Abstract
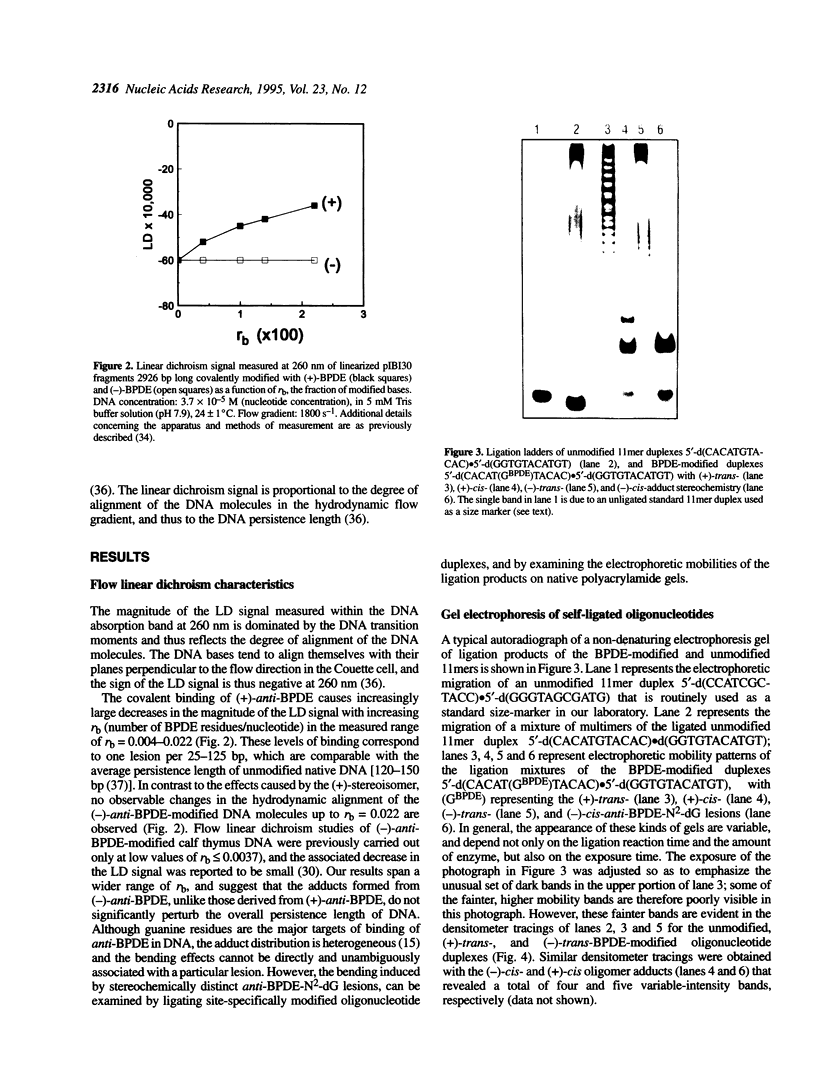
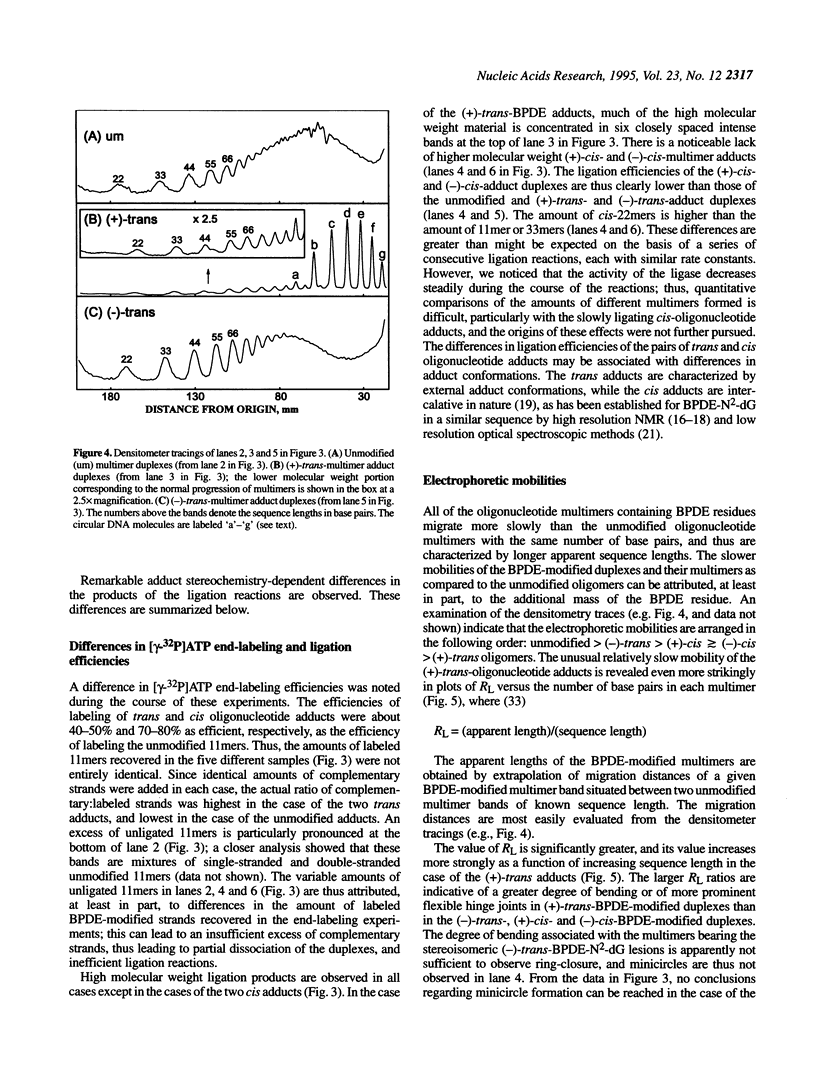
The apparent persistence length of enzymatically linearized pIBI30 plasmid DNA molecules approximately 2300 bp long, as measured by a hydrodynamic linear flow dichroism method, is markedly decreased after covalent binding of the highly tumorigenic benzo[a]pyrene metabolite 7R,8S-dihydroxy-9S,10R-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene [(+)-anti-BPDE]. In striking contrast, the binding of the non-tumorigenic, mirror-image 7S,8R,9R,10S enantiomer [(-)-anti-BPDE] to DNA has no measurable effect on its alignment in hydrodynamic flow gradients (< or = 2.2% of the DNA bases modified). In order to relate this effect to BPDE-nucleotide lesions of defined stereochemistry, the bending induced by site-specifically placed and stereochemically defined (+)- and (-)-anti-BPDE-N2-dG lesions in an 11mer deoxyoligonucleotide duplex was studied by ligation and gel electrophoresis methods. Out of the four stereochemically isomeric anti-BPDE-N2-deoxyguanosyl (dG) adducts with either (+)-trans, (-)-trans, (+)-cis, and (-)-cis adduct stereochemistry, only the (+)-trans adduct gives rise to prominent bends or flexible hinge joints in the modified oligonucleotide duplexes. Since both anti-BPDE enantiomers are known to bind preferentially to dG (> or = 85%), these observations can account for the differences in persistence lengths of DNA modified with either (+)-anti-BPDE or the chiral (-)-anti-BPDE isomer.
Full text
PDF

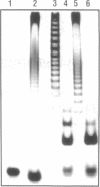
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasta L., Xu R., Geacintov N. E., Swenberg C. E., Amin S., Hecht S. S. Unwinding and hydrodynamic flow linear dichroism characteristics of supercoiled DNA covalently modified with two isomeric methylchrysene diol epoxides of different biological activities. Chem Res Toxicol. 1993 Sep-Oct;6(5):616–624. doi: 10.1021/tx00035a005. [DOI] [PubMed] [Google Scholar]
- Bellon S. F., Lippard S. J. Bending studies of DNA site-specifically modified by cisplatin, trans-diamminedichloroplatinum(II) and cis-[Pt(NH3)2(N3-cytosine)Cl]+. Biophys Chem. 1990 Apr;35(2-3):179–188. doi: 10.1016/0301-4622(90)80007-t. [DOI] [PubMed] [Google Scholar]
- Brookes P., Osborne M. R. Mutation in mammalian cells by stereoisomers of anti-benzo[a] pyrene-diolepoxide in relation to the extent and nature of the DNA reaction products. Carcinogenesis. 1982;3(10):1223–1226. doi: 10.1093/carcin/3.10.1223. [DOI] [PubMed] [Google Scholar]
- Buening M. K., Wislocki P. G., Levin W., Yagi H., Thakker D. R., Akagi H., Koreeda M., Jerina D. M., Conney A. H. Tumorigenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides in newborn mice: exceptional activity of (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5358–5361. doi: 10.1073/pnas.75.11.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carberry S. E., Geacintov N. E., Harvey R. G. Reactions of stereoisomeric non-bay-region benz[a]anthracene diol epoxides with DNA and conformations of non-covalent complexes and covalent adducts. Carcinogenesis. 1989 Jan;10(1):97–103. doi: 10.1093/carcin/10.1.97. [DOI] [PubMed] [Google Scholar]
- Cheng S. C., Hilton B. D., Roman J. M., Dipple A. DNA adducts from carcinogenic and noncarcinogenic enantiomers of benzo[a]pyrene dihydrodiol epoxide. Chem Res Toxicol. 1989 Sep-Oct;2(5):334–340. doi: 10.1021/tx00011a011. [DOI] [PubMed] [Google Scholar]
- Choi D. J., Marino-Alessandri D. J., Geacintov N. E., Scicchitano D. A. Site-specific benzo[a]pyrene diol epoxide-DNA adducts inhibit transcription elongation by bacteriophage T7 RNA polymerase. Biochemistry. 1994 Jan 25;33(3):780–787. doi: 10.1021/bi00169a020. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Cosman M., Ibanez V., Geacintov N. E., Harvey R. G. Preparation and isolation of adducts in high yield derived from the binding of two benzo[a]pyrene-7,8-dihydroxy-9,10-oxide stereoisomers to the oligonucleotide d(ATATGTATA). Carcinogenesis. 1990 Sep;11(9):1667–1672. doi: 10.1093/carcin/11.9.1667. [DOI] [PubMed] [Google Scholar]
- Cosman M., de los Santos C., Fiala R., Hingerty B. E., Singh S. B., Ibanez V., Margulis L. A., Live D., Geacintov N. E., Broyde S. Solution conformation of the major adduct between the carcinogen (+)-anti-benzo[a]pyrene diol epoxide and DNA. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1914–1918. doi: 10.1073/pnas.89.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M., Nordén B., Jernström B., Gräslund A. Binding geometries of benzo[a]pyrene diol epoxide isomers covalently bound to DNA. Orientational distribution. Biochemistry. 1988 Feb 23;27(4):1213–1221. doi: 10.1021/bi00404a022. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., Cosman M., Mao B., Alfano A., Ibanez V., Harvey R. G. Spectroscopic characteristics and site I/site II classification of cis and trans benzo[a]pyrene diolepoxide enantiomer-guanosine adducts in oligonucleotides and polynucleotides. Carcinogenesis. 1991 Nov;12(11):2099–2108. doi: 10.1093/carcin/12.11.2099. [DOI] [PubMed] [Google Scholar]
- Gräslund A., Jernström B. DNA-carcinogen interaction: covalent DNA-adducts of benzo(a)pyrene 7,8-dihydrodiol 9,10-epoxides studied by biochemical and biophysical techniques. Q Rev Biophys. 1989 Feb;22(1):1–37. [PubMed] [Google Scholar]
- Hagerman P. J. Flexibility of DNA. Annu Rev Biophys Biophys Chem. 1988;17:265–286. doi: 10.1146/annurev.bb.17.060188.001405. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J., Ramadevi V. A. Application of the method of phage T4 DNA ligase-catalyzed ring-closure to the study of DNA structure. I. Computational analysis. J Mol Biol. 1990 Mar 20;212(2):351–362. doi: 10.1016/0022-2836(90)90130-E. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991 Sep 15;51(18 Suppl):5023s–5044s. [PubMed] [Google Scholar]
- Hogan M. E., Dattagupta N., Whitlock J. P., Jr Carcinogen-induced alteration of DNA structure. J Biol Chem. 1981 May 10;256(9):4504–4513. [PubMed] [Google Scholar]
- Hruszkewycz A. M., Canella K. A., Peltonen K., Kotrappa L., Dipple A. DNA polymerase action on benzo[a]pyrene-DNA adducts. Carcinogenesis. 1992 Dec;13(12):2347–2352. doi: 10.1093/carcin/13.12.2347. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Leng M. DNA bending induced by covalently bound drugs. Gel electrophoresis and chemical probe studies. Biophys Chem. 1990 Apr;35(2-3):155–163. doi: 10.1016/0301-4622(90)80005-r. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Sun D. Y., Hurley L. H. (+)-CC-1065 produces bending of DNA that appears to resemble adenine/thymine tracts. Chem Res Toxicol. 1991 Jan-Feb;4(1):21–26. doi: 10.1021/tx00019a003. [DOI] [PubMed] [Google Scholar]
- Mackay W., Benasutti M., Drouin E., Loechler E. L. Mutagenesis by (+)-anti-B[a]P-N2-Gua, the major adduct of activated benzo[a]pyrene, when studied in an Escherichia coli plasmid using site-directed methods. Carcinogenesis. 1992 Aug;13(8):1415–1425. doi: 10.1093/carcin/13.8.1415. [DOI] [PubMed] [Google Scholar]
- Mao B., Li B., Amin S., Cosman M., Geacintov N. E. Opposite stereoselective resistance to digestion by phosphodiesterases I and II of benzo[a]pyrene diol epoxide-modified oligonucleotide adducts. Biochemistry. 1993 Nov 9;32(44):11785–11793. doi: 10.1021/bi00095a006. [DOI] [PubMed] [Google Scholar]
- Meehan T., Straub K. Double-stranded DNA steroselectively binds benzo(a)pyrene diol epoxides. Nature. 1979 Feb 1;277(5695):410–412. doi: 10.1038/277410a0. [DOI] [PubMed] [Google Scholar]
- Norden B., Kubista M., Kurucsev T. Linear dichroism spectroscopy of nucleic acids. Q Rev Biophys. 1992 Feb;25(1):51–170. doi: 10.1017/s0033583500004728. [DOI] [PubMed] [Google Scholar]
- Roche C. J., Geacintov N. E., Ibanez V., Harvey R. G. Linear dichroism properties and orientations of different ultraviolet transition moments of benzo[a]pyrene derivatives bound noncovalently and covalently to DNA. Biophys Chem. 1989 Jul;33(3):277–288. doi: 10.1016/0301-4622(89)80029-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez H., Loechler E. L. Mutagenesis by the (+)-anti-diol epoxide of benzo[a]pyrene: what controls mutagenic specificity? Biochemistry. 1993 Feb 23;32(7):1759–1769. doi: 10.1021/bi00058a009. [DOI] [PubMed] [Google Scholar]
- Rodriguez H., Loechler E. L. Mutational specificity of the (+)-anti-diol epoxide of benzo[a]pyrene in a supF gene of an Escherichia coli plasmid: DNA sequence context influences hotspots, mutagenic specificity and the extent of SOS enhancement of mutagenesis. Carcinogenesis. 1993 Mar;14(3):373–383. doi: 10.1093/carcin/14.3.373. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Marrot L., Leng M. The DNA bending by acetylaminofluorene residues and by apurinic sites. J Mol Biol. 1989 May 20;207(2):445–450. doi: 10.1016/0022-2836(89)90266-0. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Margulis L. A., Geacintov N. E., Grollman A. P. Translesional synthesis on a DNA template containing a single stereoisomer of dG-(+)- or dG-(-)-anti-BPDE (7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene). Biochemistry. 1993 Jul 27;32(29):7531–7541. doi: 10.1021/bi00080a027. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Bracken W. J., Gleason G., Levin W., Yagi H., Jerina D. M., Conney A. H. Marked differences in the skin tumor-initiating activities of the optical enantiomers of the diastereomeric benzo(a)pyrene 7,8-diol-9,10-epoxides. Cancer Res. 1979 Jan;39(1):67–71. [PubMed] [Google Scholar]
- Stevens C. W., Bouck N., Burgess J. A., Fahl W. E. Benzo[a]pyrene diol-epoxides: different mutagenic efficiency in human and bacterial cells. Mutat Res. 1985 Oct;152(1):5–14. doi: 10.1016/0027-5107(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Ulanovsky L., Bodner M., Trifonov E. N., Choder M. Curved DNA: design, synthesis, and circularization. Proc Natl Acad Sci U S A. 1986 Feb;83(4):862–866. doi: 10.1073/pnas.83.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. I., Taylor J. S. Site-specific effect of thymine dimer formation on dAn.dTn tract bending and its biological implications. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9072–9076. doi: 10.1073/pnas.88.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. W., Chang R. L., Levin W., Yagi H., Thakker D. R., Jerina D. M., Conney A. H. Differences in mutagenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1389–1396. doi: 10.1016/s0006-291x(77)80133-2. [DOI] [PubMed] [Google Scholar]
- Xu R., Birke S., Carberry S. E., Geacintov N. E., Swenberg C. E., Harvey R. G. Differences in unwinding of supercoiled DNA induced by the two enantiomers of anti-benzo[a]pyrene diol epoxide. Nucleic Acids Res. 1992 Dec 11;20(23):6167–6176. doi: 10.1093/nar/20.23.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Direct evidence for DNA bending at the lambda replication origin. Science. 1987 Apr 24;236(4800):416–422. doi: 10.1126/science.2951850. [DOI] [PubMed] [Google Scholar]
- de los Santos C., Cosman M., Hingerty B. E., Ibanez V., Margulis L. A., Geacintov N. E., Broyde S., Patel D. J. Influence of benzo[a]pyrene diol epoxide chirality on solution conformations of DNA covalent adducts: the (-)-trans-anti-[BP]G.C adduct structure and comparison with the (+)-trans-anti-[BP]G.C enantiomer. Biochemistry. 1992 Jun 16;31(23):5245–5252. doi: 10.1021/bi00138a002. [DOI] [PubMed] [Google Scholar]