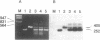Abstract
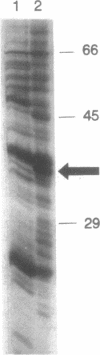
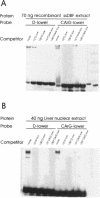
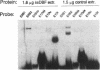
Estradiol inducible, liver-specific expression of the apoVLDL II gene is mediated through the estrogen receptor and a variety of other DNA-binding proteins. In the present study we report the cloning and characterisation of a single-strand DNA binding protein that interacts with the lower strand of a complex regulatory site, which includes the major estrogen responsive element and a site that resembles the rat albumin site D (apoVLDL II site D). Based on its binding specificity determined with electro-mobility shift assays, the protein is named single-strand D-box binding factor (ssDBF). Analysis of the deduced 302 amino acid sequence revealed that the protein belongs to the heteronuclear ribonucleoprotein A/B family (hnRNP A/B) and resembles other known eukaryotic single-strand DNA binding proteins. Transient transfection experiments in a chicken liver cell-line showed that the protein represses estrogen-induced transcription. A protein with similar binding characteristics is present in liver nuclear extract. The relevance of the occurrence of this protein to the expression of the apoVLDL II gene is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altiok S., Groner B. Interaction of two sequence-specific single-stranded DNA-binding proteins with an essential region of the beta-casein gene promoter is regulated by lactogenic hormones. Mol Cell Biol. 1993 Dec;13(12):7303–7310. doi: 10.1128/mcb.13.12.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman J. M., Wijnholds J., Schippers I. J., Pot W., Gruber M., Ab G. Regulatory elements and DNA-binding proteins mediating transcription from the chicken very-low-density apolipoprotein II gene. Nucleic Acids Res. 1991 Oct 11;19(19):5371–5377. doi: 10.1093/nar/19.19.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvoli M., Cobianchi F., Bestagno M. G., Mangiarotti A., Bassi M. T., Biamonti G., Riva S. Alternative splicing in the human gene for the core protein A1 generates another hnRNP protein. EMBO J. 1990 Apr;9(4):1229–1235. doi: 10.1002/j.1460-2075.1990.tb08230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkhoven C. F., Ab G., Wijnholds J. c/CEPB, a chicken transcription factor of the leucine-zipper C/EBP family [corrected]. Nucleic Acids Res. 1992 Aug 11;20(15):4093–4093. doi: 10.1093/nar/20.15.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Matunis M. J., Piñol-Roma S., Burd C. G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Duncan R., Bazar L., Michelotti G., Tomonaga T., Krutzsch H., Avigan M., Levens D. A sequence-specific, single-strand binding protein activates the far upstream element of c-myc and defines a new DNA-binding motif. Genes Dev. 1994 Feb 15;8(4):465–480. doi: 10.1101/gad.8.4.465. [DOI] [PubMed] [Google Scholar]
- Jansen-Dürr P., Boshart M., Lupp B., Bosserhoff A., Frank R. W., Schütz G. The rat poly pyrimidine tract binding protein (PTB) interacts with a single-stranded DNA motif in a liver-specific enhancer. Nucleic Acids Res. 1992 Mar 25;20(6):1243–1249. doi: 10.1093/nar/20.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada S., Miwa T. A protein binding to CArG box motifs and to single-stranded DNA functions as a transcriptional repressor. Gene. 1992 Oct 1;119(2):229–236. doi: 10.1016/0378-1119(92)90276-u. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Nomura K., Hirayama Y., Kitagawa T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 1987 Aug 15;47(16):4460–4464. [PubMed] [Google Scholar]
- Khan F. A., Jaiswal A. K., Szer W. Cloning and sequence analysis of a human type A/B hnRNP protein. FEBS Lett. 1991 Sep 23;290(1-2):159–161. doi: 10.1016/0014-5793(91)81249-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannigan D. A., Notides A. C. Estrogen receptor selectively binds the "coding strand" of an estrogen responsive element. Proc Natl Acad Sci U S A. 1989 Feb;86(3):863–867. doi: 10.1073/pnas.86.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery D. J., Schibler U. Circadian transcription of the cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993 Oct;7(10):1871–1884. doi: 10.1101/gad.7.10.1871. [DOI] [PubMed] [Google Scholar]
- Merrill B. M., LoPresti M. B., Stone K. L., Williams K. R. High pressure liquid chromatography purification of UP1 and UP2, two related single-stranded nucleic acid-binding proteins from calf thymus. J Biol Chem. 1986 Jan 15;261(2):878–883. [PubMed] [Google Scholar]
- Mukherjee R., Chambon P. A single-stranded DNA-binding protein promotes the binding of the purified oestrogen receptor to its responsive element. Nucleic Acids Res. 1990 Oct 11;18(19):5713–5716. doi: 10.1093/nar/18.19.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardulli A. M., Greene G. L., Shapiro D. J. Human estrogen receptor bound to an estrogen response element bends DNA. Mol Endocrinol. 1993 Mar;7(3):331–340. doi: 10.1210/mend.7.3.8483477. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Smidt M. P., Wijnholds J., Snippe L., van Keulen G., Ab G. Binding of a bZip protein to the estrogen-inducible apoVLDL II promoter. Biochim Biophys Acta. 1994 Sep 13;1219(1):115–120. doi: 10.1016/0167-4781(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tada H., Khalili K. A novel sequence-specific DNA-binding protein, LCP-1, interacts with single-stranded DNA and differentially regulates early gene expression of the human neurotropic JC virus. J Virol. 1992 Dec;66(12):6885–6892. doi: 10.1128/jvi.66.12.6885-6892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay N., Chan S. H., Ren E. C. Identification and cloning of a novel heterogeneous nuclear ribonucleoprotein C-like protein that functions as a transcriptional activator of the hepatitis B virus enhancer II. J Virol. 1992 Dec;66(12):6841–6848. doi: 10.1128/jvi.66.12.6841-6848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D., Struhl K. Functional dissection of the yeast Cyc8-Tup1 transcriptional co-repressor complex. Nature. 1994 Jun 30;369(6483):758–761. doi: 10.1038/369758a0. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Wijnholds J., Muller E., Ab G. Oestrogen facilitates the binding of ubiquitous and liver-enriched nuclear proteins to the apoVLDL II promoter in vivo. Nucleic Acids Res. 1991 Jan 11;19(1):33–41. doi: 10.1093/nar/19.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachow K. R., Conklin K. F. CArG, CCAAT, and CCAAT-like protein binding sites in avian retrovirus long terminal repeat enhancers. J Virol. 1992 Apr;66(4):1959–1970. doi: 10.1128/jvi.66.4.1959-1970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]