Abstract
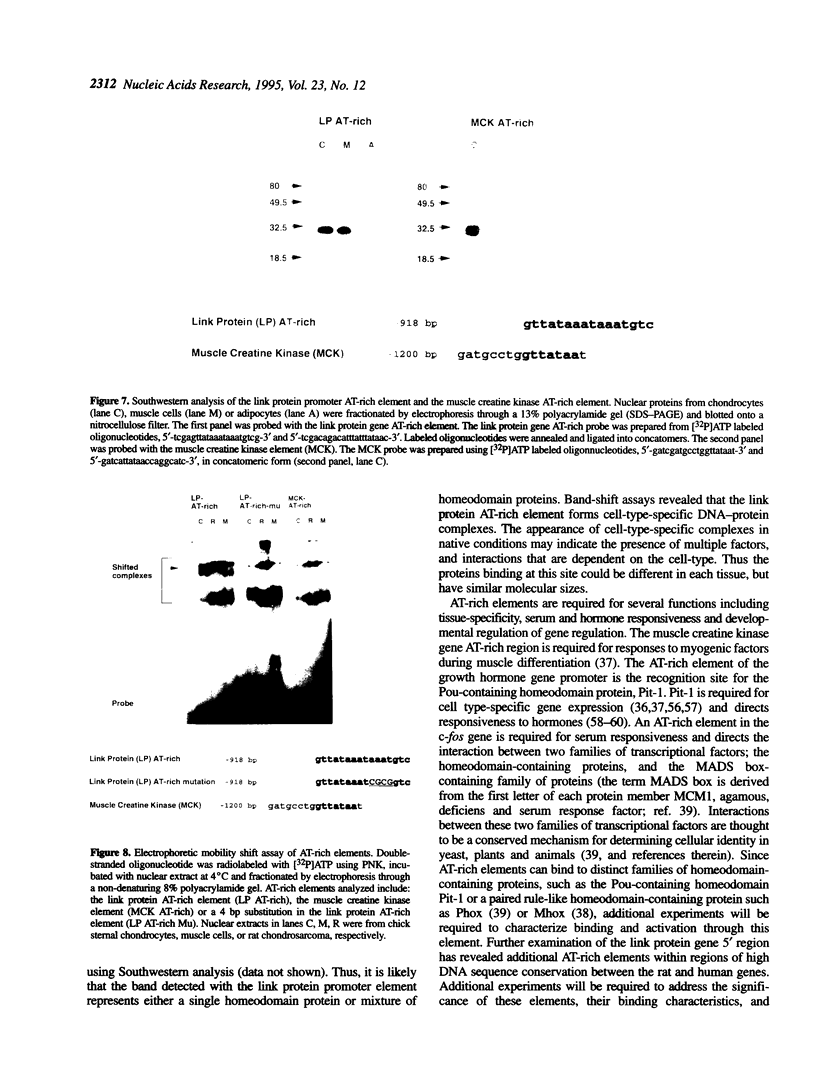
The cartilage matrix is composed of characteristic components including type II collagen, aggrecan and link protein. In this paper, we report two DNA elements that regulate the link protein gene. Using transient transfection assays with link protein gene constructs in chondrocytes, chloramphenicol acetyl transferase (CAT) assays were used to measure the transcriptional activity of the link protein gene. Previously, we identified an enhancer-like activity within the first intron of the gene. In this paper, we report an active 34 bp (+1390 to +1424) fragment within this region that contains a glucocorticoid-like response element (GRE). Both deletion of, and site-specific mutations within this sequence motif reduced the dexamethasone-inducible activity. The GRE-like sequence from the rat link protein gene, or the homologous sequence from the human link protein gene were included in vectors containing the thymidine kinase promoter linked to the CAT gene (tkCAT). Both human and rat elements transferred the ability to respond to dexamethasone and hydrocortisone with a > 10-fold induction. Deletions through the promoter from -923 to -900 identified a second site required for both glucocorticoid and serum responsiveness. A four base substitution at this site resulted in a loss of serum responsiveness. This region contains an AT-rich element, similar to the AT-rich elements involved in homeotic protein regulation of the growth hormone gene and the muscle creatine kinase gene. Southwestern analysis using oligonucleotides containing the AT-rich element from the link protein gene or the muscle creatine kinase gene, identified a 32 kDa protein band from nuclear extracts of chick chondrocytes. Using these AT-rich oligonucleotides in band-shift analyses, nuclear extracts of chick sternal muscle, rat chondrosarcoma and chick sternal chondrocytes each showed formation of different complexes suggesting cell specificity. AT-rich elements have been identified as binding sites for homeodomain-containing proteins and can contribute to gene regulation by serum response factors. The identification of an AT-rich element in the link protein gene suggests similar functions for this element.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastassiades T., Dziewiatkowski D. The effect of cortisone on the metabolism of connective tissues in the rat. J Lab Clin Med. 1970 May;75(5):826–839. [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bernier S. M., Goltzman D. Regulation of expression of the chondrocytic phenotype in a skeletal cell line (CFK2) in vitro. J Bone Miner Res. 1993 Apr;8(4):475–484. doi: 10.1002/jbmr.5650080412. [DOI] [PubMed] [Google Scholar]
- Binette F., Cravens J., Kahoussi B., Haudenschild D. R., Goetinck P. F. Link protein is ubiquitously expressed in non-cartilaginous tissues where it enhances and stabilizes the interaction of proteoglycans with hyaluronic acid. J Biol Chem. 1994 Jul 22;269(29):19116–19122. [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Böhme K., Conscience-Egli M., Tschan T., Winterhalter K. H., Bruckner P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: the role of insulin-like growth factor-I, insulin, or thyroxine. J Cell Biol. 1992 Feb;116(4):1035–1042. doi: 10.1083/jcb.116.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro A., Hascall V. C. Effects of brefeldin A on aggrecan core protein synthesis and maturation in rat chondrosarcoma cells. J Biol Chem. 1994 Sep 9;269(36):22771–22778. [PubMed] [Google Scholar]
- Calcagno M., Goyena H., Arrambide E., Arruti de Urse C. Action of cortisone and cortisol upon biosynthesis of chondroitin sulfate in femur in vitro cultures of chick embryo. Exp Cell Res. 1970 Nov;63(1):131–137. doi: 10.1016/0014-4827(70)90340-x. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Bodner M., Karin M. Purification of growth hormone-specific transcription factor GHF-1 containing homeobox. Science. 1989 Feb 10;243(4892):814–817. doi: 10.1126/science.2563596. [DOI] [PubMed] [Google Scholar]
- Chunekamrai S., Krook L. P., Lust G., Maylin G. A. Changes in articular cartilage after intra-articular injections of methylprednisolone acetate in horses. Am J Vet Res. 1989 Oct;50(10):1733–1741. [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Deák F., Barta E., Mestric S., Biesold M., Kiss I. Complex pattern of alternative splicing generates unusual diversity in the leader sequence of the chicken link protein mRNA. Nucleic Acids Res. 1991 Sep 25;19(18):4983–4990. doi: 10.1093/nar/19.18.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBattista J. A., Martel-Pelletier J., Wosu L. O., Sandor T., Antakly T., Pelletier J. P. Glucocorticoid receptor mediated inhibition of interleukin-1 stimulated neutral metalloprotease synthesis in normal human chondrocytes. J Clin Endocrinol Metab. 1991 Feb;72(2):316–326. doi: 10.1210/jcem-72-2-316. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K., Fernandez P., Hassell J. R., Sasaki M., Yamada Y. Partial cDNA sequence encoding a globular domain at the C terminus of the rat cartilage proteoglycan. J Biol Chem. 1986 Jun 25;261(18):8108–8111. [PubMed] [Google Scholar]
- Dudhia J., Bayliss M. T., Hardingham T. E. Human link protein gene: structure and transcription pattern in chondrocytes. Biochem J. 1994 Oct 1;303(Pt 1):329–333. doi: 10.1042/bj3030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Lebovitz H. E. Isolation and characterization of a serum inhibitor of cartilage metabolism. Endocrinology. 1974 Dec;95(6):1600–1607. doi: 10.1210/endo-95-6-1600. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender H. G. Role of chondrocytes in the development of osteoarthritis. Am J Med. 1987 Nov 20;83(5A):17–24. doi: 10.1016/0002-9343(87)90846-1. [DOI] [PubMed] [Google Scholar]
- Fox S. R., Jong M. T., Casanova J., Ye Z. S., Stanley F., Samuels H. H. The homeodomain protein, Pit-1/GHF-1, is capable of binding to and activating cell-specific elements of both the growth hormone and prolactin gene promoters. Mol Endocrinol. 1990 Jul;4(7):1069–1080. doi: 10.1210/mend-4-7-1069. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gray R. G., Tenenbaum J., Gottlieb N. L. Local corticosteroid injection treatment in rheumatic disorders. Semin Arthritis Rheum. 1981 May;10(4):231–254. doi: 10.1016/0049-0172(81)90001-9. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A. E., Aubin J. E., Heersche J. N. Effects of dexamethasone and vitamin D3 on cartilage differentiation in a clonal chondrogenic cell population. Endocrinology. 1989 Oct;125(4):2103–2110. doi: 10.1210/endo-125-4-2103. [DOI] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Ham J., Thomson A., Needham M., Webb P., Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res. 1988 Jun 24;16(12):5263–5276. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Itagane Y., Inada H., Fujita K., Isshiki G. Interactions between steroid hormones and insulin-like growth factor-I in rabbit chondrocytes. Endocrinology. 1991 Mar;128(3):1419–1424. doi: 10.1210/endo-128-3-1419. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Shapiro I. M., Yagami K., Boskey A. L., Leboy P. S., Adams S. L., Pacifici M. Retinoic acid induces rapid mineralization and expression of mineralization-related genes in chondrocytes. Exp Cell Res. 1993 Aug;207(2):413–420. doi: 10.1006/excr.1993.1209. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Kato Y., Gospodarowicz D. Stimulation by glucocorticoid of the synthesis of cartilage-matrix proteoglycans produced by rabbit costal chondrocytes in vitro. J Biol Chem. 1985 Feb 25;260(4):2364–2373. [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Langille R. M., Paulsen D. F., Solursh M. Differential effects of physiological concentrations of retinoic acid in vitro on chondrogenesis and myogenesis in chick craniofacial mesenchyme. Differentiation. 1989 May;40(2):84–92. doi: 10.1111/j.1432-0436.1989.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Lebovitz H. E., Eisenbarth G. S. Hormonal regulation of cartilage growth and metabolism. Vitam Horm. 1975;33:575–648. doi: 10.1016/s0083-6729(08)60973-5. [DOI] [PubMed] [Google Scholar]
- McCumbee W. D., McCarty K. S., Jr, Lebovitz H. E. Hormone responsiveness of a transplantable rat chondrosarcoma. II. Evidence for in vivo hormone dependence. Endocrinology. 1980 Jun;106(6):1930–1940. doi: 10.1210/endo-106-6-1930. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Suh B. J., Kühnel B., Hutchison C. A., 3rd Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990 Dec;4(12):1866–1873. doi: 10.1210/mend-4-12-1866. [DOI] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J. The therapeutic effects of NSAID and corticosteroids in osteoarthritis: to be or not to be. J Rheumatol. 1989 Mar;16(3):266–269. [PubMed] [Google Scholar]
- Rhodes C., Savagner P., Line S., Sasaki M., Chirigos M., Doege K., Yamada Y. Characterization of the promoter for the rat and human link protein gene. Nucleic Acids Res. 1991 Apr 25;19(8):1933–1939. doi: 10.1093/nar/19.8.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schaufele F., West B. L., Baxter J. D. Synergistic activation of the rat growth hormone promoter by Pit-1 and the thyroid hormone receptor. Mol Endocrinol. 1992 Apr;6(4):656–665. doi: 10.1210/mend.6.4.1584227. [DOI] [PubMed] [Google Scholar]
- Strähle U., Klock G., Schütz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessler R. H., Salmon W. D., Jr Glucocorticoid inhibition of sulfate incorporation by cartilage of normal rats. Endocrinology. 1975 Apr;96(4):898–902. doi: 10.1210/endo-96-4-898. [DOI] [PubMed] [Google Scholar]
- Tsonis P. A. 1,25-Dihydroxyvitamin D3 stimulates chondrogenesis of the chick limb bud mesenchymal cells. Dev Biol. 1991 Jan;143(1):130–134. doi: 10.1016/0012-1606(91)90060-g. [DOI] [PubMed] [Google Scholar]
- Wong M., Lawton T., Goetinck P. F., Kuhn J. L., Goldstein S. A., Bonadio J. Aggrecan core protein is expressed in membranous bone of the chick embryo. Molecular and biomechanical studies of normal and nanomelia embryos. J Biol Chem. 1992 Mar 15;267(8):5592–5598. [PubMed] [Google Scholar]
- Wynne J., Treisman R. SRF and MCM1 have related but distinct DNA binding specificities. Nucleic Acids Res. 1992 Jul 11;20(13):3297–3303. doi: 10.1093/nar/20.13.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Ye Z. S., Forman B. M., Aranda A., Pascual A., Park H. Y., Casanova J., Samuels H. H. Rat growth hormone gene expression. Both cell-specific and thyroid hormone response elements are required for thyroid hormone regulation. J Biol Chem. 1988 Jun 5;263(16):7821–7829. [PubMed] [Google Scholar]