Abstract
The importance of nitric oxide in mammalian physiology has been known for nearly 30 years. Similar attention for other nitrogen oxides such as nitroxyl (HNO) has been more recent. While there has been speculation as to the biosynthesis of HNO, its pharmacological benefits have been demonstrated in several pathophysiological settings such as cardiovascular disorders, cancer, and alcoholism. The chemical biology of HNO has been identified as related to, but unique from, that of its redox congener nitric oxide. A summary of these findings as well as a discussion of possible endogenous sources of HNO is presented in this review. Antioxid. Redox Signal. 14, 1659–1674.
Introduction
Nitrogen oxides are important components of the biochemistry and physiology of many organisms. The actions of these species range from serving as primary sources of ammonia through nitrate/nitrite reductases to regulating vascular tone and neuronal function. Since the seminal discoveries of nitric oxide (NO) as the endothelium-derived relaxing factor (EDRF) (72, 116, 127) and as an antitumor and antipathogen in the immune response (61, 119), the field of NO biology has expanded greatly over the last three decades. Much of the focus has been on the biological effects of NO as well as the interactions of NO with reactive oxygen species (ROS). Since the chemistry of nitrogen oxides is the primary determinant of biological activity (105, 159, 174), an understanding of their mechanisms of action under specific biological conditions is crucial. Due to the high complexity of nitrogen oxide chemistry, producing a comprehensible framework for the chemical biology of nitrogen oxides is an evolving process.
Although nitroxyl (HNO) has long been suspected to be an intermediate in the nitrogen cycle, particularly denitrification, it is a relative latecomer to the class of nitrogen oxides that are important to mammalian biology (106). HNO was originally a candidate for the EDRF (50), but was largely ignored once NO was identified as this species. However, over the last decade discovery of unique biological properties in the cardiovascular system (128, 129) and in receptor response (56) has led to a significant expansion in HNO-related research. Interestingly, many of the biological effects of HNO are distinct and at times opposite to NO (173). The basis for the unique effects of these nitrogen oxides is distinct targets (112). For example, HNO preferentially reacts with thiols and ferric complexes, whereas NO prefers radicals and ferrous complexes (174). The interesting pharmacological effects of HNO and the relationship to NO suggest the possibility that HNO is a component of redox signaling (44, 112, 130). In this review, the chemistry that makes HNO unique among the nitrogen oxides is described.
Donors of HNO
Endogenous production of HNO both in bacteria and mammals has been a matter of speculation for decades. Although there are a number of potential routes to HNO biosynthesis, as discussed below, to date data on the effects of HNO on biomolecules, in cultured cells and in mammalian systems, have been acquired with HNO donors. Such species are required because HNO is metastable due to dimerization to hyponitrous acid followed by irreversible and rapid dehydration [Eq. 1 (84, 89)] [8×106 M−1 s−1 (145)].
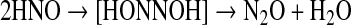 |
(1) |
The two most commonly used donors of HNO are Angeli's salt (sodium trioxodinitrate, Na2N2O3) and derivatives of sulfohydroxamic acid, particularly Piloty's acid (benzenesulfohydroxamic acid, C6H5SO2NHOH). Several clinically used compounds such as cyanamide and hydroxyurea can also be bioactivated to HNO [for reviews on HNO donors, see (70, 79, 80, 110)].
Angeli's salt decomposes spontaneously to produce HNO and nitrite [Eq. 2 (32, 71)] [t½ of 3 min under physiological conditions (100)].
Decomposition of Piloty's acid (131) is base-catalyzed (Eq. 3) (15), which allows for investigation of NO− as well as HNO.
Fundamental Chemistry of HNO with Respect to NO
The relationship of NO to NO− and NO+ is central to the chemical biology of NO since all three species have distinct reaction profiles (45). Early pulse radiolysis studies suggested a pKa for HNO of 4.7 (57), indicating that NO− would be the predominant species under physiological conditions. Based on this pKa, the reduction potential for the NO/NO− couple was calculated to be 0.39 V [vs. normal hydrogen electrode (154)]. Thus, NO would be predicted to be rapidly reduced under biological conditions to NO−. Moreover, the rapid (145) reaction of NO− with O2 would form peroxynitrite (ONOO−), which can lead to modification of biomolecules. A number of inconsistencies with this scenario were evident in the literature, however, such as the more facile reduction of O2 compared with NO (40, 171) and the observation of distinct effects of NO and HNO donors (112). These discrepancies led to numerous studies focused on determining the fundamental relationships of NO to NO− and HNO.
In 2002, the reduction potential for NO was determined by a variety of techniques to be −0.8 V (8, 145). From this potential the pKa for HNO was derived to exceed 11, indicating that NO− is actually a minor species at physiological pH. Although the reduction potential for NO is predicted to be increased to −0.5 V at neutral pH (8, 145), outer sphere one-electron reduction of free NO is likely to be nearly inaccessible in mammalian systems. In certain prokaryotes, reduction of NO may be possible (92), which is a plausible reason as to why bacteria and mammals respond differently to NO.
That NO− readily undergoes outer sphere electron transfer was important to experimentally verify the reduction potential for NO. Decomposition of an alkyl sulfohydroxamic acid in the presence of a series of viologens at high pH suggested that NO− had a potential close to −0.7 V (8). Conversely, at pH 7, the HNO donor Angeli's salt did not reduce methyl viologen, which has a potential of −0.44 V. Thus, either HNO does not readily participate in outer sphere electron transfer or the potential is less negative than that of methyl viologen. Moreover, the rate of the reduction of ferricytochrome c by HNO (91, 112) is at least several orders of magnitude slower than that by 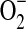 (13), suggesting a more positive potential for the NO, H+/HNO couple than the
(13), suggesting a more positive potential for the NO, H+/HNO couple than the 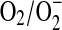 couple [−0.33 V (154)]. HNO has been shown to be converted to NO by a variety of other oxidants (106) as well, including ferricyanide (172, 177), Tempol (172), and methylene blue (50).
couple [−0.33 V (154)]. HNO has been shown to be converted to NO by a variety of other oxidants (106) as well, including ferricyanide (172, 177), Tempol (172), and methylene blue (50).
Assuming that HNO is a poor reductant, oxidation to NO could be considered to occur through the intermediacy of NO−. Given that the ground states of HNO and NO− are singlet and triplet, respectively, the acid–base relationship is interesting [for detailed discussion, see ref. (106)], and proton transfer is inordinately slow due to spin forbiddenness [at neutral pH, deprotonation of HNO occurs with a kobs∼5×10−3 s−1, whereas protonation of NO− is 10-fold slower (145)]. A large reorganization energy may also be required. Thermodynamic and kinetic impediments to interconversion of HNO and NO are supported by the many studies that now show distinct effects of HNO and NO donors (106, 112, 125, 173).
HNO Reactivity
Given the fact that NO is a free radical, its chemistry is unexpectedly limited. In biological systems, NO primarily reacts with ferrous systems, oxygen species, and free radicals (174). Furthermore, NO is neither readily reduced [NO/NO− potential of −0.8 V, (8, 145)] nor oxidized [NO+/NO potential of 1.2 V, (85)]. Conversely, the reactivity of HNO is quite extensive (Table 1). The reactants are generally oxidants, reductants, nucleophiles, or metalloproteins and their analogs. The reactions are varied, but many lead to the formal oxidation of HNO to NO. Such reactions have often been invoked as the basis for the vasoactive properties of HNO donors. In fact, detection of NO in the presence of oxidants, particularly ferricyanide, has long been used as an indirect indicator of HNO production (110). Two-electron oxidants such as flavin adenine dinucleotide (FAD) can also oxidize HNO to NO (52).
Table 1.
Rate Constants for Reaction with Nitroxyl or Nitric Oxide
| |
k (M−1s−1) |
|
|---|---|---|
| Reactant | HNO | NO |
| HNO | 8×106 (22°C)a | 6×106 (23°C)a |
| NO | 6×106 (22°C)a | NA |
| 8×106 (23°C)b | ||
| O2 | 3×103 (37°C)c | 6×106 (23°C)l |
| 1×104 (23°C)d | ||
| NH2OH | 4×103 (23°C)e | 7×10−3 (25°C)m |
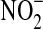 |
1×103 (23°C)d | NR |
| selenomethionine | 9×103 (23°C)e | NR |
| NADPH | 1×104 (23°C)e | NR |
| NADH | 1×104 (23°C)e | NR |
| Thiosulfate | 2×104 (23°C)e | NR |
| Trolox | 2×104 (23°C)e | NR |
| Tempol | 8×104 (37°C)c | NR |
| Tempo | 6×104 (23°C)e | NA |
| Ascorbate | 1×105 (23°C)e | NR |
| Ferricyanide | >104 (23°C)* | NR |
| GSH | 2×106 (37°C)c | NR |
| 8×106 (23°C)e | ||
| N-acetyl-l-cysteine | 5×105 (37°C)c | NR |
| GAPDH | ∼109 (37°C)f | NR |
| Triscarboxyethylphosphine | 8×106 (23°C)e | NR |
| Cu,Zn SOD | 1×106 (37°C)c | NR |
| 9×104 (23°C)g,† | ||
| MnSOD | 7×105 (37°C)c | NA |
| Ferricytochrome c | 4×104 (37°C)c | 7×102 (20°C)n |
| 2×104 (23°C)g,† | ||
| Ferric Mb | 6–8×105 (37°C)c,h | 2×105 (20°C)n |
| Ferrous Mb | 1×104 (37°C)i | 2×107 (37°C)o |
| MbO2 | 1×107 (37°C)c | 4×107 (37°C)p |
| Catalase | 3×105 (37°C)c | 3×107 (37°C)n |
| HRP | 2×106 (37°C)c | 2×105 (20°C)q |
| Fe(III)TPPS | 3×105 (25°C)j,† | 5×105 (25°C)r |
| Mn(III)TPPS/Mn(II)(TPP)Cl | 4×104 (25°C)k | NAs |
Preliminary data from David A. Wink.
Recalculated using the revised dimerization rate constant of 8×106 M−1 s−1 from (145).
(145), b(82), c(112) d(92), e(76), f(94), (95), g(91), h(10), i(158), j(6), k(101), l(170), m(16), n(68), o(124), p(35), q(83), r(87), s(168).
TPPS, meso-tetra(4-sulfonatophenyl)porphyrinato; NR, no reaction; NA, rate constant not available; HNO, nitroxyl; NO, nitric oxide; NH2OH, hydroxylamine; GSH, glutathione; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SOD, superoxide dismutase; HRP, horseradish peroxidase; Mb, myoglobin.
Reduction of HNO has received recent interest. Calculations suggest that both one- and two-electron reduction of HNO is favorable [0.6 and 0.8 V to NHOH and hydroxylamine (NH2OH), respectively (39)]. Analysis of two-electron reduction of HNO is complicated by the reaction of HNO with NH2OH (14, 26). While ascorbate induces simple two-electron reduction (76), NH2OH was not observed in the presence of NAD(P)H (76, 136). Covalent interactions or O2-dependent reactions may influence the products. Since reduction of HNO may influence the pharmacological actions of HNO donors, further analysis of such reactions is required.
The chemical biology of NO is defined by direct and indirect effects (174). In general, direct interaction of NO with biological targets, particularly ferrous heme or non-heme iron proteins and free radicals, leads to physiological effects or to removal of strong oxidants. Indirect effects arise when NO is oxidized by oxygen species. The resulting nitrogen oxides can lead to oxidative, nitrosative, and nitrative modifications of proteins and DNA, which in turn can have pathological consequences. Given the broader reactivity of HNO (106), such classification may not be as straightforward, but the important direct and indirect reactions of HNO are presented below.
Direct reactions
Based on high rate constants relative to the interactions with redox reagents (typically ≤105 M−1 s−1) and reactant abundance, cellular consumption of HNO is assumed to primarily involve reduction of ferrihemes or oxidation of thiols and oxyhemes.
Thiols
HNO has been demonstrated to function as an electrophile in analogy to formaldehyde (7). Association with thiols is one of the most facile reactions of HNO (Table 1). In contrast, NO does not react directly with thiols (169). The majority of early analysis of HNO was performed by Nagasawa et al., who were interested in the inhibition of aldehyde dehydrogenase with compounds such as cyanamide, which are used clinically to deter alcohol consumption. Metabolism of cyanamide to HNO (Eq. 4) was determined to inhibit a critical thiol on the enzyme (30, 118).
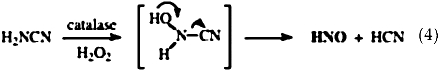 |
Association of HNO with thiols produces an N-hydroxysulfenamide, which is unstable (Eq. 5). In the presence of excess thiol, this intermediate reacts with a second thiol to produce disulfide and NH2OH [Eq. 6 (37)]. Otherwise, rearrangement generates the sulfinamide [Eq. 7 (152, 175)].
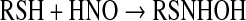 |
(5) |
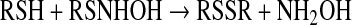 |
(6) |
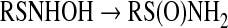 |
(7) |
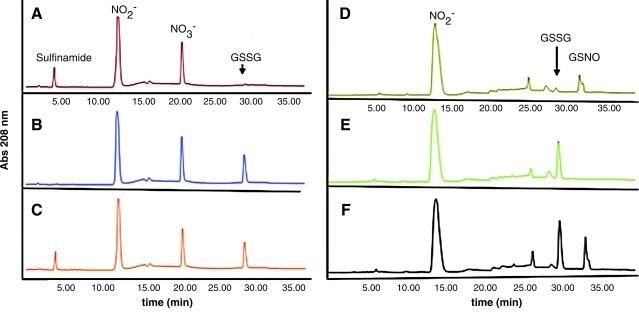
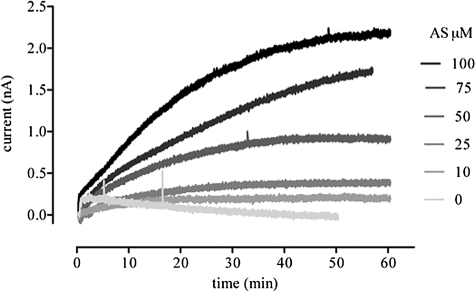
Recent experimental and computational analyses of these interactions have clarified mechanistic details (60, 148, 149). High-performance liquid chromatography detection of sulfinamide has also been offered as an indicator of HNO production (34). Figure 1 shows the specificity of sulfinamide production when glutathione (GSH) is exposed to an equimolar concentration of Angeli's salt compared with the structurally related NO donor diethylamine NONOate. Conversely, production of NH2OH can be observed electrochemically as 1 mM GSH is exposed to increasing concentrations of Angeli's salt (Fig. 2).
FIG. 1.
Indirect high-performance liquid chromatography detection of HNO formation by sulfinamide production. The reaction was performed in phosphate-buffered saline (pH 7.4) with 50 μM of the metal chelator diethylene triamine pentaacetic acid (DTPA) for 10 min at 37°C. All reagents were present at 100 μM. Samples were filtered prepared and analyzed using the methodology described in (34). Briefly, samples (250 μL) were separated on a Kromasil C-18 column (250×4.6 mm, 5 μm particle size, equipped with a guard column; Phenomenex, St. Torrance, CA) by a step gradient from buffer A (pairing reagent 10 mM tetrabutylammonium hydroxide, 10 mM KH2PO4, and 0.25% methanol, pH 7.00) to buffer B (2.8 mM tetrabutylammonium hydroxide, 100 mM KH2PO4, and 30% methanol, pH 5.50) as follows: 10 min of 100% buffer A, 3 min at up to 80% buffer A, 10 min at up to 70% buffer A, and 12 min at up to 55% buffer A. A flow rate of 1.2 ml/min, column temperature of 18°C, and sample temperature of 4°C were employed. The wavelength of detection was 208 nm except for GSNO, which was 334 nm. Ultrapure standards of GSH, GSSG, nitrite, and nitrate were freshly prepared and used to identify eluted peaks by comparison of retention times and absorption spectra. All samples were filtered through a Centricon MW 3000 cutoff filter before high-performance liquid chromatography analysis. (A) Angeli's salt and GSH, (B) Angeli's salt and oxidized glutathione (GSSG), (C) Angeli's salt and a mixture of GSH/GSSG, (D) DEA/NO and GSH, (E) DEA/NO and GSSG, and (F) DEA/NO and a mixture of GSH/GSSG. Sulfinamide formation was observed only in samples containing Angeli's salt and GSH (A, C). DEA/NO reacted with GSH to produce GSNO (D, F). HNO, nitroxyl; GSH, glutathione; GSNO, S-nitrosogluathione; GSSG, glutathione disulfide; DEA/NO, diethylamine NONOate. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 2.
Indirect electrochemical detection of HNO formation by hydroxylamine production. Real-time detection of NH2OH was performed using an H2O2 electrode from WPI on an Apollo 4000 series instrument. We found that this electrode is far more sensitive to NH2OH than to H2O2 (data not shown). NH2OH was detected in the presence of 100 μM catalase to eliminate interference from H2O2. The reaction conditions were as described in Figure 1 except that 1 mM GSH was exposed to varied concentrations of Angeli's salt (0–100 μM). NH2OH, hydroxylamine; H2O2, hydrogen peroxide.
Posttranslational modification of thiols by NO has been the subject of intense interest for years. Although NO does not react directly with thiols under biological conditions (169), oxidative products can induce thiol nitrosation (48, 88), which is reversible upon exposure to dithiothreitol (DTT). Detailed analysis of inhibition of the cysteine proteases cathepsin B (163) and papain (161) by HNO has demonstrated that the modifications by HNO are at least partially irreversible by addition of DTT, which also reduces disulfides. This difference was attributed to selective formation of sulfinamide by HNO. Several pathways exist to convert glutathione disulfide (GSSG) to GSH [e.g., thioredoxin (65), and glutathione reductase (102)], but sulfinamide may represent a terminal modification. Oxidation to the sulfenic acid, which has the same sulfur oxidation state as the sulfinamide, can be amended enzymatically by sulfiredoxin (77), glutaredoxin (103), or peroxiredoxins (176), but a comparable enzyme for sulfinamides has yet to be identified.
A recent mass spectral study examined HNO-induced modifications in human platelet proteins and found that cysteine-containing tryptic peptides were modified to sulfinamides and, to a lesser extent, disulfide linkages with no other long-lived intermediates or side products (63). Storage or sample preparation led to conversion of the sulfinamide to the sulfinic acid. This study identified 10 proteins with stable sulfinamide bonds such as integrin β3 and actin. The highest sensitivity to exogenous HNO was observed with surface receptors. Interestingly, exposure of bovine serum albumin (BSA) to HNO results in an as yet unidentified amine cross-link (148), indicating that the initial N-hydroxysulfenamide may also undergo nucleophilic attack from another protein residue. Such results suggest that the protein environment is an important determinant in the biological activity of HNO with thiols.
As seen in Table 1, the rate constant for reaction of HNO with GSH is greater than that for N-acetyl-L-cysteine. We have estimated a slight increase in the rate constant with BSA (8×106 M−1 s−1, preliminary data). This trend implies that protein thiols may be more susceptible to attack by HNO than peptides or free amino acids. Furthermore, a number of protein thiols, including aldehyde dehydrogenase (118) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (94, 95), have been observed to be modified by HNO despite high cellular concentrations of GSH. Based on the low cellular concentration of GAPDH [2 μM in yeast (94)], this could imply a rate constant of >109 M−1 s−1. The basis for such selectivity has been offered to be protein-induced reduction in thiol pKa (34). Given the electrophilic nature of HNO (7), the enhanced nucleophilicity of thiolates is likely to impact the reaction rate over sulfhydryls.
Zinc finger proteins such as poly(ADP-ribose) polymerase (PARP) (153) have also been shown to interact with HNO. In contrast, thioethers such as methione do not react directly with HNO but as discussed below are modified by the product of HNO autoxidation (109). Interestingly, selenomethione, which a is softer nucleophile than methione, does react with HNO (104 M−1 s−1) (76), implying that selenoproteins may be targets of HNO under certain conditions.
Metalloproteins
The primary target of NO is the ferrous heme of soluble guanylyl cyclase (sGC). The nitrosyl complex induces a conformational change in the protein that leads to increased activity and eventually vasodilation. Conversely, reaction of NO with oxyhemoglobin and oxymyoglobin is assumed to be a major consumption pathway for NO (Eq. 8), forming the stable endproduct nitrate and oxidizing the heme.
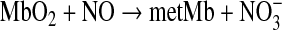 |
(8) |
The stability of ferric porphyrin nitrosyl systems is much lower than that of ferrous nitrosyl species (47). However, NO is able to reductively nitrosylate ferric porphyrins upon nucleophilic attack [Eqs. 9, 10 (3, 58, 167)].
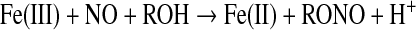 |
(9) |
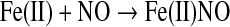 |
(10) |
Binding of NO to non-heme systems also has some biological importance [e.g., (18)] that will not be discussed here.
That HNO donors induce vasorelaxation has led to much speculation about whether HNO can interact with sGC in a similar manner to NO. A brief history of the evolution of this question was given above. A complicating factor in the discussion is that very few stable HNO complexes are known, in contrast to the many characterized nitrosyl complexes, which include the formal oxidation states of NO, NO+ and NO−. Additionally, sGC is a large protein that includes ∼30 cysteines, and Fukuto and coworkers (104) have recently suggested that both the heme and at least a subset of the thiols may be important to the effects of HNO on sGC activity. Whether the vasoactivity of HNO donors in vivo is directly due to HNO or occurs upon reduction to NO remains unknown. That MbHNO is highly unstable in air suggests that if HNO complexes do form in biological systems, they will typically be short-lived.
As with NO, the reaction of HNO with oxyhemes can be considered to be a consumption pathway. Nitrate and oxidized heme are produced with both HNO and NO with similar rate constants [107 M−1 s−1 (35, 38, 112)], but the stoichiometry with respect to the heme is increased for HNO [Eq. 11 (36, 37, 147)].
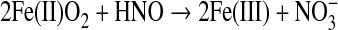 |
(11) |
The mechanism of this reaction has yet to be fully elucidated (106) for discussion.
Like NO, the association with ferric hemes can lead to reductive nitrosylation, but in a single step [Eq. 12 (11, 37)].
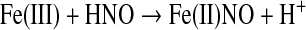 |
(12) |
Unlike NO, the association of HNO is generally more favorable with ferric [>105 M−1 s−1, (112)] rather than ferrous [<104 M−1 s−1 (86)] hemes. That the ferrous nitrosyl complex can be formed by both NO and HNO illustrates the related nature of these two redox congeners. However, reaction with metMb in air is one of the most common methods to detect HNO (10, 110). Other ferric proteins, including cytochromes and peroxidases, also undergo reductive nitrosylation by HNO (9, 37, 67, 111, 138, 146, 150).
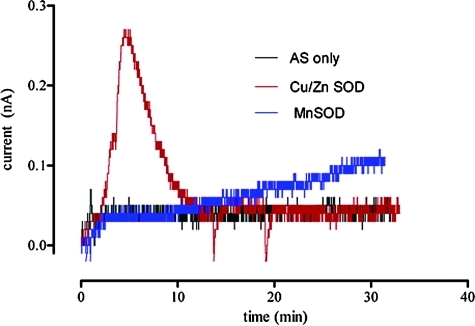
Whether reductive nitrosylation results in free NO depends upon the identity of the metal complex. For example, occupation of the axial sites in ferricytochrome c both inhibits the reaction (112) and reduces the stability of the resulting nitrosyl complex (47, 66, 67), such that free NO can be detected (36, 68). Free NO is also produced by the interaction of HNO with Cu,Zn superoxide dismutase (SOD) (90, 117) since Cu(I) does not bind NO due to the d10 configuration metal. Figure 3 shows the rapid increase in detectable signal from an NO-specific electrode when Cu,Zn SOD is incubated with Angeli's salt. Conversely, MnSOD, which forms a stable nitrosyl complex (46), releases NO much more slowly.
FIG. 3.
Reactivity of HNO with Cu,Zn SOD and MnSOD. Real-time measurement of NO produced from Angeli's salt (5 μM) and upon the addition of Cu,Zn SOD or MnSOD (20 μM) in the presence of GSH (100 μM), as in Figure 1 using an NO-specific electrode (ISO-NOP, WPI Apollo 4000 series instrument). In the presence of Cu,Zn SOD, there was rapid conversion of HNO to NO. In contrast, the HNO-MnSOD complex slowly degraded. SOD, superoxide dismutase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Since reductive nitrosylation of a synthetic ferric porphyrin (6) has a similar rate constant to ferric proteins such as metMb (112) [see ref. (106) for full discussion], the rate-limiting step for reductive nitrosylation appears to be addition of HNO to the metal with little structural influence from the protein. For NO, the dissociation of water from ferric porphyrins is the rate-limiting step (68), and this is likely the case for HNO as well. Thus, the stability of the resulting nitrosyl complex determines the profile of NO release and the resulting biological effect of conversion of HNO to NO by interaction with metal complexes.
Indirect reactions
As stated above, the direct effects of NO are typically regulatory or protective in nature, whereas oxidized products are typically deleterious. For HNO, posttranslational modification of thiols in particular can be envisioned to inhibit a variety of enzymes, with the impact dependent on function. However, oxidation of HNO induces neurotoxicity and can lead to double-strand DNA breaks.
Reaction of HNO and O2
Decomposition of HNO donors consumes O2 in aerobic solution (25, 52, 109) without appreciable formation of NO (41, 50, 97, 132, 177). Some caution is therefore required when using a high concentration of HNO donor to evaluate biological properties in closed systems or in containers with low surface areas. Autoxidation of HNO is relatively slow [∼103 M−1 s−1 (76, 92, 112)] compared with reactions with metal complexes and thiols, suggesting limited importance in the cytoplasm. However, in cell membranes where the concentrations of neutral gases are higher (93) and scavengers such as thiols are less abundant, autoxidation of HNO may become relevant.
Although it is logical to assume that ONOO− or peroxynitrous acid is the species generated from the reaction of HNO with O2, there are several important lines of evidence that show distinct differences between synthetic ONOO− and HNO donors in aerobic solution (32, 42, 91, 109, 113, 122, 136, 145, 165, 166, 172). Both synthetic ONOO− and the HNO/O2 product readily oxidize dihydrohrhodamine 123 (DHR) by two electrons to rhodamine 123 (109, 172). However, where ONOO− readily oxidizes phenols by one electron, this reactivity is not observed with aerobic HNO. Conversely, hydroxylation is more efficient with HNO. Perhaps, most importantly, while ONOO− is readily scavenged by CO2, the chemistry of aerobic HNO is not altered in carbonate buffer (113). Both ONOO− and HNO/O2 are efficiently quenched by urate and ascorbate (109). Despite experimental and computational efforts (92, 107, 109, 113, 145), the structure of the HNO autoxidation product is currently unknown. The mechanism may involve either direct, spin-forbidden association of O2 and HNO or nucleophilic addition of HNO to a solvent or target molecule followed by reaction with O2 with this HNO adduct.
Autoxidation of HNO can be readily distinguished from that of NO by the dyes DHR and 4,5-diaminofluorescein (DAF), which are both converted to fluorescent products much more efficiently by donors of HNO than NO (42, 109). Although NO autoxidation can lead to detrimental modification in proteins and to point mutations in DNA (137, 174), HNO autoxidation can induce DNA double-strand breaks (19, 113, 123, 172), suggesting the potential for higher toxicity.
Reaction of HNO and NO
Conversion of HNO to NO either by simple oxidation or by reductive nitrosylation can lead to formation of reactive intermediates (Eqs. 13–15) (57, 142, 143, 145).
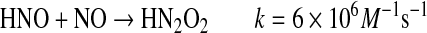 |
(13) |
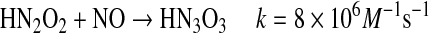 |
(14) |
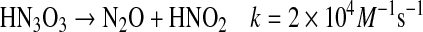 |
(15) |
The coexistence of HNO and NO is certainly conceivable in a cell, although at low concentrations. The rate constant of Eq. 13 (145) is similar to that of HNO dimerization and to reaction with GSH and metalloproteins. Thus, for Eq. 13 to be competitive, biologically the concentrations of efficient scavengers must be low.
The hyponitrous radical is predominantly deprotonated at physiological pH [pKa of 5.5 (96)]. The reduction potential of the 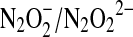 couple has been experimentally determined to be 0.96 V (134), whereas a continuum solvation model has predicted a value of 0.34 V (76). Both potentials support the experimental observation that the NO/HNO reaction produces an oxidant, capable for instance of oxidizing NADH with a rate constant of ∼106 M−1 s−1. Proton-coupled reduction of
couple has been experimentally determined to be 0.96 V (134), whereas a continuum solvation model has predicted a value of 0.34 V (76). Both potentials support the experimental observation that the NO/HNO reaction produces an oxidant, capable for instance of oxidizing NADH with a rate constant of ∼106 M−1 s−1. Proton-coupled reduction of 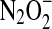 to
to 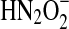 (pKas of H2N2O2 of 7.04, 11.4) is predicted to be more favorable by ∼1 V (76, 133).
(pKas of H2N2O2 of 7.04, 11.4) is predicted to be more favorable by ∼1 V (76, 133).
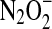 can also react with a second NO, ultimately resulting in formation of nitrous oxide (N2O) and nitrite [Eqs. 16, 17 (57, 96, 142, 143, 145)].
can also react with a second NO, ultimately resulting in formation of nitrous oxide (N2O) and nitrite [Eqs. 16, 17 (57, 96, 142, 143, 145)].
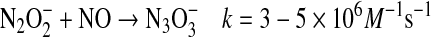 |
(16) |
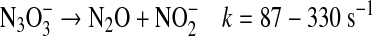 |
(17) |
Pharmacological and Biological Behavior
NO donors are currently in clinical use for treatment of hypertension and angina. In addition to cardiovascular diseases, NO donors are being considered for treatment of a variety of other conditions such as organ transplantation, cancer therapeutics, osteoarthritis, osteoporosis, urinary tract infections, preeclampsia, neurodegenerative diseases, and sickle cell disease. Similarly, significant pharmacological effects of HNO donors have been demonstrated in alcohol metabolism, vasodilation, myocardial contractility, platelet aggregation, neuronal function, angiogenesis, and cancer proliferation. For NO, the most heavily studied effects are in the cardiovascular, immune, and neuronal systems, as illustrated by the names of the three types of NO synthase (endothelial, inducible, and neuronal NOS). Here, the effects of HNO donors on the cardiovascular system, cancer therapy, and neuronal function are described.
The cardiovascular system
Both NO and HNO are vasoactive in tissue preparations and in vivo (50, 52, 53, 117, 164). HNO donors like NO donors induce relaxation in both capacitance and resistance vascular beds in dogs and several rodent species (50, 129). In canine systems, HNO donors behave much as classical nitrosovasodilators such as organic nitrates (50, 73, 129). Indeed, HNO relaxes venous capacitance beds as well as arteries and arterioles. In contrast, spontaneous donors of NO have a more prominent action on afterload (e.g., vascular resistance) (129). Thus, both direct and metabolized NO donors such as nitroglycerin are good unloading agents, decreasing both left ventricle dimensions and filling pressures. Consequently, organic nitrates have clinical indications in conditions such as angina, acute myocardial infarction, congestive heart failure (systo- and diastolic dysfunction), and hypertension (1). Since HNO-induced vasodilation is fully preserved in canine failing preparations (128) when compared with controls (129), they may also prove to be useful in treating altered vascular tone in several chronic cardiac diseases. In fact HNO donors have several intriguing features that are unique from clinical NO donors. For instance, initial studies suggest that unlike nitrates (1), HNO donors do not seem to induce tolerance with chronic exposure (74), at least in isolated tissue. Moreover, HNO donors are more amenable for hypertensive crises, or hypertension in general, bringing high blood pressure back to normal values without the risk of severe hypotension such as in the case of certain NO-based agents (4).
Like NO, HNO-induced vasodilation is seemingly a result of activation of a variety of effectors. The primary target for NO is sGC, which converts GTP into cGMP. The initial observations of vasodilation by HNO donors led to speculation about whether HNO could also react with the ferrous heme of sGC. Early studies on partially purified protein suggested that HNO was not able to directly activate sGC (31). However, later demonstrations by Farmer and Sulc (43) that HNO could bind to ferrous myoglobin (Mb) led to further analysis of the interaction of HNO with sGC. Fukuto and coworkers (104) have recently demonstrated that two structurally unrelated HNO donors are able to activate purified sGC. Mayer and coworkers (178) also recently reported that a reductant such as SOD is required for activation by HNO donors. Thus, the question remains open, and the role of the numerous cysteine residues of sGC may be critical (104).
HNO donors can activate voltage-gated potassium channels (Kv) leading to vascular smooth muscle cell hyperpolarization, which is at least in part sGC/cGMP dependent (73, 74). While delineating the mechanistic intricacies of HNO-induced vasorelaxation requires further investigation, the interesting vasodilatative profile in combination with studies showing that HNO donors are anti-aggregating agents (12) renders HNO-releasing compounds as attractive alternatives to more traditional nitrosovasodilators.
In addition to vasodilation, another striking pharmacological feature of HNO is the ability to enhance myocardial contraction and accelerate relaxation (i.e., positive inotropy and lusitropy, respectively). First described in a canine model under both normal and failing conditions (128, 129), the positive impact of HNO on myocardial function was then mechanistically dissected at the cellular level. Cheong et al. (21) showed that HNO donated by Angeli's salt can activate the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) pump and ryanodine receptors, which are critical structures in the control of calcium cycling within heart cells. Tocchetti et al. then coupled these observations with functional observation in intact myocytes, showing that Angeli's salt increased whole myocyte inotropy/lusitropy in a manner that is cAMP/protein kinase A (PKA) and cGMP/protein kinase G (PKG) independent but sensitive to thiols such as DTT (160). Similar results were obtained in intact rat cardiac muscle in which Angeli's salt increased force development more than the whole calcium transient, also suggesting a prominent impact of HNO directly on the myofilament (27). Again, this effect was offset by pre-treatment or co-infusion of DTT at the peak of force generation. Together, these findings suggest that HNO can target critical cysteine residues in various proteins that are crucial for cardiac electro-contraction coupling.
Froehlich et al. have suggested that HNO specifically interacts with cysteine residues present in the transmembrane domain of phospholamban (49). When the inhibitory activation of phospholamban on SERCA2a is relieved, for instance, by PKA-induced phosphorylation of phospholamban (99), SERCA2a activity is enhanced. Thus, despite different mechanisms, β-agonists and HNO donors are able to remove the phospholamban brake on SERCA2a, accelerating the re-uptake of calcium from the cytosol into the lumen of the cardiac sarcoplasmic reticulum by SERCA2a (27). That the positive inotropy and lusitropy induced by HNO is not diminished in models of congestive heart failure renders HNO donors as an attractive new avenue to provide inotropic support (at least in the short term) to failing hearts in which inotropic agents such as β-agonists not only lose their efficacy, but are also contraindicated in most cases (115).
Finally, in addition to enhancing cardiac function, HNO donors are protective against myocardial structural and functional disarrangement following global ischemia and reperfusion. NO donors are well known to confer early and late protection against this type of myocardial insult, and have been shown to afford beneficial effects even when given directly at reperfusion (98). This is not the case, however, of HNO donated by Angeli's salt, which is only beneficial when administered before ischemia in a pre-condition type of fashion (126). When Angeli's salt is infused directly at reperfusion, it is both structurally and functionally detrimental. While this apparent adverse effect may reflect a dose issue, it also points to the intriguing possibility that in ischemia/reperfusion injury, HNO in analogy to its effects on inotropy/lusitropy, may require redox intact switches to provide myocardial protection. Such availability may be greatly compromised in the heart at reperfusion where reintroduction of O2 leads to uncontrolled formation of ROS. Under such conditions modification of critical or protective thiols by HNO may be an aggravating factor (21). Additional studies are needed to precisely delineate whether, when, and how HNO donors are beneficial in countermanding myocardial damage during ischemia/reperfusion. However, evidence showing that HNO donors are powerful preconditioning agents is another potential factor for fully deploying HNO donating agents as cardiovascular therapeutics.
Cancer therapy
In addition to cardiovascular effects, HNO donors have shown promise as anti-cancer agents. Angeli's salt is cytotoxic to V79 cells and neurons only at millimolar concentrations (172), which is typical for the diazeniumdiolate class of nitrogen oxide donors. In cell culture or with purified DNA, Angeli's salt can induce DNA double-strand breaks (19, 113, 123, 172). It should be noted that DNA damage is induced with millimolar concentrations of donor, whereas the cardiovascular effects require micromolar doses. The oxygen dependence of such damage suggests that the autoxidation product of HNO is the active species. Whether infusion of HNO donors into animals results in DNA cleavage has yet to be determined. However, autoxidation of HNO is assumed to be a minor pathway as discussed above.
Irreversible thiol modification of GAPDH by HNO affects glycolysis (94, 95). Since most solid tumors must survive in a hypoxic environment, glycolysis is the major energy pathway. Thus, GAPDH and other critical thiol enzymes that facilitate cancer progression may be targets by HNO-donating anti-cancer agents. Consistent with this idea, HNO has been shown to inhibit breast (120) and neuroblastoma (155) cancer proliferation in mouse xenografts as well as in culture. Reduced blood vessel density with the tumors was accompanied by reduced levels of circulating vascular endothelial growth factor and in total hypoxia-inducible factor 1α protein (120). As a result, an increase in apoptosis and the apoptotic factor caspase-9 were observed.
In addition to the direct effects of HNO on cancer cells, HNO donors may be useful adjuvant agents to cancer chemotherapy. HNO has been shown to inhibit PARP in a breast cancer cell line (153). As an important component of the DNA repair machinery, PARP is a major target in the design of anti-cancer agents. Also, since a number of chemotherapies and radiation therapy are based on inducing DNA damage in cancer cells, inhibition of PARP by HNO donors may increase the efficacy of these treatments.
Neurotoxicity
Although HNO donors show substantial promise in the treatment of alcoholism, cardiovascular conditions and cancer, the toxicological effects, particularly as a result of thiol reactivity or autoxidation, remain to be fully elucidated. As stated above, DNA strand breakage in particular needs to be assessed in whole organisms. Administration of 10 μmol Angeli's salt into rats has induced neurotoxicity. For instance, injection into cerebral spinal fluid led to motor neuron injury, although sensory neurons were not affected. Similarly, 400 nmol of Angeli's salt reduced striatal dopamine 7 after treatment (162). Since GSH appears to play important roles in neuroprotection and neurotoxicity (114), direct injection of an HNO donor into the cerebrospinal fluid may critically alter GSH levels.
Similarly, modification of thiols on receptors may impact neurotoxicity. For instance, HNO has been shown to modify a thiol on N-methyl-D-aspartate (NMDA) receptors (78), which are pivotal in the regulation of neuronal communication and synaptic function in the central nervous system. Influx of calcium through NMDA receptors is both glutamate and voltage dependent. Overstimulation of the NMDA receptor, for example, during ischemia/reperfusion injury, leads to excitotoxicity (23). The impact of HNO on NMDA channel function has been shown to be oxygen dependent (25), with augmentation observed under aerobic conditions, whereas attenuation occurred under hypoxia. GSH may also play a role in excitotoxicity via NMDA receptor activation (114). Thus, HNO may protect against neuronal damage, for instance, during stroke, similarly to the above-described protective effect of HNO on myocardial damage during infarct. However, a recent examination of the effects on infarct size in mouse brain of Angeli's salt infusion before cerebral artery occlusion suggests that HNO is detrimental due to oxidative damage (22). It is difficult to correlate doses and animal size between mice, rats, rabbits, and dogs, but further analysis of the potential neurotoxicity of HNO donors is required.
Pathways for Formation of Endogenous HNO
NO is primarily biosynthesized by NOSs (157). There is growing interest in the reduction of nitrite as a secondary source of NO (28). Although significant nitrite can be provided dietarily, endogenous nitrite largely originates from NO. Thus, the 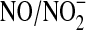 cycle is often assumed to be a storage mechanism to extend the temporal response to NO production. Conversely, despite the significant pharmacological effects of HNO donors, endogenous production of HNO has yet to be demonstrated. However, the diversity of these pharmacological effects suggests at least the potential for biosynthesis of HNO. Recently, Kemp-Harper and coworkers proposed that HNO is an endothelium-derived relaxing and hyperpolarizing factor in rodent resistance arteries, which supports this hypothesis (4). The metastability and higher reactivity of HNO compared with NO severely complicates definitively detecting HNO in biological systems. Sulfinamide formation (Eq. 7) in particular is of significant interest in the pursuit of selective detection of HNO (34, 63, 148). To date, there are three primary mechanisms proposed for HNO generation: decomposition of S-nitrosothiols (RSNO), oxidation of L-arginine by NOS under certain conditions, and oxidation of NH2OH.
cycle is often assumed to be a storage mechanism to extend the temporal response to NO production. Conversely, despite the significant pharmacological effects of HNO donors, endogenous production of HNO has yet to be demonstrated. However, the diversity of these pharmacological effects suggests at least the potential for biosynthesis of HNO. Recently, Kemp-Harper and coworkers proposed that HNO is an endothelium-derived relaxing and hyperpolarizing factor in rodent resistance arteries, which supports this hypothesis (4). The metastability and higher reactivity of HNO compared with NO severely complicates definitively detecting HNO in biological systems. Sulfinamide formation (Eq. 7) in particular is of significant interest in the pursuit of selective detection of HNO (34, 63, 148). To date, there are three primary mechanisms proposed for HNO generation: decomposition of S-nitrosothiols (RSNO), oxidation of L-arginine by NOS under certain conditions, and oxidation of NH2OH.
Formation of HNO from RSNO occurs through nucleophilic attack by a sulfhydryl leading to the release of HNO and concurrent formation of the corresponding disulfide [Eq. 18 (5, 64, 121, 141)].
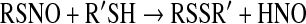 |
(18) |
Low-molecular-weight RSNO such as S-nitrosogluathione (GSNO) as well as protein RSNOs that do not contain juxtaposed thiols can be long lived. For example, decomposition of GSNO is on the order of hours, whereas the half-lives of S-nitrosated DTT or dihydrolipoic acid are ∼9 min and <20 s, respectively (5). Furthermore, significant HNO has been trapped only with dithiols. Under cellular conditions, a dithiol can metabolize low-molecular-weight RSNOs through a transnitrosation reaction, which may provide a means to both produce HNO and modulate protein function by disulfide formation. The cellular dithiols thioredoxin and dihydrolipoic acid have recently been shown to catabolize protein RSNOs (144, 156). These species thus may be involved in the regulation of cellular RSNO and in protein function.
RSNOs are commonly detected by the biotin-switch assay (17). This method involves reduction of RSNOs by ascorbate followed by biotin labeling. Interestingly, the reduction of RSNOs by ascorbate leads to significant production of HNO, which can impede detection (81).
Another alternative for HNO generation is during NOS catalysis (2, 4, 50, 55, 62, 135, 140). Detection of N2O and NH2OH and dependence of NO production on Cu,Zn SOD (62, 75, 140) all suggest that HNO could be an intermediate in NOS metabolism, although this has yet to be conclusively established. Again, detection of HNO is critical to elucidate this pathway.
The NOS cofactor tetrahydrobiopterin (BH4) has also been indicated to be critical for NO formation (24, 135, 138, 140). That the oxidation state of the iron nitrosyl complex produced during NOS turnover was BH4 dependent (2) suggested that the function of BH4 is to enable NOS to generate NO instead of HNO. Oxidation of NOS metabolites may also generate HNO. Nω-hydroxy-L-arginine (NOHA), which can be uncoupled from NOS at high levels (59, 138), can be oxidized to release HNO by a variety of oxidants (54, 55). Coupled NOHA (135, 138) can also produce HNO under low BH4 conditions (2, 135, 138, 140). Again, such reactivity has yet to be shown in vivo, but condition-dependent products are intriguing, for example, in the control of channel function (25).
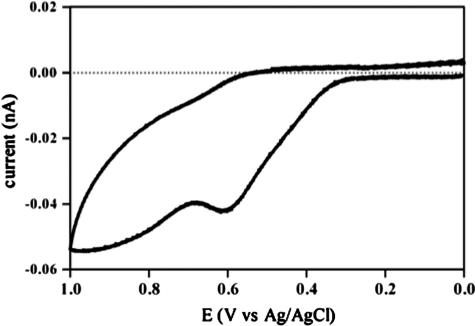
NOHA is a derivative of NH2OH, which is two electrons more reduced than HNO. NH2OH is also a product of the reaction of HNO with thiols (Eq. 6). Thus, oxidation of NH2OH can be considered to be a recycling mechanism for HNO as nitrite reduction is for NO. NH2OH has been shown to induce vasodilation, indicating that it is metabolized to NO or HNO (29). The two-electron reduction of HNO to NH2OH has a potential of 0.3 V at pH 7 (145). Successive one-electron reduction of HNO to NH2OH via NHOH is also favorable at pH 7 [0.1 and 0.5 V, respectively (51)]. NOHA and other hydroxamic acids such as suberoylanilide hydroxamic acid (SAHA, an anticancer agent via inhibition of histone deacetylase) and hydroxyurea (clinically used for treatment of sickle cell crisis) can be oxidized to mixtures of NO and N2O (54, 55, 69, 139). Cyclic voltammetry of NH2OH shows a single two-electron process (data not shown), whereas NOHA and SAHA are oxidized in two one-electron steps (Fig. 4). Similarly, hydroxyurea has been shown to be oxidized by one-electron to the nitroxide radical (69).
FIG. 4.
Cyclic Voltammetry measurements of suberoylanilidehydroxamic acid. The voltammogram was obtained with an EG Potensiostat/Galvanostat Model 273A from AMETEK Princeton Applied Research (Oak Ridge, TN). Measurements were performed on 2 mM suberoylanilidehydroxamic acid under aerobic conditions at room temperature in acetonitrile (2 mM) using the platinum auxiliary electrode and Ag/AgCl (saturated KCl) reference electrode.
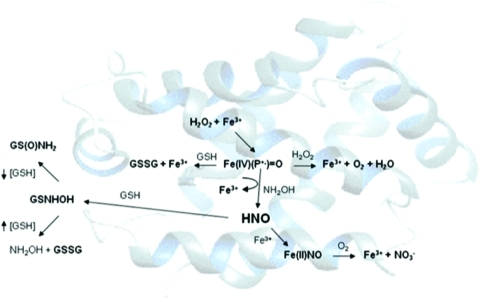
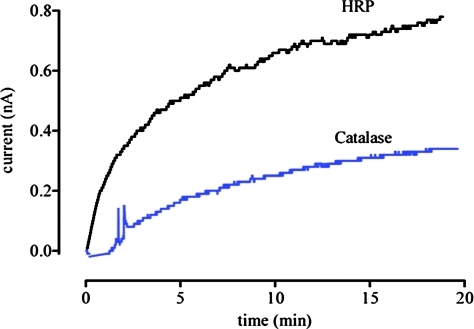
Both NH2OH and hydroxyurea can be oxidized by peroxidases (33, 69) [Fig. 5 for NH2OH; a related two step process is suggested for hydroxyurea (69)]. Production of HNO is accompanied by reduction of high-valent iron-oxo intermediates to the ferric state, suggesting that reductive nitrosylation (Eq. 12) may occur. Whether HNO will be capable of escaping the heme pocket seems to be protein dependent. A survey of heme proteins (33) showed that proteins such as Mb and myeloperoxidase, which have proximal histidine residues, can generate free HNO, as indicated by formation of sulfinamide. A lower affinity of the ferric heme for HNO may also correlate to a lower affinity for NO upon reductive nitrosylation. Figure 6 indicates that horseradish peroxidase (HRP), which produces a higher level of sulfinamide than catalase, also produces a higher amount of NO as measured by NO-specific electrode. The yield of NO is quite low, however, indicating that this is a minor pathway compared with release of HNO or reductive nitrosylation.
FIG. 5.
Peroxidation of NH2OH by heme proteins [adapted from (33)]. 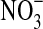 , nitrate, GSNHOH, glutathione N-hydroxysulfenamide; GS(O)NH2, sulfinamide. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
, nitrate, GSNHOH, glutathione N-hydroxysulfenamide; GS(O)NH2, sulfinamide. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 6.
Reactivity of HNO with HRP and catalase. Detection of NO produced from the aerobic reaction of NH2OH (500 μM) and H2O2 (50 μM, added last to initiate the reaction) in the presence of HRP (100 μM) or catalase (100 μM) as in Figure 1 using an NO-specific electrode (ISO-NOP, WPI Apollo 4000 series instrument). The two profiles show the higher production of NO from HRP (500 nM at 20 min) compared with catalase (250 nM at 20 min). HRP, horseradish peroxidase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Oxidation of NOHA (33) by HRP did not lead to detection of HNO, indicating some specificity for hydroxamic acid identity in this reaction. HNO has been assumed to be an intermediate of SAHA oxidation by a peroxidase mechanism (139). Addition of HRP/H2O2 /NH2OH to cells loaded with DAF resulted in an increase in fluorescence (33), indicating that HNO produced by this reaction can migrate into cells (42). Interestingly, HNO pharmacology has been shown to be effective with cardiovascular conditions in which inflammation will produce H2O2.
Another potential source of HNO is from the metal-catalyzed dismutation of NO, for example by MnSOD (46). Production of NH2OH and GSNO in the presence of GSH suggests generation of both HNO and NO+ upon sustained exposure of MnSOD to NO. This reactivity has been suggested to represent a mechanism to protect cells from the deleterious effects associated with overproduction of NO. Furthermore, the interrelated nature of HNO and NO and the impact of biomolecules on fundamental reactivity are highlighted by such a reaction.
Conclusion
As with other nitrogen oxides of biological interest, the chemical biology of HNO must be analyzed in the context of other biomolecules as well as derivative products. That is, the biological effects associated with HNO can be the result of either direct interactions with specific targets (e.g., thiols or metalloproteins) or indirect effects associated with reaction for example with O2 and NO. As redox congeners, NO and HNO have similar targets but produce different products through distinct mechanisms (e.g., direct modification of thiols by HNO vs. nitrosation of thiols upon NO autoxidation). Additionally, HNO and NO can produce identical products from different targets or by unique mechanisms (e.g., interaction with heme systems to produce ferrous nitrosyl complexes).
At this juncture, protein thiols should be considered to be the primary target of endogenous HNO. Inhibition of enzymes containing critical thiols by HNO can affect alcohol metabolism (30, 118) as well as DNA repair, apoptosis, and glycolysis (95, 120, 153, 155), which affect tumor progression. The cardiovascular effects of HNO donors are driven by thiol-modifications, particularly of calcium channels such as the ryanodine receptor and SERCA2a calcium pump (20, 160). Modification of calcium channels such as the NMDA receptor may also attenuate neurotoxicity (25). However, the neurotoxicity observed in rats upon infusion of low levels of Angeli's salt has been attributed to thiol modification (162). Clearly, the effects of broad-spectrum modification of thiols by HNO donors need to be assessed in detail.
Although stable association of HNO with metals is assumed to be limited in organisms, reductive nitrosylation may be significant pharmacologically. Release of NO upon reductive nitrosylation may explain some of the similarities observed for NO and HNO donors, such as vasodilation. Conversely, formation of a reasonably stable ferrous nitrosyl complex from a ferric resting state may lead to HNO-specific inhibition of enzymes such as cytochrome P450s (108), peroxidases (9), and the mitochondrial respiration complexes I and II (151).
Given the slow reaction of HNO with O2 relative to GSH, metalloproteins, and other scavengers, autoxidation of HNO is expected to be minimal in whole organisms, in contrast to exposure to purified biomolecules or in cultured cells, where antioxidant systems can be readily overwhelmed. For instance, although Angeli's salt increased lipid peroxidation in brain homogenates at concentrations below 100 μM, such modifications were not observed upon intranigral infusion of rats with up to 400 nmol of Angeli's salt (162). A recent study in mice showing that HNO can exacerbate oxidative stress (22) emphasizes the need for further study of the potential toxicological impacts of HNO administration. This also includes the potential for oxidative modifications when HNO and NO are produced proximally. Such studies are necessary in order to fully realize the potential of HNO donors as pharmacological agents.
Abbreviations Used
- BH4
tetrahydrobiopterin
- BSA
bovine serum albumin
- DAF
4,5-diaminofluorescein
- DEA/NO
diethylamine NONOate
- DHR
dihydrorhodamine 123
- DTT
dithiothreitol
- EDRF
endothelium-derived relaxing factor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- GSNHOH
glutathione N-hydroxysulfenamide
- GSNO
S-nitrosogluathione
- GS(O)NH2
sulfonamide
- GSSG
glutathione disulfide
- HNO
nitroxyl
- H2O2
hydrogen peroxide
- HRP
horseradish peroxidase
- Mb
myoglobin
- N2O
nitrous oxide
- NH2OH
hydroxylamine
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- NO3−
nitrate
- NOHA
NG-hydroxy-L-arginine
- NOS
nitric oxide synthase
- ONOO−
peroxynitrite
- PARP
poly(ADP-ribose) polymerase
- PKA
protein kinase A
- PKG
protein kinase G
- ROS
reactive oxygen species
- RSNO
S-nitrosothiol
- SAHA
suberoylanilidehydroxamic acid
- SERCA2A
sarcoplasmic reticulum Ca2+-ATPase
- sGC
soluble guanylyl cyclase
- SOD
superoxide dismutase
- TPPS
meso-tetra(4-sulfonatophenyl)porphyrinato
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (to D.A.W.), by NIH grants (R01-GM076247 to K.M.M.; NIAAA 1F31AA018069-01A1 to D.J.S.), by the National Science Foundation (CHE-0645818 to K.M.M.), by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme (PIEF-GA-2008-221666 to S.D.), and by the Forschungsförderungsfonds NWF-08/04 (S.D.)
References
- 1.Abrams J. Frishman WH. The organic nitrates and nitroprusside. In: Frishman WH, editor; Sonnenblick EH, editor; Sica DA, editor. Cardiovascular Pharmacotherapeutics. Second. New York: McGraw-Hill Medical Publishing Division; 2003. pp. 186–200. [Google Scholar]
- 2.Adak S. Wang Q. Stuehr DJ. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase - implications for mechanism. J Biol Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 3.Addison AW. Stephanos JJ. Nitrosyliron(III) hemoglobin: autoreduction and spectroscopy. Biochemistry. 1986;25:4104–4113. doi: 10.1021/bi00362a018. [DOI] [PubMed] [Google Scholar]
- 4.Andrews KL. Irvine JC. Tare M. Apostolopoulos J. Favaloro JL. Triggle CR. Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnelle DR. Stamler JS. NO+, NO, and NO− donation by S-nitrosothiols - implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 6.Bari SE. Marti MA. Amorebieta VT. Estrin DA. Doctorovich F. Fast nitroxyl trapping by ferric porphyrins. J Am Chem Soc. 2003;125:15272–15273. doi: 10.1021/ja036370f. [DOI] [PubMed] [Google Scholar]
- 7.Bartberger MD. Fukuto JM. Houk KN. On the acidity and reactivity of HNO in aqueous solution and biological systems. Proc Natl Acad Sci USA. 2001;98:2194–2198. doi: 10.1073/pnas.041481598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartberger MD. Liu W. Ford E. Miranda KM. Switzer C. Fukuto JM. Farmer PJ. Wink DA. Houk KN. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc Natl Acad Sci USA. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastian NR. Foster MJP. Ballantune M. Lu Y. Nitrogen oxides, the good, the bad and the ugly. Curr Topics Biophys. 2002;26:115–127. [Google Scholar]
- 10.Bazylinski DA. Hollocher TC. Evidence from the reaction between trioxodinitrate(II) and (15N)NO that trioxodinitrate(II) decomposes into nitrosyl hydride and nitrite in neutral aqueous-solution. Inorg Chem. 1985;24:4285–4288. [Google Scholar]
- 11.Bazylinski DA. Hollocher TC. Metmyoglobin and methemoglobin as efficient traps for nitrosyl hydride (nitroxyl) in aqueous solution. J Am Chem Soc. 1985;107:7982–7986. [Google Scholar]
- 12.Bermejo E. Sáenz DA. Alberto F. Rosenstein RE. Bari SE. Lazzari MA. Effect of nitroxyl on human platelets function. Thromb Haemost. 2005;94:578–584. doi: 10.1160/TH05-01-0062. [DOI] [PubMed] [Google Scholar]
- 13.Bielski BHJ. Cabelli DE. Arudi RL. Ross AB. Reactivity of HO2/O2− radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1071–1072. [Google Scholar]
- 14.Bonner FT. Dzelzkalns LS. Bonucci JA. Properties of nitroxyl as intermediate in nitric oxide hydroxylamine reaction and in trioxodinitrate decomposition. Inorg Chem. 1978;17:2487–2494. [Google Scholar]
- 15.Bonner FT. Ko YH. Kinetic, isotopic, and N15 NMR-study of N-hydroxybenzenesulfonamide decomposition - an HNO source reaction. Inorg Chem. 1992;31:2514–2519. [Google Scholar]
- 16.Bonner FT. Wang NY. Reduction of nitric oxide by hydroxylamine. 1. Kinetics and mechanism. Inorg Chem. 1986;25:1858–1862. [Google Scholar]
- 17.Burgoyne JR. Eaton P. A rapid approach for the detection, quantification, and discovery of novel sulfenic acid or S-nitrosothiol modified proteins using a biotin-switch method. Methods Enzymol. 2010;473:281–303. doi: 10.1016/S0076-6879(10)73015-9. [DOI] [PubMed] [Google Scholar]
- 18.Butler AR. Megson IL. Non-heme iron nitrosyls in biology. Chem Rev. 2002;102:1155–1165. doi: 10.1021/cr000076d. [DOI] [PubMed] [Google Scholar]
- 19.Chazotte-Aubert L. Oikawa S. Gilibert I. Bianchini F. Kawanishi S. Ohshima H. Cytotoxicity and site-specific DNA damage induced by nitroxyl anion (NO-) in the presence of hydrogen peroxide - implications for various pathophysiological conditions. J Biol Chem. 1999;274:20909–20915. doi: 10.1074/jbc.274.30.20909. [DOI] [PubMed] [Google Scholar]
- 20.Cheong E. Tumbev V. Abramson J. Salama G. Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. doi: 10.1016/j.ceca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Cheong E. Tumbev V. Stoyanovsky D. Salama G. Effects of pO2 on the activation of skeletal muscle ryanodine receptors by NO: a cautionary note. Cell Calcium. 2005;38:481–488. doi: 10.1016/j.ceca.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Choe CU. Lewerenz J. Fischer G. Uliasz TF. Espey MG. Hummel FC. King SB. Schwedhelm E. Böger RH. Gerloff C, et al. Nitroxyl exacerbates ischemic cerebral injury and oxidative neurotoxicity. J Neurochem. 2009;110:1766–1773. doi: 10.1111/j.1471-4159.2009.06266.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi D. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- 24.Clague MJ. Wishnok JS. Marletta MA. Formation of N-delta-cyanoornithine from NG-hydroxy-L-arginine and hydrogen peroxide by neuronal nitric oxide synthase: implications for mechanism. Biochemistry. 1997;36:14465–14473. doi: 10.1021/bi971024u. [DOI] [PubMed] [Google Scholar]
- 25.Colton CA. Gbadegesin M. Wink DA. Miranda KM. Espey MG. Vicini S. Nitroxyl anion regulation of the NMDA receptor. J Neurochem. 2001;78:1126–1134. doi: 10.1046/j.1471-4159.2001.00509.x. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JN. Chilton JE. Powell RE. Reaction of nitric oxide with alkaline hydroxylamine. Inorg Chem. 1970;9:2303–2304. [Google Scholar]
- 27.Dai T. Tian Y. Tocchetti CG. Katori T. Murphy AM. Kass DA. Paolocci N. Gao WD. Nitroxyl increases force development in rat cardiac muscle. J Physiol. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejam A. Hunter CJ. Schechter AN. Gladwin MT. Emerging role of nitrite in human biology. Blood Cells Mol Dis. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 29.DeMaster EG. Raij L. Archer SL. Weir EK. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem Biophys Res Commun. 1989;163:527–533. doi: 10.1016/0006-291x(89)92169-4. [DOI] [PubMed] [Google Scholar]
- 30.DeMaster EG. Redfern B. Nagasawa HT. Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent agent cyanamide. Biochem Pharmacol. 1998;55:2007–2015. doi: 10.1016/s0006-2952(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 31.Dierks EA. Burstyn JN. Nitric oxide (NO), the only nitrogen monoxide redox form capable of activating soluble guanylyl cyclase. Biochem Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- 32.Donald CE. Hughes MN. Thompson JM. Bonner FT. Photolysis of the N=N bond in trioxodinitrate - reaction between triplet NO− and O2 to form peroxonitrite. Inorg Chem. 1986;25:2676–2677. [Google Scholar]
- 33.Donzelli S. Espey MG. Flores-Santana W. Switzer CH. Yeh GC. Huang J. Stuehr DJ. King SB. Miranda KM. Wink DA. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radical Biol Med. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donzelli S. Espey MG. Thomas DD. Mancardi D. Tocchetti CG. Ridnour LA. Paolocci N. King SB. Miranda KM. Lazzarino G, et al. Discriminating HNO formation from that of other reactive nitrogen oxide species. Free Radical Biol Med. 2006;40:1056–1066. doi: 10.1016/j.freeradbiomed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 35.Doyle MP. Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 36.Doyle MP. Mahapatro SN. Nitric oxide dissociation from trioxodinitrate(II) in aqueous solution. J Am Chem Soc. 1984;106:3678–3679. [Google Scholar]
- 37.Doyle MP. Mahapatro SN. Broene RD. Guy JK. Oxidation and reduction of hemoproteins by trioxodinitrate(II): the role of nitrosyl hydride and nitrite. J Am Chem Soc. 1988;110:593–599. [Google Scholar]
- 38.Doyle MP. Pickering RA. Cook BR. Oxidation of oxymyoglobin by nitric oxide through dissociation from cobalt nitrosyls. J Inorg Biochem. 1983;19:329–338. [Google Scholar]
- 39.Dutton AS. Fukuto JM. Houk KN. Theoretical reduction potentials for nitrogen oxides from CBS-QB3 energetics and (C)PCM solvation calculations. Inorg Chem. 2005;44:4024–4028. doi: 10.1021/ic048734q. [DOI] [PubMed] [Google Scholar]
- 40.Ehman DL. Sawyer DT. Electrochemistry of nitric oxide and of nitrous acid at a mercury electrode. J Electroanal Chem. 1968;16:541–549. [Google Scholar]
- 41.Ellis A. Lu H. Li CG. Rand MJ. Effects of agents that inactivate free radical NO on nitroxyl anion-mediated relaxations, and on the detection of NO center dot released from the nitroxyl anion donor Angeli's salt. Br J Pharmacol. 2001;134:521–528. doi: 10.1038/sj.bjp.0704287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espey MG. Miranda KM. Thomas DD. Wink DA. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radical Biol Med. 2002;33:827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 43.Farmer PJ. Sulc F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J Inorg Biochem. 2005;99:166–184. doi: 10.1016/j.jinorgbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Favaloro JL. Kemp-Harper BK. Redox variants of NO (NO and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am J Physiol-Heart Circul Physiol. 2009;296:H1274–H1280. doi: 10.1152/ajpheart.00008.2009. [DOI] [PubMed] [Google Scholar]
- 45.Feelisch M. Stamler JS. Donors of nitrogen oxides. In: Feelisch M, editor; Stamler JS, editor. Methods in Nitric Oxide Research. New York: John Wiley & Sons; 1996. pp. 71–115. [Google Scholar]
- 46.Filipović MR. Stanić D. Raicević S. Spasić M. Niketić V. Consequences of MnSOD interactions with nitric oxide: nitric oxide dismutation and the generation of peroxynitrite and hydrogen peroxide. Free Radic Res. 2007;41:62–72. doi: 10.1080/10715760600944296. [DOI] [PubMed] [Google Scholar]
- 47.Ford PC. Lorkovic IM. Mechanistic aspects of the reactions of nitric oxide with transition-metal complexes. Chem Rev. 2002;102:993–1018. doi: 10.1021/cr0000271. [DOI] [PubMed] [Google Scholar]
- 48.Foster MW. McMahon TJ. Stamler JS. S-Nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 49.Froehlich JP. Mahaney JE. Keceli G. Pavlos CM. Goldstein R. Redwood AJ. Sumbilla C. Lee DI. Tocchetti CG. Kass DA, et al. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 50.Fukuto JM. Chiang K. Hszieh R. Wong P. Chaudhuri G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 51.Fukuto JM. Dutton AS. Houk KN. The chemistry and biology of nitroxyl (HNO): a chemically unique species with novel and important biological activity. Chembiochem. 2005;6:612–619. doi: 10.1002/cbic.200400271. [DOI] [PubMed] [Google Scholar]
- 52.Fukuto JM. Hobbs AJ. Ignarro LJ. Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems—the role of physiological oxidants and relevance to the biological activity of HNO. Biochem Biophys Res Commun. 1993;196:707–713. doi: 10.1006/bbrc.1993.2307. [DOI] [PubMed] [Google Scholar]
- 53.Fukuto JM. Hszieh R. Gulati P. Chiang KT. Nagasawa HT. N,O-Diacylated-N-hydroxyarylsulfonamides: nitroxyl precursors with potent smooth muscle relaxant properties. Biochem Biophys Res Commun. 1992;187:1367–1373. doi: 10.1016/0006-291x(92)90453-r. [DOI] [PubMed] [Google Scholar]
- 54.Fukuto JM. Stuehr DJ. Feldman PL. Bova MP. Wong P. Peracid oxidation of an N-hydroxyguanidine compound—a chemical model for the oxidation of N-omega-hydroxy-L-arginine by nitric oxide synthase. J Med Chem. 1993;36:2666–2670. doi: 10.1021/jm00070a010. [DOI] [PubMed] [Google Scholar]
- 55.Fukuto JM. Wallace GC. Hszieh R. Chaudhuri G. Chemical oxidation of N-hydroxyguanidine compounds: release of nitric oxide, nitroxyl and possible relationship to the mechanism of biological nitric oxide generation. Biochem Pharmacol. 1992;43:607–613. doi: 10.1016/0006-2952(92)90584-6. [DOI] [PubMed] [Google Scholar]
- 56.Gbadegesin M. Vicini S. Hewett SJ. Wink DA. Espey M. Pluta RM. Colton CA. Hypoxia modulates nitric oxide-induced regulation of NMDA receptor currents and neuronal cell death. Am J Physiol-Cell Physiol. 1999;277:C673–C683. doi: 10.1152/ajpcell.1999.277.4.C673. [DOI] [PubMed] [Google Scholar]
- 57.Gratzel M. Taniguchi S. Henglein A. A pulse radiolytic study of short-lived byproducts on nitric oxide reduction in aqueous solution. Ber Bunsenges Phys Chem. 1970;74:1003–1010. [Google Scholar]
- 58.Gwost D. Caulton KG. Reductive nitrosylation of group VIIIB compounds. Inorg Chem. 1973;12:2095–2099. [Google Scholar]
- 59.Hecker M. Schott C. Bucher B. Bussi R. Stoclet JC. Increase in serum N-hydroxy-L-arginine in rats treated with bacterial lipopolysaccharide. Eur J Pharmacol. 1995;275:R1–R3. doi: 10.1016/0014-2999(95)00046-n. [DOI] [PubMed] [Google Scholar]
- 60.Hedberg JJ. Griffiths WJ. Nilsson SJ. Hoog JO. Reduction of S-nitrosoglutathione by human alcohol dehydrogenase 3 is an irreversible reaction as analysed by electrospray mass spectrometry. Eur J Biochem. 2003;270:1249–1256. doi: 10.1046/j.1432-1033.2003.03486.x. [DOI] [PubMed] [Google Scholar]
- 61.Hibbs JBJ. Synthesis of nitric oxide from L-arginine: a recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res Immunol. 1991;142:565–569. doi: 10.1016/0923-2494(91)90103-p. [DOI] [PubMed] [Google Scholar]
- 62.Hobbs AJ. Fukuto JM. Ignarro LJ. Formation of free nitric oxide from L-arginine by nitric oxide synthase—direct enhancement of generation by superoxide dismutase. Proc Natl Acad Sci USA. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffman MD. Walsh GM. Rogalski JC. Kast J. Identification of nitroxyl-induced modifications in human platelet proteins using a novel mass spectrometric detection method. Mol Cell Proteomics. 2009;8:887–903. doi: 10.1074/mcp.M800230-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hogg N. Singh RJ. Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett. 1996;382:223–228. doi: 10.1016/0014-5793(96)00086-5. [DOI] [PubMed] [Google Scholar]
- 65.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 66.Hoshino M. Laverman LE. Ford PC. Nitric oxide complexes of metalloporphyrins: an overview of some mechanistic studies. Coord Chem Rev. 1999;187:75–102. [Google Scholar]
- 67.Hoshino M. Maeda M. Konishi R. Seki H. Ford PC. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J Am Chem Soc. 1996;118:5702–5707. [Google Scholar]
- 68.Hoshino M. Ozawa K. Seki H. Ford PC. Photochemistry of nitric oxide adducts of water-soluble iron(III) porphyrin and ferrihemoproteins studied by nanosecond laser photolysis. J Am Chem Soc. 1993;115:9568–9575. [Google Scholar]
- 69.Huang JM. Sommers EM. Kim-Shapiro DB. King SB. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J Am Chem Soc. 2002;124:3473–3480. doi: 10.1021/ja012271v. [DOI] [PubMed] [Google Scholar]
- 70.Hughes MN. Cammack R. Synthesis, chemistry, and applications of nitroxyl ion releasers sodium trioxodinitrate or Angeli's salt and Piloty's acid. Methods Enzymol. 1999;301:279–287. doi: 10.1016/s0076-6879(99)01092-7. [DOI] [PubMed] [Google Scholar]
- 71.Hughes MN. Wimbledon PE. The chemistry of trioxodinitrates. 2. Effect of added nitrite on stability of sodium trioxodinitrate in aqueous solution. J Chem Soc Dalton Trans. 1977;17:1650–1653. [Google Scholar]
- 72.Ignarro LJ. Buga GM. Wood KS. Byrns RE. Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irvine JC. Favaloro JL. Kemp-Harper BK. NO− activates soluble guanylate cyclase and K+ channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 74.Irvine JC. Favaloro JL. Widdop RE. Kemp-Harper BK. Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aorta. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 75.Ishimura Y. Gao YT. Panda SP. Roman LJ. Masters BS. Weintraub ST. Detection of nitrous oxide in the neuronal nitric oxide synthase reaction by gas chromatography-mass spectrometry. Biochem Biophys Res Commun. 2005;338:543–549. doi: 10.1016/j.bbrc.2005.07.202. [DOI] [PubMed] [Google Scholar]
- 76.Jackson MI. Han TH. Serbulea L. Dutton A. Ford E. Miranda KM. Houk KN. Wink DA. Fukuto JM. Kinetic feasibility of nitroxyl reduction by physiological reductants and biological implications. Free Radical Biol Med. 2009;47:1130–1139. doi: 10.1016/j.freeradbiomed.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeong W. Park SJ. Chang TS. Lee DY. Rhee SG. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. J Biol Chem. 2006;281:14400–14407. doi: 10.1074/jbc.M511082200. [DOI] [PubMed] [Google Scholar]
- 78.Kim WK. Choi YB. Rayudu PV. Das P. Asaad W. Arnelle DR. Stamler JS. Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO−. Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 79.King SB. N-hydroxyurea and acyl nitroso compounds as nitroxyl (HNO) and nitric oxide (NO) donors. Curr Top Med Chem. 2005;5:665–673. doi: 10.2174/1568026054679362. [DOI] [PubMed] [Google Scholar]
- 80.King SB. Nagasawa HT. Chemical approaches toward generation of nitroxyl. Methods Enzymol. 1999;301:211–220. doi: 10.1016/s0076-6879(99)01084-8. [DOI] [PubMed] [Google Scholar]
- 81.Kirsch M. Büscher AM. Aker S. Schulz R. de Groot H. New insights into the S-nitrosothiol-ascorbate reaction. The formation of nitroxyl. Org Biomol Chem. 2009;7:1954–1962. doi: 10.1039/b901046g. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi K. Miki M. Tagawa S. Kinetic behavior of nitric oxide studied by pulse radiolysis. Furi Rajikaru no Rinshu. 1996;10:78–84. [Google Scholar]
- 83.Kobayashi K. Tamura M. Hayashi K. Kinetic analysis of the recombination of NO with ferrihemoproteins by the flash photolysis method. Biochemistry. 1982;21:729–732. doi: 10.1021/bi00533a022. [DOI] [PubMed] [Google Scholar]
- 84.Kohout FC. Lampe FW. On the role of the nitroxyl molecule in the reaction of hydrogen atoms with nitric oxide. J Am Chem Soc. 1965;87:5795–5796. [Google Scholar]
- 85.Koppenol WH. Thermodynamics of reactions involving nitrogen-oxygen compounds. Methods Enzymol. 1996;268:7–12. doi: 10.1016/s0076-6879(96)68005-7. [DOI] [PubMed] [Google Scholar]
- 86.Kumar MR. Pervitsky D. Chen L. Poulos T. Kundu S. Hargrove MS. Rivera EJ. Diaz A. Colon JL. Farmer PJ. Nitrosyl hydride (HNO) as an O2 analogue: long-lived HNO adducts of ferrous globins. Biochemistry. 2009;48:5018–5025. doi: 10.1021/bi900122r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laverman LE. Ford PC. Mechanistic studies of nitric oxide reactions with water soluble iron(II), cobalt(II), and iron(III) porphyrin complexes in aqueous solutions: implications for biological activity. J Am Chem Soc. 2001;123:11614–11622. doi: 10.1021/ja0113910. [DOI] [PubMed] [Google Scholar]
- 88.Li J. Billiar TR. Talanian RV. Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 89.Lin MC. He YS. Melius CF. Theoretical interpretation of the kinetics and mechanisms of the HNO+HNO and HNO+2NO reactions with a unified model. Int J Chem Kinet. 1992;24:489–516. [Google Scholar]
- 90.Liochev SI. Fridovich I. Copper, zinc superoxide dismutase as a univalent NO-oxidoreductase and as a dichlorofluorescin peroxidase. J Biol Chem. 2001;276:35253–35257. doi: 10.1074/jbc.M104237200. [DOI] [PubMed] [Google Scholar]
- 91.Liochev SI. Fridovich I. Nitroxyl (NO−): a substrate for superoxide dismutase. Arch Biochem Biophys. 2002;402:166–171. doi: 10.1016/S0003-9861(02)00074-7. [DOI] [PubMed] [Google Scholar]
- 92.Liochev SI. Fridovich I. The mode of decomposition of Angeli's salt (Na2N2O3) and the effects thereon of oxygen, nitrite, superoxide dismutase, and glutathione. Free Radical Biol Med. 2003;34:1399–1404. doi: 10.1016/s0891-5849(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 93.Liu XP. Miller MJS. Joshi MS. Thomas DD. Lancaster JR. Accelerated reaction of nitric oxide with O2− within the hydrophobic interior of biological membranes. Proc Natl Acad Sci USA. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez BE. Rodriguez CE. Pribadi M. Cook NM. Shinyashiki M. Fukuto JM. Inhibition of yeast glycolysis by nitroxyl (HNO): mechanism of HNO toxicity and implications to HNO biology. Arch Biochem Biophys. 2005;442:140–148. doi: 10.1016/j.abb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Lopez BE. Wink DA. Fukuto JM. The inhibition of glyceraldehyde-3-phosphate dehydrogenase by nitroxyl (HNO) Arch Biochem Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 96.Lymar SV. Shafirovich V. Poskrebyshev GA. One-electron reduction of aqueous nitric oxide: a mechanistic revision. Inorg Chem. 2005;44:5212–5221. doi: 10.1021/ic0501317. [DOI] [PubMed] [Google Scholar]
- 97.Ma XL. Gao F. Liu GL. Lopez BL. Christopher TA. Fukuto JM. Wink DA. Feelisch M. Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proc Natl Acad Sci USA. 1999;96:14617–14622. doi: 10.1073/pnas.96.25.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma XL. Weyrich AS. Lefer DJ. Lefer AM. Diminished basal nitric oxide release after mycardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 99.MacLennan DH. Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 100.Maragos CM. Morley D. Wink DA. Dunams TM. Saavedra JE. Hoffman A. Bove AA. Isaac L. Hrabie JA. Keefer LK. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide - vasorelaxant effects. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 101.Marti MA. Bari SE. Estrin DA. Doctorovich F. Discrimination of nitroxyl and nitric oxide by water-soluble Mn(III) porphyrins. J Am Chem Soc. 2005;127:4680–4684. doi: 10.1021/ja044632n. [DOI] [PubMed] [Google Scholar]
- 102.Meister A. On the cycles of glutathione metabolism and transport. Curr Topics Cell Regul. 1981;18:21–58. doi: 10.1016/b978-0-12-152818-8.50009-8. [DOI] [PubMed] [Google Scholar]
- 103.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller TW. Cherney MM. Lee AJ. Francoleon NE. Farmer PJ. King SB. Hobbs AJ. Miranda KM. Burstyn JN. Fukuto JM. The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols. J Biol Chem. 2009;284:21788–21796. doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miranda K. Espey MG. Jourd'heuil D. Grisham MB. Fukuto M. Feelisch M. Wink DA. The chemical biology of nitric oxide. In: Ignarro LJ, editor. Nitric Oxide: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 41–55. [Google Scholar]
- 106.Miranda KM. The chemistry of nitroxyl (HNO) and implications in biology. Coord Chem Rev. 2005;249:433–455. [Google Scholar]
- 107.Miranda KM. Dutton AS. Ridnour LA. Foreman CA. Ford E. Paolocci N. Katori T. Tocchetti CG. Mancardi D. Thomas DD, et al. Mechanism of aerobic decomposition of Angeli's salt (sodium trioxodinitrate) at physiological pH. J Am Chem Soc. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 108.Miranda KM. Espey MG. Wink DA. A discussion of the chemistry of oxidative and nitrosative stress in cytotoxicity. J Inorg Biochem. 2000;79:237–240. doi: 10.1016/s0162-0134(99)00241-x. [DOI] [PubMed] [Google Scholar]
- 109.Miranda KM. Espey MG. Yamada K. Krishna M. Ludwick N. Kim S. Jourd'heuil D. Grisham MB. Feelisch M. Fukuto JM, et al. Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem. 2001;276:1720–1727. doi: 10.1074/jbc.M006174200. [DOI] [PubMed] [Google Scholar]
- 110.Miranda KM. Nagasawa HT. Toscano JP. Donors of HNO. Curr Top Med Chem. 2005;5:649–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 111.Miranda KM. Nims RW. Thomas DD. Espey MG. Citrin D. Bartberger MD. Paolocci N. Fukuto JM. Feelisch M. Wink DA. Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins. A chemical discussion of the differential biological effects of these redox related products of NOS. J Inorg Biochem. 2003;93:52–60. doi: 10.1016/s0162-0134(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 112.Miranda KM. Paolocci N. Katori T. Thomas DD. Ford E. Bartberger MD. Espey MG. Kass DA. Feelisch M. Fukuto JM, et al. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miranda KM. Yamada K. Espey MG. Thomas DD. DeGraff W. Mitchell JB. Krishna MC. Colton CA. Wink DA. Further evidence for distinct reactive intermediates from nitroxyl and peroxynitrite: effects of buffer composition on the chemistry of Angeli's salt and synthetic peroxynitrite. Arch Biochem Biophys. 2002;401:134–144. doi: 10.1016/S0003-9861(02)00031-0. [DOI] [PubMed] [Google Scholar]
- 114.Monks TJ. Ghersi-Egea JF. Philbert M. Cooper AJ. Lock EA. Symposium overview: the role of glutathione in neuroprotection and neurotoxicity. Toxicol Sci. 1999;51:161–177. doi: 10.1093/toxsci/51.2.161. [DOI] [PubMed] [Google Scholar]
- 115.Mudd JO. Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 116.Murad F. The nitric oxide cyclic-GMP signal-transduction system for intracellular and intercellular communication. Recent Prog Hormone Res. 1994;49:239–248. doi: 10.1016/b978-0-12-571149-4.50016-7. [DOI] [PubMed] [Google Scholar]
- 117.Murphy ME. Sies H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:10860–10864. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagasawa HT. DeMaster EG. Redfern B. Shirota FN. Goon DJ. Evidence for nitroxyl in the catalase-mediated bioactivation of the alcohol deterrent agent cyanamide. J Med Chem. 1990;33:3120–3122. doi: 10.1021/jm00174a001. [DOI] [PubMed] [Google Scholar]
- 119.Nathan CF. Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 120.Norris AJ. Sartippour MR. Lu M. Park T. Rao JY. Jackson MI. Fukuto JM. Brooks MN. Nitroxyl inhibits breast tumor growth and angiogenesis. Int J Cancer. 2008;122:1905–1910. doi: 10.1002/ijc.23305. [DOI] [PubMed] [Google Scholar]
- 121.Oae S. Kim YH. Fukushima D. Shinhama K. New syntheses of thionitrites and their chemical reactivities. J Chem Soc Perkin Trans. 1978;1:913–917. [Google Scholar]
- 122.Ohshima H. Celan I. Chazotte L. Pignatelli B. Mower HF. Analysis of 3-nitrotyrosine in biological fluids and protein hydrolyzates by high-performance liquid chromatography using a postseparation, on-line reduction column and electrochemical detection: results with various nitrating agents. Nitric Oxide. 1999;3:132–141. doi: 10.1006/niox.1999.0216. [DOI] [PubMed] [Google Scholar]
- 123.Ohshima H. Gilibert I. Bianchini F. Induction of DNA strand breakage and base oxidation by nitroxyl anion through hydroxyl radical production. Free Radical Biol Med. 1999;26:1305–1313. doi: 10.1016/s0891-5849(98)00327-x. [DOI] [PubMed] [Google Scholar]
- 124.Olson JS. Phillips GN. Kinetic pathways and barriers for ligand binding to myoglobin. J Biol Chem. 1996;271:17596. [PubMed] [Google Scholar]
- 125.Pagliaro P. Differential biological effects of products of nitric oxide (NO) synthase: it is not enough to say NO. Life Sci. 2003;73:2137–2149. doi: 10.1016/s0024-3205(03)00593-9. [DOI] [PubMed] [Google Scholar]
- 126.Pagliaro P. Mancardi D. Rastaldo R. Penna C. Gattullo D. Miranda KM. Feelisch M. Wink DA. Kass DA. Paolocci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radical Biol Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 127.Palmer RMJ. Ferrige AG. Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 128.Paolocci N. Katori T. Champion HC. St. John ME. Miranda KM. Fukuto JM. Wink DA. Kass DA. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from β-adrenergic signaling. Proc Natl Acad Sci USA. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paolocci N. Saavedra WF. Miranda KM. Martignani C. Isoda T. Hare JM. Espey MG. Fukuto JM. Feelisch M. Wink DA, et al. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci USA. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paolocci N. Wink DA. The shy Angeli and his elusive creature: the HNO route to vasodilation. Am J Physiol-Heart Circul Physiol. 2009;296:H1217–H1220. doi: 10.1152/ajpheart.00243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Piloty O. Ueber eine oxydation des hydroxylamins durch benzolsulfochlorid. Ber Dtsch Chem Ges. 1896;29:1559–1567. [Google Scholar]
- 132.Pino RZ. Feelisch M. Bioassay discrimination between nitric oxide (NO) and nitroxyl (NO−) using L-cysteine. Biochem Biophys Res Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 133.Poskrebyshev GA. Shafirovich V. Lymar SV. Disproportionation pathways of aqueous hyponitrite radicals (HN2O2/N2O2−) J Phys Chem A. 2008;112:8295–8302. doi: 10.1021/jp803230c. [DOI] [PubMed] [Google Scholar]
- 134.Poskrebyshev GA. Shafirovich V. Lymar SV. Hyponitrite radical, a stable adduct of nitric oxide and nitroxyl. J Am Chem Soc. 2004;126:891–899. doi: 10.1021/ja038042l. [DOI] [PubMed] [Google Scholar]
- 135.Pufahl RA. Wishnok JS. Marletta MA. Hydrogen peroxide-supported oxidation of NG-hydroxy-L-arginine by nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 136.Reif A. Zecca L. Riederer P. Feelisch M. Schmidt HHHW. Nitroxyl oxidizes NADPH in a superoxide dismutase inhibitable manner. Free Radical Biol Med. 2001;30:803–808. doi: 10.1016/s0891-5849(01)00477-4. [DOI] [PubMed] [Google Scholar]
- 137.Ridnour LA. Thomas DD. Mancardi D. Paolocci N. Espey MG. Miranda KM. Feelisch M. Fukuto J. Wink DA. The chemistry of nitrosative stress by nitric oxide and reactive nitrogen oxide species: putting perspective on stressful biological situations. Biol Chem. 2004;385:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 138.Rusche KM. Spiering MM. Marletta MA. Reactions catalyzed by tetrahydrobiopterin-free nitric oxide synthase. Biochemistry. 1998;37:15503–15512. doi: 10.1021/bi9813936. [DOI] [PubMed] [Google Scholar]
- 139.Samuni Y. Flores-Santana W. Krishna MC. Mitchell JB. Wink DA. The inhibitors of histone deacetylase suberoylanilide hydroxamate and trichostatin A release nitric oxide upon oxidation. Free Radical Biol Med. 2009;47:419–423. doi: 10.1016/j.freeradbiomed.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmidt HHHW. Hofmann H. Schindler U. Shutenko ZS. Cunningham DD. Feelisch M. No NO from NO synthase. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schulz U. McCalla DR. Reactions of cysteine with N-methyl-N-nitroso-P-toluenesulfonamide and N-methyl-N'-nitro-N-nitrosoguanidine. Can J Chem. 1969;47:2021–2027. [Google Scholar]
- 142.Seddon WA. Fletcher JW. Sopchyshyn FC. Pulse radiolysis of nitric oxide in aqueous solution. Can J Chem. 1973;51:1123–1130. [Google Scholar]
- 143.Seddon WA. Young MJ. Pulse radiolysis of nitric oxide in aqueous solution. Can J Chem. 1970;48:393–394. [Google Scholar]
- 144.Sengupta R. Ryter SW. Zuckerbraun BS. Tzeng E. Billiar TR. Stoyanovsky DA. Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiol. Biochemistry. 2007;46:8472–8483. doi: 10.1021/bi700449x. [DOI] [PubMed] [Google Scholar]
- 145.Shafirovich V. Lymar SV. Nitroxyl and its anion in aqueous solutions: spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc Natl Acad Sci USA. 2002;99:7340–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shahidullah M. Duncan A. Strachan PD. Rafique KM. Ball SL. McPate MJW. Nelli S. Martin W. Role of catalase in the smooth muscle relaxant actions of sodium azide and cyanamide. Eur J Pharmacol. 2002;435:93–101. doi: 10.1016/s0014-2999(01)01540-0. [DOI] [PubMed] [Google Scholar]
- 147.Sharpe MA. Cooper CE. Reactions of nitric oxide with mitochondrial cytochrome c: a novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem J. 1998;332:9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shen B. English AM. Mass spectrometric analysis of nitroxyl-mediated protein modification: comparison of products formed with free and protein-based cysteines. Biochemistry. 2005;44:14030–14044. doi: 10.1021/bi0507478. [DOI] [PubMed] [Google Scholar]
- 149.Sherman MP. Grither WR. McCulla RD. Computational investigation of the reaction mechanisms of nitroxyl and thiols. J Org Chem. 2010;75:4014–4024. doi: 10.1021/jo100172t. [DOI] [PubMed] [Google Scholar]
- 150.Shibata Y. Sato H. Sagami I. Shimizu T. Interaction of Angeli's salt with cytochrome P450 1A2 distal mutants: an optical absorption spectral study. Biochim Biophys Acta-Protein Struct Mol Enzym. 1997;1343:67–75. doi: 10.1016/s0167-4838(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 151.Shiva S. Crawford JH. Ramachandran A. Ceaser EK. Hillson T. Brookes PS. Patel RP. Darley-Usmar VM. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379:359–366. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shoeman DW. Shirota FN. DeMaster EG. Nagasawa HT. Reaction of nitroxyl, an aldehyde dehydrogenase inhibitor, with N-acetyl-L-cysteine. Alcohol. 2000;20:55–59. doi: 10.1016/s0741-8329(99)00056-7. [DOI] [PubMed] [Google Scholar]
- 153.Sidorkina O. Espey MG. Miranda KM. Wink DA. Laval J. Inhibition of poly(ADP-ribose) polymerase (PARP) by nitric oxide and reactive nitrogen oxide species. Free Radical Biol Med. 2003;35:1431–1438. doi: 10.1016/j.freeradbiomed.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 154.Stanbury DM. Reduction potentials involving inorganic free radicals in aqueous solution. Adv Inorg Chem. 1989;33:69–136. [Google Scholar]
- 155.Stoyanovsky DA. Schor NF. Nylander KD. Salama G. Effects of pH on the cytotoxicity of sodium trioxodinitrate (Angeli's salt) J Med Chem. 2004;47:210–217. doi: 10.1021/jm030192j. [DOI] [PubMed] [Google Scholar]
- 156.Stoyanovsky DA. Tyurina YY. Tyurin VA. Anand D. Mandavia DN. Gius D. Ivanova J. Pitt B. Billiar TR. Kagan VE. Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J Am Chem Soc. 2005;127:15815–15823. doi: 10.1021/ja0529135. [DOI] [PubMed] [Google Scholar]
- 157.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 158.Sulc F. Immoos CE. Pervitsky D. Farmer PJ. Efficient trapping of HNO by deoxymyoglobin. J Am Chem Soc. 2004;126:1096–1101. doi: 10.1021/ja0376184. [DOI] [PubMed] [Google Scholar]
- 159.Thomas DD. Ridnour LA. Isenberg JS. Flores-Santana W. Switzer CH. Donzelli S. Hussain P. Vecoli C. Paolocci N. Ambs S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radical Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tocchetti CG. Wang W. Froehlich JP. Huke S. Aon MA. Wilson GM. Di Benedetto G. O'Rourke B. Gao WD. Wink DA, et al. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Väänänen AJ. Kankuri E. Rauhala P. Nitric oxide-related species-induced protein oxidation: reversible, irreversible, and protective effects on enzyme function of papain. Free Radical Biol Med. 2005;38:1102–1111. doi: 10.1016/j.freeradbiomed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 162.Väänänen AJ. Moed M. Tuominen RK. Helkamaa TH. Wiksten M. Liesi P. Chiueh CC. Rauhala P. Angeli's salt induces neurotoxicity in dopaminergic neurons in vivo and in vitro. Free Radical Biol Med. 2003;37:381–389. doi: 10.1080/1071576031000061011. [DOI] [PubMed] [Google Scholar]
- 163.Väänänen AJ. Salmenperä P. Hukkanen M. Miranda KM. Harjula A. Rauhala P. Kankuri E. Persistent susceptibility of cathepsin B to irreversible inhibition by nitroxyl (HNO) in the presence of endogenous nitric oxide. Free Radical Biol Med. 2008;45:749–755. doi: 10.1016/j.freeradbiomed.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 164.Vanin AF. Vedernikov YI. Galagan ME. Kubrina LN. Kuzmanis YA. Kalvinsh IY. Mordvintsev PI. Angeli salt as a producer of nitrogen oxide in the animal organism. Biochem-Moscow. 1990;55:1048–1052. [PubMed] [Google Scholar]
- 165.VanUffelen BE. Van der Zee J. de Koster BM. VanSteveninck J. Elferink JGR. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem J. 1998;330:719–722. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vidwans AS. Uliasz TF. Hewett JA. Hewett SJ. Differential modulation of prostaglandin H synthase-2 by nitric oxide-related species in intact cells. Biochemistry. 2001;40:11533–11542. doi: 10.1021/bi0108960. [DOI] [PubMed] [Google Scholar]
- 167.Wayland BB. Olson LW. Spectroscopic studies and bonding model for nitric oxide complexes of iron porphyrins. J Am Chem Soc. 1974;96:6037–6041. doi: 10.1021/ja00826a013. [DOI] [PubMed] [Google Scholar]
- 168.Wayland BB. Olson LW. Siddiqui ZU. Nitric oxide complexes of manganese and chromium tetraphenylporphyrin. J Am Chem Soc. 1976;98:94–98. [Google Scholar]
- 169.Williams DL. Aldred SE. Inhibition of nitrosation of amines by thiols, alcohols and carbohydrates. Food Chem Toxicol. 1982;20:79–81. doi: 10.1016/s0278-6915(82)80013-6. [DOI] [PubMed] [Google Scholar]
- 170.Wink DA. Darbyshire JF. Nims RW. Saavedra JE. Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media—determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993;6:23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 171.Wink DA. Feelisch M. Feelisch M. Stamler JS. Methods in Nitric Oxide Research. New York: John Wiley & Sons; 1996. Formation and detection of nitroxyl and nitrous oxide; pp. 403–412. [Google Scholar]
- 172.Wink DA. Feelisch M. Fukuto J. Christodoulou D. Jourd'heuil D. Grisham MB. Vodovotz Y. Cook JA. Krishna M. DeGraff WG, et al. The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 173.Wink DA. Miranda KM. Katori T. Mancardi D. Thomas DD. Ridnour LA. Espey MG. Feelisch M. Colton CA. Fukuto JM, et al. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: a novel redox paradigm. Am J Physiol-Heart Circul Physiol. 2003;285:H2264–H2276. doi: 10.1152/ajpheart.00531.2003. [DOI] [PubMed] [Google Scholar]
- 174.Wink DA. Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radical Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 175.Wong PSY. Hyun J. Fukuto JM. Shirota FN. DeMaster EG. Shoeman DW. Nagasawa HT. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 176.Woo HA. Chae HZ. Hwang SC. Yang KS. Kang SW. Kim K. Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 177.Zamora R. Grzesiok A. Weber H. Feelisch M. Oxidative release of nitric oxide accounts for guanylyl cyclase stimulating, vasodilator and anti-platelet activity of Piloty's acid: a comparison with Angeli's salt. Biochem J. 1995;312:333–339. doi: 10.1042/bj3120333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeller A. Wenzl MV. Beretta M. Stessel H. Russwurm M. Koesling D. Schmidt K. Mayer B. Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli's salt. Mol Pharmacol. 2009;76:1115–1122. doi: 10.1124/mol.109.059915. [DOI] [PubMed] [Google Scholar]