 5;218(1):129–140. doi: 10.1016/0022-2836(91)90879-b. [DOI] [PubMed] [Google Scholar]
- Sofia H. J., Burland V., Daniels D. L., Plunkett G., 3rd, Blattner F. R. Analysis of the Escherichia coli genome. V. DNA sequence of the region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994 Jul 11;22(13):2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenmaier H., Van Hove B., Yaraghi Z., Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989 May;171(5):2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T., Sasakawa C., Okada N., Honma Y., Yoshikawa M. vacB, a novel chromosomal gene required for expression of virulence genes on the large plasmid of Shigella flexneri. J Bacteriol. 1992 Oct;174(20):6359–6367. doi: 10.1128/jb.174.20.6359-6367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H. C., Zhao G., Feng G., Leung H. C., Winkler M. E. The mutL repair gene of Escherichia coli K-12 forms a superoperon with a gene encoding a new cell-wall amidase. Mol Microbiol. 1994 Jan;11(1):189–202. doi: 10.1111/j.1365-2958.1994.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Gene regulation by phosphate in enteric bacteria. J Cell Biochem. 1993 Jan;51(1):47–54. doi: 10.1002/jcb.240510110. [DOI] [PubMed] [Google Scholar]
- Westenberg D. J., Gunsalus R. P., Ackrell B. A., Cecchini G. Electron transfer from menaquinol to fumarate. Fumarate reductase anchor polypeptide mutants of Escherichia coli. J Biol Chem. 1990 Nov 15;265(32):19560–19567. [PubMed] [Google Scholar]
- Wolfe S. A., Smith J. M. Nucleotide sequence and analysis of the purA gene encoding adenylosuccinate synthetase of Escherichia coli K12. J Biol Chem. 1988 Dec 15;263(35):19147–19153. [PubMed] [Google Scholar]
- Yim H. H., Villarejo M. osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in Escherichia coli. J Bacteriol. 1992 Jun;174(11):3637–3644. doi: 10.1128/jb.174.11.3637-3644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Mori H., Nagai H., Nagata T., Ishihama A., Fujita N., Isono K., Mizobuchi K., Nakata A. Systematic sequencing of the Escherichia coli genome: analysis of the 0-2.4 min region. Nucleic Acids Res. 1992 Jul 11;20(13):3305–3308. doi: 10.1093/nar/20.13.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Roth J. R. The nadI region of Salmonella typhimurium encodes a bifunctional regulatory protein. J Bacteriol. 1991 Feb;173(3):1302–1310. doi: 10.1128/jb.173.3.1302-1310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]


