Abstract
The lymphotropic herpesvirus KSHV principally infects B cells in vivo and is linked to several human B cell lymphoproliferative syndromes. Here we examine the susceptibility of primary tonsillar lymphocytes to infection by a recombinant KSHV (rKSHV.219) that constitutively expresses GFP. At an MOI of ~ 1, ca 5–10% of CD19+ B cells became GFP-positive. Surprisingly, in the same culture many more T cells became infected. However, in contrast to isolated B cells, isolated infected T cells did not support correct viral transcription and did not produce infectious virus, indicating the presence of one or more post-entry blocks to lytic KSHV replication in T cells. No immortalization or transformation has yet been observed in either B or T cells. These results affirm the feasibility of studying KSHV infection in primary lymphoid cells, and help to rationalize the detection of KSHV DNA in rare human T cell lymphomas in vivo.
Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV) is the newest addition to human herpesvirus family. Initially identified in 1994 on the basis of its association with Kaposi’s sarcoma, an endothelial neoplasm ((Chang et al., 1994; Chuck et al., 1996) and reviewed in (Ganem, 2006)), it is now classified as a member of the lymphotropic (γ) herpesvirus subfamily together with Epstein-Barr virus (EBV) and its simian relatives, Herpesvirus saimiri (HVS) and Rhesus rhadinovirus (RRV). Consistent with this classification, KSHV DNA can be found in circulating B cells in infected patients (Ambroziak et al., 1995; Mesri et al., 1996). Moreover, the virus also causes two B cell lymphoproliferative disorders: primary effusion lymphoma (PEL) and multicentric Castleman’s diseases (MCD) (Carbone and Gloghini, 2008; Cesarman et al., 1995; Cesarman et al., 1996; Nador et al., 1995). B cells derived from PEL were the first cultured cells in which KSHV latency was observed, and remain the most widely studied experimental system for the molecular analysis of latent gene expression. (Mesri et al., 1996; Renne et al., 1996). However, despite this clear evidence of lymphotropism, all established B cell lines tested to date are resistant to KSHV infection in culture (Bechtel et al., 2003; Blackbourn et al., 2000; Renne et al., 1998), as are resting peripheral blood mononuclear cells. This failure to infect B cells is all the more striking given the ready infection of most other adherent cell lines in vitro (Bechtel et al., 2003; Vieira et al., 2001). Why this is so remains a mystery, and one that has greatly impeded our understanding of the relationship of KSHV infection to B cell function, survival and transformation.
Primary B cells from peripheral blood have recently been shown to be susceptible to KSHV infection if they are first activated by CD40L and IL-4 (Rappocciolo et al., 2008). We set out to develop a different system for the infection of primary lymphoid elements by KSHV. We chose to focus on cells derived from human tonsils, for two reasons: (i) they can be directly accessed by the oral route and their surgical removal is frequently carried out, assuring adequate tissue availability; and (ii) the main source of infectious KSHV in infected humans is saliva, and tonsillar infection in vivo is presumed to be one important source of this infectivity (infected pharyngeal epithelium being the other). Therefore, tonsillar infection has likely relevance to the in vivo setting. Moreover, primary cells derived from tonsils have been successfully employed to cultivate other lymphotropic viruses, including HIV (Glushakova et al., 1995; Kreisberg, Yonemoto, and Greene, 2006), HHV-6 (Grivel et al., 2003; Roush et al., 2001), and EBV (Aman et al., 1985; Babcock and Thorley-Lawson, 2000; Strowig et al., 2008);.
Two types of culture systems can be derived from human tonsils. Blocks of intact tonsils, which retain the three dimensional structure of the lymphoid organ, can be cultured (Glushakova et al., 1995; Penn et al., 1999), but it is difficult to expose all cells in this type of explant to an infectious inoculum, and quantitation of the resulting infection can be problematic. Alternatively, dispersed cell suspensions can be made from the tonsil and placed directly into culture (to form so-called human lymphoid aggregate culture, or HLAC) (Chiu et al., 2005; Eckstein et al., 2001). These HLAC cultures preserve most of the cell types of the organ in their correct ratios and are more readily infected ex vivo. More importantly, they are amenable to experimental manipulations: e.g. deletion of cell subsets, activation of cell subsets with appropriate ligands, staining with antibodies, analysis of apoptosis, etc. For this reason, we have chosen to employ HLAC explants to explore lymphoid infection by KSHV. We show here that this system allows efficient and reproducible infection of lymphocytes by KSHV, and treatment of the cultures with HDAc inhibitors results in enhanced production of infectious virus. To our surprise, we found that T cells as well as B cells can be infected, with both CD4 and CD8 subsets proving susceptible to KSHV entry. Virus entry into B cells is inefficient, with only 5–10% of B cells displaying GFP expression; by contrast, large numbers of T cells can support viral entry. However, infected T cells do not support production of infectious virus, either spontaneously or after treatment with HDAc inhibitors, indicating that T cell infection is abortive. Neither B nor T cells are immortalized following KSHV exposure, though infected B cells do show modest prolongation of their lifespan in culture.
Materials and Methods
Cells and human lymphoid aggregate culture (HLAC)
QBI293A cells, a subcloned 293 cell line, were purchased from Q-Biogene (Carlsbad, CA) for the titration of rKSHV.219 stocks. Stable Vero cells, harboring rKSHV.219 genome, were a kind gift from Dr. Jeffrey Vieira (Vieira and O’Hearn, 2004). Following approval from the UCSF Committee on Human Research, tonsillar tissue from routine tonsillectomies was obtained (via the Cooperative Human Tissue Network) and processed for HLAC as described previously (Eckstein et al., 2001; Kreisberg, Yonemoto, and Greene, 2006). In brief, tonsils were minced in tonsil medium (RPMI 1640 supplemented with 15% FCS, 100 μg/ml gentamicin, 100 μg/ml ampicillin, 1 mM sodium pyruvate, 1% nonessential amino acids (Mediatech), 2 mM L-glutamine, and 1% fungizone (Invitrogen)). Tonsil blocks were passed through a 40-μm cell strainer, and cultured in 96-well U-bottomed polystyrene plates (2×106 cells/well) in tonsil medium (200 μl/well).
Reagents and antibodies
All antibodies were purchased from Biolegend except streptavidin-QDot 605 (Invitrogen). Lyophilized PHA was purchased from Sigma and reconstituted in PBS and aliquots were stored at −80°C. All cytokines including Monomeric CD40L, IL-4, and IL-10 was purchased from Peprotech.
Cell purification using magnetic beads
Untouched B or T cells were isolated negatively by employing B cell isolation kit II and Pan T cell isolation kit II, respectively, according to manufacturer’s instruction. Briefly, 108 cells were labeled with 100 μl of biotin-antibody cocktail for 10 min at 4°C followed by 200 μl of anti-biotin MicroBeads for 15 min at 4°C. Unwanted cells were removed through a column and eluents were washed twice in tonsil medium before use. When required, CD4+ or CD8+ T cells were positively selected by CD4 and CD8 T MicroBeads, respectively. All reagents used for cell purification were purchased from Miltenyi Biotec.
Virus preparation
rKSHV.219 stocks were prepared from stable Vero cells as described elsewhere with minor modifications (Vieira and O’Hearn, 2004). Briefly, stable Vero cells were infected with recombinant adenovirus expressing RTA (Bechtel et al., 2003) at MOI 100 in a minimum volume for 2–3 hours before wash. Cells were then induced for 22 hours with 1200 μM valproate. The medium with valproate was removed and fresh DMEM without puromycin but supplemented with 2% FBS and penicillin & streptomycin, was added. Induced cells were further cultured for another 72 hours before culture supernatant was collected. Virus was purified as described previously (Grossmann and Ganem, 2008). In brief, supernatants were cleared of cells by centrifugation (2,000 RPM for 10 min) with subsequent filtration through a 0.45 μm filter, and the virions were pelleted at 27,000×g for 2 hours. Viral pellets were resuspended in 1/125 of the original volume using tonsil medium. Infectious units (IU) of virus stocks were determined on QBI293A cells as described previously (Vieira and O’Hearn, 2004). Briefly, virus stock was serially diluted and infected into QBI293A cells for 6 hours in a 24-well plate. After fresh media was added, cells were further cultured for 48hours before the percentage of GFP+ cells was determined by FACS Calibur. IU’s were calculated by multiplying the percentage of GFP+ cells by the total number of QBI293A cells in a well at the time of flow cytometric analysis.
Stimulation of tonsillar cells and virus infection
2×106 tonsillar cells were stimulated with PHA (Sigma) for various duration of time and washed extensively before use. PHA-stimulated cells were infected at MOI 0.2–3 in a 96-well U-bottomed plate in total 200 μl/well for 48 hours before cells were stained for flow cytometric analysis (1 MOI is defined by 1 IU per cell). To examine effects of inflammatory cytokines and immunomodulatory molecules on KSHV infectivity in B cells, 2×106 tonsillar cells were stimulated by monomeric soluble CD40L (sCD40L), IL-4, IL-10 or in combinations for 24 hours prior to virus infection. Cells were washed extensively before use.
Conditioned Medium from PHA- and mixed lymphocyte reaction (MLR)- stimulated tonsillar cell cultures
Tonsillar cells were stimulated with PHA (10 μg/ml) for 12 hours and washed extensively in complete tonsil medium. Cells were further cultured in fresh medium and culture supernatants were collected and stored at −20°C until use after filtration through a 0.45-μm filter. To obtain MLR-stimulated culture supernatants, tonsillar cells from two different individuals were mixed: 5×106 responder cells were mixed with 2.5×106 stimulator cells. Culture supernatants were collected at various times and kept at −20°C until use after filtration through a 0.45-μm filter. To confirm that PHA- and MLR-stimulated culture supernatants contain immuno-modulatory molecules (cytokines, chemokines, etc) from activated immune cells, levels of IL-2 were assessed using ELISA MAX® kit for human IL-2 (Biolegend). Conditioned media were added at v/v 40% to fresh tonsillar cultures for 24 hr prior to viral infection.
Flow cytometry
rKSHV.219-infected tonsil cells were stained with antibodies for 30 min at 4°C. Cells were washed twice with 1ml PBS containing 4% FBS before fixed with 1% paraformaldehyde. Fixed cells were washed twice with PBS and resuspended in PBS/4% FBS. Stained cells were analyzed on FACS Calibur or LSR II (Beckton Dickinson). FACS data were analyzed using FlowJo software.
Viral DNA extraction and quantitative Real-time PCR
Viral DNA was extracted from culture supernatants as described previously (Grossmann and Ganem, 2008). In brief, culture supernatants were collected from induced cells and filtered through a 0.45 μM filter. To remove free viral DNA in the medium, 20 U/ml DNase I was added and incubated at 37 °C for 1 h. Virions were pelleted down at 27,000×g for 2 h at 4 °C. Virus pellet was resuspended in 600 μl lysis buffer (20 mM Tris–HCl at pH 8, 10 mM EDTA, 100 mM NaCl, 0.5% SDS) and incubated at room temperature for 10 min. 0.7 mg/ml Proteinase K and linearized plasmid encoding an unrelated malaria gene (cysteine protease gene of Plasmodium falciparum) were diluted in 100 μl lysis buffer and added to the 600 μl resuspended virions. The 700 μl total volume was incubated at 37 °C overnight; this was then extracted with phenol/chloroform/isoamyl. Viral DNA was precipitated and resuspended in 85 μl of water. 2 μl of this was used in the subsequent Taqman and Sybergreen assays for PAN promoter and spiked-in malaria gene as a normalizing factor as described previously (Grossmann and Ganem, 2008) using BAC36 as a standard (Zhou et al., 2002). Taqman (for Pan promoter) and Sybergreen (for malaria DNA) reactions were performed in triplicates using Taqman Universal Master Mix (Applied Biosystems) and SybergreenER mastermix (Invitrogen), respectively, according to the manufacturer’s instructions.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from 2*106 cells by RNA-Bee (Tel Test Inc., TX) according to the manufacturer’s instruction. 50 ng of total RNA, treated with DNase I, was used to synthesize cDNA using gene-specific primers for ORF59 and vCyclin. Primers used were; ORF59, forward: 5′-GTTTACCCCCGGGCTGAT-3′, reverse: 5′-GGGCACACC TTCCACTTCTAAT-3′, probe: 5′-FAM-TGGCACTCCAACGAA-MGB-3′; vCyclin, forward: 5′-CGCGGCATAGCAAAGTGAA-3′, reverse: 5′-GCCTGTTAGTGGCC AGTAAGCT-3′, probe: 5′-FAM-TGGTAGAAATAGGCGTGAGGCTTCT-MGB-3′. Reverse primers were used to generate gene-specific cDNA in reverse transcriptase reactions using SuperScript® III Reverse Transcriptase (invitrogen, CA). Quantitative real-time PCR was done on each cDNA by empolying TaqMan® Universal PCR master mix from Applied Biosystems with plasmids encoding each gene as a standard according to the manufacturer’s instruction.
Microarray analysis on viral gene expression in infected T cells
Total RNA was extracted from tonsillar cells using RNA-Bee (Tel-Test, inc.) after a wash with cold incomplete PBS according to the manufacturer’s protocol. Tonsillar RNA’s were repurified twice using the RNeasy minikit (QIAgen) according to the manufacturer’s instruction. The quality of the purified RNA’s was analyzed using the RNA 6000 Pico total RNA kit and the 2100 Bioanalyzer (Agilent). All RNA samples displayed RIN value of at least 9, which indicates good integrity of RNA’s, and RNA samples were quantified using the ND1000 spectrophotometer (nanodrop). Labeled cRNA from 250ng of total RNA was generated using the two-color Quick Amp Labeling (Agilent) according to the manufacturer’s protocol. Experimental samples (labeled with Cyanine 5-CTP) and reference samples (labeled with Cyanine 3-CTP) were competitively hybridized to custom KSHV microarrays (Agilent) according to the manufacturer’s instruction. Cyanine 3-CTP and Cyanine 5-CTP were purchased from Perkin Elmer. Hybridizations and washes were conducted according to the manufacturer’s protocol. Washed arrays were scanned using the GenePix 400B Scanner (Axon Instruments) and feature intensities extracted using GenePix Pro 6.0 software. TIFF images of scanned slides were analyzed using the Feature Extraction Software, Ver 9.5.3 (Agilent). A graphical presentation of processed data (heat map) was generated by Java Treeview.
Statistical analysis
Data are shown either as the means ± standard deviations (SD) of at least three independent experiments or as one representative example from at least three independent experiments. The significance of differences in the mean values was determined by the Student’s t test. P < 0.05 was considered statistically significant.
Results
rKSHV.219 infects primary lymphoid cells
To investigate whether rKSHV.219 infects tonsillar cells, recombinant virus stocks were prepared and titrated on QBI293A cells as described in Materials and Methods. To study specific infection of KSHV in tonsillar cells, polybrene was not included in infection cultures as it is known to enhance KSHV infection non-specifically (Inoue et al., 2003; Lagunoff et al., 2002). We prepared 2 million tonsillar cells as an HLAC culture as described in Materials and Methods; these cells were then infected with 1.4×106 IU (MOI=0.7) of rKSHV.219. Cells were analyzed at 48 hours post infection (hpi) for GFP expression by flow cytometry, gating on CD19-positive (B) cells or CD3-positive (T) cells. In a given tonsil, CD19+ B cells account for 60–80% of the cells, while CD3+ T cells represent 20–40% of the cells, respectively (Fig. 1, left panel). All other cell types, including NK (CD56+), granulocytes (CD66b+), monocytes (CD14+), and dendritic cells (CD11c+ or CD123+), constitute altogether less than 1% of total tonsillar cells in a given HLAC preparation, making it difficult to study specific KSHV infection in these populations. Therefore, examination of KSHV infectivity in tonsillar cells was focused on B and T cells.
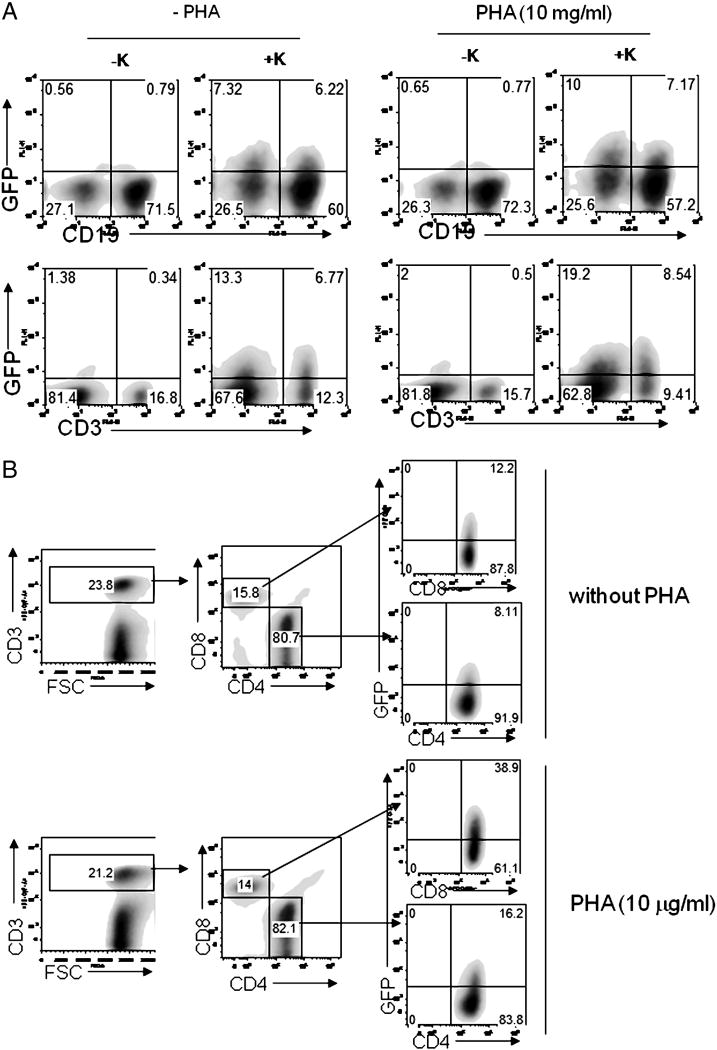
Figure 1. Ex vivo tropism of rKSHV.219 in human lymphoid aggregate culture.
(A) 2×106 homogenated tonsillar cells were pre-treated with PHA (10 μg/ml) for 12 hours and infected with 1.5*106 infectious units (IU) rKSHV.219 for 48 hours (MOI=0.7), after which cells were stained with either anti-CD19 (top panels) or anti-CD3 antibodies (bottom panels). KSHV infectivity in each cell type was determined by examining GFP expression by flow cytometry. (B) 2×106 tonsillar cells were either treated or untreated with PHA (10 μg/ml) for 12 hours. Cells were infected at MOI 0.04 for 48 hours with subsequent staining with anti-CD3, anti-CD4, and anti-CD8 antibodies. KSHV infectivity in CD4+ or CD8+ T cells was analyzed in live CD3+ cells. A representative plot (n=14) is shown.
As shown in Fig. 1A (top left panel), under standard infection conditions 9.4% of CD19+ cells became GFP+, indicating that they were infected by rKSHV.219. Surprisingly, primary CD3+ T cells seemed to be even more susceptible to rKSHV.219 infection than CD19+ B cells. In the absence of PHA, fully 35.5% of CD3+ T cells were GFP+ at 48 hpi (bottom left panel). [Note: this assay only measures viral entry, uncoating and reporter gene expression, and does not directly address whether the infected cells produce infectious viral progeny; that issue will be addressed in Fig. 6]. When the cultures were treated with the polyclonal T cell activator PHA, the fraction of CD3 cells that scored for GFP increased to 47.6% ((Fig. 1A, bottom right panel),. (PHA treatment, as might be expected, did not enhance susceptibility of B cells to infection; top right panel). Given that at an MOI =0.7 some 48% of the culture is expected from Poisson statistics to be uninfected, this means that approximately 70% of T cells and 18% of B cells in this experiment were susceptible to infection by KSHV ex vivo. In other experiments, the percentage of infectable B cells in tonsillar explants varied from 4–10% (Fig. 2 and data not shown).
Figure 6. Infected tonsillar cells support full replication of rKSHV.219.

(A) Tonsillar cells were treated with PHA (5 μg/ml) for 12 hours and infected with rKSHV.219 at MOI 0.1 for 6 hours. Cells were extensively washed with warm complete media and induced with valproate (300 μM) for 5 days. 100 μl of induced culture supernatants was added on 1×105 QBI293A cells and cultured for another 48 hours before infectious units were determined by enumerating GFP+ cells by flow cytometry as described in Materials and Methods. Histograms represent mean (±SD) from 3 independent experiments. Statistical analysis was performed by Student t-test. The dotted line denotes detection limit. (B) Infected tonsillar cells as described in Figure 6A were induced with various stimuli as indicated for 5 days. Viral DNA was extracted from 100 μl induced culture supernatant and used as template for real-time PCR. Histograms represent mean (±SD) of duplicated samples. A representative plot is shown from 2 independent experiments. Val denotes valproic acid.
Figure 2. Reproducibility of rKSHV.219 infectivity in tonsillar B and T cells.

Tonsillar cells were left untreated or treated with PHA (10 mg/ml) for 6–12 hours prior to virus infection (MOI=0.04). Virus infectivity in various cell types was determined at 48 hr post-infection by examining GFP expression by flow cytometry. Each dot represents rKSHV.219 infectivity in each cell type from individual tonsils. Mean of 10 independent experiments is provided. Statistical analysis was performed by Student t-test.
To analyze which subsets of T cells are infected by rKSHV.219, tonsillar cells infected at a lower MOI (0.04) were stained with anti-CD3, anti-CD4 and anti-CD8 antibodies. As shown in Fig. 1B, both CD4 and CD8 T cells became infected. In the absence of PHA stimulation, ca 8% of CD4 cells and 12% of CD8 cells were GFP-positive, and in the presence of PHA these percentages increased 2- and 3-fold, respectively. This increased GFP expression reflects an enhanced susceptibility to KSHV infection triggered by PHA exposure: when PHA was added to tonsillar cells at the time of virus inoculation or 24 hours post-inoculation, GFP-positivity was not significantly affected compared to that of the untreated group (data not shown).
The MOI used in these experiments was based on viral infectivity titers measured in 293 cells, a standard non-lymphoid line known to be permissive for KSHV (Bechtel et al., 2003). The Poisson distribution predicts that at MOI=0.04, ca. 4% of cells exposed to virus should receive one or more infectious virus particles. In the absence of PHA, we observe that ~2.5% of total lymphoid cells became infected, as judged by GFP expression. (In the presence of T cell activation, fully 4–5% of the cells in the culture became infected). At an MOI of 0.7, conditions under which 48% of cells are expected to be infected, 16–20% of the cells in the HLAC culture became GFP-positive. We conclude that the specific infectivity of KSHV for primary lymphoid cells is ca 33–50% that observed for cultured 293 cells. Activated T cells are fully as susceptible to KSHV infection as 293 cells, but B cells are infected only ~ 20% as efficiently as 293 cells in vitro (at best). Moreover, we note that the levels of virus-encoded gene expression per infected cell is lower in primary lymphocytes compared to established nonlymphoid cell lines. For example, when analyzed by flow cytometry, the mean fluorescence intensity of GFP in rKSHV.219-infected 293 cells is typically 20–30 fold higher than that observed in infected primary tonsillar B or T cells.
Reproducibility of lymphoid infection in HLAC cultures
One limitation of HLAC cultures is that, of necessity, they derive from diverse, genetically unrelated individuals. To determine how much individual variation in the patterns of KSHV infection may exist from prep to prep, we systematically examined the lymphoid targets of infection in tonsillar cells from 10 individuals, whether untreated or treated with PHA (10 μg/ml) for 6–12 hours. Cells were infected with rKSHV.219 for 48 hours, at MOI=0.04. As shown in Figure 2, even in the absence of PHA stimulation, CD8+ T cells were generally infected at higher levels (13.2%) than CD4+ T (8.3%) or CD19+ B cells (4.6%) (CD8+ vs CD4+ T cells, p=0.032). PHA stimulation reproducibly enhanced infectivity in both T cell subsets, with the enhancement being characteristically larger in CD8-positive cells. Thus, although cultures do vary somewhat from individual to individual in the absolute number of cells infected following exposure, the distribution of infection among the major B and T cell populations is remarkably reproducible.
KSHV infection does not immortalize either tonsillar B or T cells in HLAC cultures
To examine the potential consequences of KSHV infection in lymphocytes, we infected cells and periodically examined them for several weeks following inoculation. In no case was immortalization of the culture observed; no stable lines of latently infected cells have yet emerged from KSHV-infected HLAC cultures. To increase the sensitivity of detection of rare immortalizing events, we took advantage of the fact that rKSHV.219 also carries a selectable marker (for puromycin -resistance). Tonsillar cells were infected with rKSHV.219, and puromycin used to select stable transductants – but again, no stable puromycin-resistant lines emerged. Next, in search of more subtle phenotypes linked to KSHV infection, we examined each cell type in the culture for GFP expression as a function of time following infection in 6 independent infections of different tonsils (Fig. 3). Without PHA pre-stimulation, the proportion of GFP+ CD4+ T cells (expressed as a percentage of the total number of CD4 cells) at day13 post-infection was significantly lower than that at day 2: 3.4% vs 9.4% (p=0.015). The same was true of infected CD8 T cells, which declined from being 16.7% of the initial CD8 population to 5.2% on day 13. By contrast, at day13 PI, the percentage of GFP+ B cells was significantly higher compared to that at day 2: 4.6% vs 14% (p=0.0009). The overall viability of uninfected HLAC cultures was 30–50% at day 2 p.i. and waned to 5– 10% at day13, without changes in B/T cell ratio (data not shown). These data could suggest that KSHV infection provides a transient survival advantage in infected B cells (see Discussion). However, this extension of survival is not indefinite, at least in vitro: by day 60 p.i. most infected tonsillar cells were dead. PHA pre-stimulation increased KSHV infectivity in T cells on day 2 (Fig. 4; cf. also Fig. 2). But levels of GFP+ T cells at day 13 were significantly lower and furthermore, their levels were reduced to the levels of cells without PHA pre-stimulation at d13 p.i., suggesting PHA enhances susceptibility of T cells to KSHV infection, but not the survival of infected T cells. Not unexpectedly, PHA pre-treatment did not influence the time-dependent enrichment of GFP+ CD19+ B cells.
Figure 3. GFP+ B cells are enriched in human lymphoid aggregate culture at later stage of infection.

Tonsillar cells were treated with PHA and infected as described in Figure 2. Infected culture was examined by flow cytometry for GFP expression in each cell type at day 2 and day 13. Mean of 6 independent experiments is provided. Statistical analysis was performed by Student t-test.
Figure 4. Cytokine-rich supernatants from activated T cells cannot enhance KSHV infection of tonsillar T cells or B cells.

Culture supernatants from mock-, PHA- or MLR-stimulated cells were collected at the indicated time points (1~4 days post-stimulation) and used as conditioned medium after filtered through 0.2-μm nylon filter. Cells from fresh tonsils were treated with medium, PHA (10 μg/ml) or conditioned medium, added at 40% v/v, for 24 hours and washed extensively before virus infection. At 48 hours post-infection, cells were stained and virus infectivity was analyzed on flow by assessing GFP expression in CD4+ (A), CD8+ (B), or CD19+ (C) cells. One representative data of 3 independent experiments is shown.
Activation signals that enhance lymphocyte infection
It is very surprising that T cells are, in general, more susceptible to KSHV entry (and GFP expression) than B cells, and that their infection is significantly enhanced by PHA treatment (Fig.1 and Fig. 2). Time-course experiments (data not shown) indicate that PHA enhancement of KSHV infectivity in T cells is very rapid; treatment for as little as 2 hours results in near-maximal infectivity enhancement. The fact that the extent of GFP expression in B cells in mixed HLAC cultures was unaffected by PHA stimulation of T cells suggested that T cells activated in this fashion were not producing soluble mediators that influence KSHV entry into B cells. This inference was sustained by studies in which HLAC cultures were pretreated with conditioned medium from PHA-treated tonsillar cells, then exposed to rKSHV.219. As shown in Fig. 4, no enhancement of viral entry and GFP expression was seen in either B or T cells by PHA-conditioned medium. These results also suggest that the stimulation of T cell infectivity by PHA is largely cell-autonomous.
We also examined whether a still more potent T cell activation reaction could generate paracrine factors that might affect lymphoid cell infectivity. Accordingly, we set up a mixed lymphocyte reaction by admixing HLAC cultures from two unrelated donors, then used conditioned medium from such co-cultures to pretreat uninfected tonsil cells; these cells were then exposed to rKSHV.219. Assays of the MLR supernatants for IL-2 affirmed that a robust MLR had indeed occurred (data not shown). Again, however, no stimulation of viral entry and GFP expression was observed in either B or T cells under these conditions (Fig. 4). Furthermore, MLR-stimulated cells themselves did not show higher susceptibility to KSHV entry (data not shown).
Next, we asked if stimuli that activate B cells could increase the susceptibility of the B cells in HLAC cultures to KSHV infection (Rappocciolo et al., 2008). However, we found that pretreatment of tonsillar cells with, monomeric CD40L (≤10 μg/ml) alone or monomeric CD40L in combination with IL-4 (≤50 ng/ml) had no effect on the proportion of B cells expressing GFP after KSHV exposure (Suppl. Fig. 1), despite clear upregulation of CD86 by these treatments (data not shown). LPS treatment of tonsillar HLAC cultures similarly failed to enhance B cell infectivity (data not shown)
Finally, we asked whether KSHV-infected T cells display upregulation of activation markers. To examine whether KSHV infection in T cells up-regulates CD69 (immediate early activation marker) and CD25 (early activation marker), infected tonsillar cells were gated for CD4+ (Fig. 5A and C)) or CD8+ (Fig. 5B and D) and the surface expression of CD69 (Fig. 5A and B) or CD25 (Fig. 5C and D) was examined by flow cytometry. One representative FACS plot is shown. Of note, mock-infected CD4+ and CD8+ T cell populations showed CD69 expression on their surface in many cells even in the absence of PHA pre-treatment: 46.3% and 20.1%, respectively. This reflects the fact that tonsillectomies are only performed on patients with hyperplastic (i.e. activated) tonsils. Nonetheless, CD69 expression was detected in still larger numbers of KSHV-infected (GFP+) CD4+ and CD8+ (middle panels) T cells compared to their uninfected (GFP-) counterparts (top panels): 67.8% vs 36.5% (CD4+ T cells) and 49.5% vs 8.45% (CD8+ T cells). As expected, PHA pre-treatment significantly increased surface expression of CD69 and CD25 (Fig. 5, right panels). KSHV infection in CD4+ and CD8+ T cells further enhanced CD69 and CD25 expression on target cells, indicating T cells undergo activation following infection by KSHV.
Figure 5. rKSHV.219 infection activates susceptible T cells.
Tonsillar cells were treated and infected as described in Figure 5. Cells were mock infected (top panels) or infected with rKSHV.219 (bottom panels). At 48 hours post-infection, CD3+ T cells were gated and expression of activation markers, CD69 (A and B) or CD25 (C and D), in CD4+ (A and C) or CD8+ (B and D) T cells was analyzed by flow cytometry. GFP- or GFP+ cells were gated for the expression of activation markers in each cell type. A representative plot out of 4 independent experiments is shown.
Infected mixed (T+B) cultures, but not isolated T cells, support the full replicative cycle of KSHV
Since the preceding experiments scored only for GFP expression, they effectively only measure viral entry, uncoating and delivery of the viral genome to the nucleus; they do not examine whether such cells can undergo lytic replication. To analyze whether such cells can be lytically reactivated, PHA-treated tonsillar cells were infected at MOI 0.1 for 6 hours. After extensive wash, lytic replication was induced by Valproic acid (300 μM). At 5 days post-induction (Fig. 3A), induced supernatants were collected and tested for infectious virus on QBI293A cells, as assayed by enumeration of GFP-positive cells. Infectious units were calculated by multiplying the percentage of GFP+ cells by the number of total cells (Fig. 6A). When infected cells were induced in the presence of Valproic acid, significantly higher levels of infectious units were detected (p=0.046), suggesting that latent infection in lymphocytes was reversible and that the lytic cycle could progress to completion, with the production of infectious progeny virus.
To further understand the extent of lytic reactivation of rKSHV.219 from infected tonsillar cells, infected PHA-treated tonsillar cells were reactivated by various stimuli, such as PMA, PMA plus ionomycin, PHA, and increasing amount of valproic acid (Fig. 6B). Culture supernatants were harvested, treated with DNase I to remove nonencapsidated DNA and examined by quantitiative real-time PCR for virion DNA. Each of these stimuli resulted in enhanced virion production, with induction ratios varying from 11–65 fold. Comparison of the viral DNA copy number with the titer of infectious virus produced under these circumstances leads us to estimate a particle: infectivity ratio of ca 2000:1 – a number similar to that seen in unpurified stocks of other herpesviruses (Benyesh-Melnick et al., 1966; Dargan, Patel, and Subak-Sharpe, 1995), but which is somewhat higher than observed for rKSHV.219 grown in adherent Vero or SLK cells (particle:infectivity ~800; J.M, unpublished data).
In addition to its constitutive GFP marker, rKSHV.219 also carries an RFP gene under the control of a delayed-early lytic promoter (for PAN). As such, lytic reactivation in most cultured cells is accompanied by RFP expression. However, it is interesting to note that tonsillar cells infected with rKSHV.219 did not show increase in RFP expression following lytic induction with valproic acid (Suppl Fig. 2). We do not know the reason for this behavior; some possibilities include that transcription, RNA processing or translation of the chimeric RFP reporter gene is weak or aberrant in lymphoid cells, or the resulting mRNA or protein is unstable in the presence of lytically replicating virus.
Since the ability of T cells to support entry was unexpected, we asked if isolated T cells (purified away from B cells) could support lytic virus replication following infection. We infected either a mixed (T+B) culture or a pure T cell culture with rKSHV.219, washed away free inoculum, then examined culture supernatants for infectious KSHV, before and after valproate induction (Fig. 7). As shown in Fig. 7, under conditions in which the mixed (T+B) culture showed valproate-inducible infectivity, purified T cells released no infectious virus. We conclude that the infection of T cells by KSHV is abortive, with one or more post-entry blocks preventing the release of infectious virions. By inference, the infectious virus released by the mixed culture must be coming from infected B cells. We have affirmed this inference by direct examination of infection of purified primary B cells; these experiments revealed robust production of KSHV by B cells (Fig 7). Interestingly, this replication was not further enhanced by valproate, suggesting that lytic replication of KSHV in B cells is either constitutive, or that reactivation from latency in isolated B cells is very efficient. The fact that spontaneous production of KSHV infectivity in the mixed culture is limited (Fig 7) suggests that reactivation is regulated in a complex fashion by T cells; this regulation is the subject of a detailed study to be published elsewhere (Myoung and Ganem, submitted). Consistent with this, quantitative RT-PCR analysis reveals that infected T cells harbor only very low levels of v-cyclin mRNA and no detectable ORF 59 mRNA (a classical marker of lytic infection), even after lytic induction; by contrast, infected mixed cultures, or isolated infected B cells, harbor abundant ORF 59 mRNA (Fig. 8). To further affirm the defective nature of infection in T cells, we examined transcription in such cells more extensively, by hybridizing labeled RNA from infected T cells to a custom KSHV genomic tiling array whose sequences span the entire KSHV coding region (save for the noncoding terminal repeats). As shown in Fig 8B, no detectable transcription was observed by expression profiling in infected primary T cells; as a positive control for the extraction and hybridization procedures, induced PEL cells displayed robust viral transcription, as expected.
Fig. 7. Mixed cultures, but not purified T cells, support production of infectious virus.

CD3+ T cells or CD19+ B cells were isolated as described in Materials and Methods. Mixed, CD3+ T, or CD19+ B cells were infected with rKSHV.219 for 6–12 hr and infected cultures were left untreated or induced with valproic acid (300 μM) for 5 days. 100 μl of culture supernatants were infected on QBI293A cells by low speed centrifugation (2000 RPM for 90 min). GFP+ 293 cells were enumerated by FACS at d2 post-infection and infectious units (IU) were calculated by multiplying total number of 293 cells by the percentage of GFP+ cells in a given culture. Data from 3 individual tonsils are plotted with standard deviation. VAL denotes valproic acid and the dotted line indicates detection limit.
Fig 8. Viral gene expression in infected lymphocytes.

(A) Mixed or purified cells were prepared, infected, and induced as described in Figure 7. At d2 and d4 post-induction, induced infected cultures were harvested and total RNA was extracted with 50 μl of RNA used for cDNA synthesis using gene-specific primers. Quantitative real-time PCR was performed as described in Materials and Methods with plasmids containing each gene used as standard control. Data plotted are from 3 individual tonsils. Statistical significance was analyzed by Student t-test. (B) Viral gene expression profile in purified T cells, either uninfected (lane 1) or infected (lane 2), was assessed by microarray analysis. RNA was extracted from T cells at d2 post-infection. Array analysis was done as described in Materials and Methods. Zero-transformation was done against uninfected T cells. For comparison, RNA was extracted from either BJAB cells (lane 3) or induced BCBL-1 (lane 4) at d2 post-induction by valproate (600 μM). Zero-transformation was done against BJAB cells. KSHV tiling microarray data (ordered by genome position) are displayed for 13444 unique probes. The color bar indicates the fold change relative to the level for the uninfected T cells or BJAB cells.
Discussion
The inability of KSHV to infect established lymphoblastoid cell lines in culture has been a major impediment to the study of the pathogenesis of the several lymphoproliferative diseases linked to this virus infection. To date, we are aware of only a single report of infection by KSHV of primary lymphoid elements (Rappocciolo et al., 2008). That study focused primarily on peripheral blood B cells pre-purified from other mononuclear cells prior to infection. It showed that resting B cells were poorly infectable, but that susceptibility was enhanced by B cell activation. While our results do not directly address the role of B cell activation in KSHV infection, they are certainly compatible with the notion that the activation state of the B cell contributes to infectivity, since all our cells are derived from tonsils with reactive hyperplasia in vivo. Taken together, these two studies indicate that the longstanding barrier to growth of KSHV in lymphoid cells has now been overcome. The question before the field now is: what can these systems tell us about KSHV infection of these cells?
Our work establishes several new findings that raise interesting questions about KSHV infection in the lymphoid compartment. First, only a subset of B cells (typically 5–10%) can be infected ex vivo (cf. Figs. 2, 3, and 4). Even though we were limited (by the modest achievable titers of rKSHV.219) to infections at MOI = 0.7 or less, this number is well below the expected level of infection (~50%) under such conditions, assuming all B cells in the culture are equally susceptible. Why do relatively few B cells become infected? Of course it is possible that this merely reflects the absence of some important condition or cofactor from our growth media. Alternatively, and perhaps more interestingly, it may be that only certain subpopulations of B cells are truly susceptible to KSHV infection. It seems unlikely that activation state alone accounts for this result, since we observed no further enhancement of infection in the presence of powerful activators like LPS (data not shown) or CD40L plus IL4 (Suppl. Fig. 2). We are currently examining cellular mRNA expression profiles and cell surface markers in the GFP-positive and GFP-negative B cells that emerge post KSHV infection, in an effort to shed light on phenotypic differences that might relate to susceptibility.
Despite their relatively modest numbers, KSHV-infected B cells are the only infected elements to be enriched in number after 2 weeks in culture (Fig. 3). We do not know the basis of this phenomenon, which could reflect reduced sensitivity to apoptosis or the spread of infectious virus from infected B cells to other B cells in the culture. The abundant production of virus by infected B cells (Fig 8) might favor the latter explanation. Even if there is a survival advantage in culture, however, it is transient, and no immortalization in infected tonsillar B cells is observed – in contrast to the powerfully immortalizing effects of the EBV latency III program, which leads to the regular and reproducible outgrowth of immortalized B lymphoblasts from PBMCs (Altmann et al., 2006; Faumont et al., 2009; Martin and Gutkind, 2008). A third possibility is that infected B cells may undergo limited rounds of proliferation, as B cells display activated phenotype upon infection (unpublished observations). But even if this is the case, the transient increase in numbers of infected B cells is ultimately offset by B cell death in culture (Suppl. Figure 3).
The minimal phenotype of infected B cells in culture is consistent with everything we know about KSHV infection in humans. For example, no clinical syndrome of lymphoproliferation is regularly linked to primary KSHV infection in vivo (Ganem, 2006), in contrast to the dramatic illness of mononucleosis induced in many adults experiencing primary infection by EBV (Thorley-Lawson, 2001; Thorley-Lawson and Gross, 2004). Similarly, latent infection of endothelial cells in KS produces an indolent lesion in immunocompetent hosts, with few fatalities, long periods of relative quiescence (Ganem, 2006), and even occasional spontaneous remissions (Gambassi et al., 2005; Lospalluti et al., 1995). Cells cultured from KS lesions typically are not transformed, display enhanced rather than reduced sensitivity to exogenous growth factors (Ensoli et al., 1989; Ensoli et al., 2001; Ensoli and Sturzl, 1998), and are nontumorigenic in nude mice (Ensoli et al., 1994; Ensoli and Sturzl, 1998; Fiorelli et al., 1995). Thus, nothing in the human biology of KSHV infection should lead us to expect a dramatic in vitro phenotype in latency. Nonetheless, we acknowledge that KSHV infection is indeed linked to very rare lymphoproliferative diseases in vivo, and note that our results are entirely based on cell cultures, which may not reproduce the conditions necessary for B cell immortalization.
Perhaps the most surprising result to emerge from this work was that T cells were very efficiently infected ex vivo (Figs. 1,2, 4 and 5) – in fact, a greater proportion of T cells than B cells became KSHV positive, despite their smaller absolute numbers in the culture. Both CD4 and CD8 subsets are infectable, and infectivity was substantially enhanced by T cell activation in a cell-autonomous fashion (Figs. 1, 2, and 5), implying that signaling within the activated cell was responsible. However, these infected T cells display very minimal KSHV gene expression (Fig 8) and do not produce infectious viral progeny (Fig. 7), indicating that infection is abortive. These data indicate that GFP expression driven by the constitutive cellular promoter (EF1α) does not always serve as an accurate indicator of genuine KSHV latency. The fact that very little authentic KSHV latent gene expression proceeds in infected T cells suggests that either (i) T cells lack permissive factors for KSHV latent promoters; or (ii) T cells actively repress latent viral gene expression, at either the genetic or epigenetic level. Additionally, post-transcriptional mechanisms (e.g. accelerated viral mRNA turnover) could also be operating on some transcripts. Further work will be required to sort out these mechanisms in detail. Obviously, these factors are not mutually exclusive, and identification of blocks to viral transcription in T cells could shed light on novel forms of host regulation of viral gene expression that influence KSHV tissue tropism.
What is the significance of tonsillar T cell infection? Most studies of KSHV tropism, which have focused on the distribution of KSHV genomes in cell populations purified from PBMCs, have concluded that infection is limited to CD19+ B cells, and possibly to monocytes (Blasig et al., 1997; Wu et al., 2006). Similar conclusions have been drawn from limited studies of lymph nodes from patients with MCD (Dupin et al., 1999). Moreover, it is important to acknowledge that many prior studies of in vitro infection by KS have noted infection of cell types in culture that are not infected in vivo. For example, fibroblasts and keratinocytes are readily infected by KSHV in vitro (Bechtel et al., 2003; Cerimele et al., 2001; Renne et al., 1998), but studies of dermal KS show KSHV antigens localized only to endothelial cells, and not in fibroblasts or epithelium (Boshoff et al., 1995a; Ganem, 2006; Herndier and Ganem, 2001). So it is entirely possible that our finding that T cells can support viral entry is merely another example of this phenomenon – whose molecular basis, parenthetically, remains a mystery. But since tonsillar T cell infection is clearly abortive, secondary spread of virus among T cells is predicted to be negligible, which may likewise account for the negative studies in peripheral blood and secondary nodes distal to the portal of entry of KSHV.
Interestingly, there are hints from the human biology of KSHV that the virus can enter T cells in vivo. The most compelling of these are three reports of T cell lymphomas in which KSHV DNA was found; all were PEL-like in that they originated from body cavities (Coupland et al., 2005; Lechapt-Zalcman et al., 2001; Said et al., 1999). Although rare, the existence of such tumors bears witness to the fact that T cell infection can occur in humans, at least at a low level. In addition, Harrington et al. (Harrington et al., 1996) demonstrated that T cells purified by immune-magnetic beads from PBMC of a KS patient are infected by KSHV, albeit to a lesser degree than B cells. These results were confirmed by an independent group (Sirianni et al., 1997), who showed that clonal TCR αβ+CD8+ T cells, derived from 3 patients, harbored KSHV genomes when assessed by nested PCR (Boshoff et al., 1995b). Furthermore, these KSHV-infected CD8+ T cells were functional in terms of cytokine release (e.g., interferon-γ production). Thus, while it is likely that the ex vivo HLAC system exaggerates the susceptibility of T cells to KSHV compared to infection in vivo, these results suggest that the conventional wisdom that T cells are never targets of KSHV may be an oversimplification.
Supplementary Material
Tonsillar cells were stimulated with monomeric soluble CD40L (sCD40L), IL-10, IL-4 (A) or in combination of CD40L (10 μg/ml) and IL-4/IL-10 (B) at various concentrations as indicated for 24 hours before virus infection (MOI=0.7). At 48 hours post-infection, cells were stained with anti-CD19 and subjected to flow cytometry. KSHV infectivity in tonsillar B cells was not affected with/without cytokine stimulation when viruses were infected at lower MOI (0.1 or 0.04) (data not shown).
Infected tonsillar cells were induced by valproate at 300 μM for 5 days as described in Figure 3. Cells were subjected to FACS analysis for infectivity and RFP expression in CD8+ (top), CD4+ (middle), and CD19+ (bottom).
The number of surviving infected lymphocytes in tonsillar cultures. The number of infected cells were calculated by multiplying total number of cells in a given culture at the indicated days post-infection with percentage of specific lymphocyte subsets (CD4+ or CD8+ T cells and CD19 B cells). Experiments were performed on 2 individual tonsils. Percentage of lymphocyte subsets is given on top of each histogram.
Acknowledgments
We thank Dr. Jeffrey Vieira for his kind gift of stable Vero cells harboring rKSHV.219. This work was supported by a grant from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann M, Pich D, Ruiss R, Wang J, Sugden B, Hammerschmidt W. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV’s transforming genes. Proc Natl Acad Sci U S A. 2006;103(38):14188–93. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman P, Gordon J, Lewin N, Nordstrom M, Ehlin-Henriksson B, Klein G, Carstensson A. Surface marker characterization of EBV target cells in normal blood and tonsil B lymphocyte populations. J Immunol. 1985;135(4):2362–7. [PubMed] [Google Scholar]
- Ambroziak JA, Blackbourn DJ, Herndier BG, Glogau RG, Gullett JH, McDonald AR, Lennette ET, Levy JA. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268(5210):582–3. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- An J, Sun Y, Sun R, Rettig MB. Kaposi’s sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-kappaB and JNK/AP1 pathways. Oncogene. 2003;22(22):3371–85. doi: 10.1038/sj.onc.1206407. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci U S A. 2000;97(22):12250–5. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel JT, Liang Y, Hvidding J, Ganem D. Host range of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 2003;77(11):6474–81. doi: 10.1128/JVI.77.11.6474-6481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyesh-Melnick M, Probstmeyer F, McCombs R, Brunschwig JP, Vonka V. Correlation between infectivity and physical virus particles in human cytomegalovirus. J Bacteriol. 1966;92(5):1555–61. doi: 10.1128/jb.92.5.1555-1561.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbourn DJ, Lennette E, Klencke B, Moses A, Chandran B, Weinstein M, Glogau RG, Witte MH, Way DL, Kutzkey T, Herndier B, Levy JA. The restricted cellular host range of human herpesvirus 8. Aids. 2000;14(9):1123–33. doi: 10.1097/00002030-200006160-00009. [DOI] [PubMed] [Google Scholar]
- Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Tschachler E, Colombini S, Ensoli B, Sturzl M. Monocytes in Kaposi’s sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71(10):7963–8. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, McGee JO, Weiss RA, O’Leary JJ. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995a;1(12):1274–8. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Whitby D, Hatziioannou T, Fisher C, van der Walt J, Hatzakis A, Weiss R, Schulz T. Kaposi’s-sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995b;345(8956):1043–4. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- Brown HJ, Song MJ, Deng H, Wu TT, Cheng G, Sun R. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol. 2003;77(15):8532–40. doi: 10.1128/JVI.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008;140(1):13–24. doi: 10.1111/j.1365-2141.2007.06879.x. [DOI] [PubMed] [Google Scholar]
- Cerimele F, Curreli F, Ely S, Friedman-Kien AE, Cesarman E, Flore O. Kaposi’s sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J Virol. 2001;75(5):2435–43. doi: 10.1128/JVI.75.5.2435-2443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86(7):2708–14. [PubMed] [Google Scholar]
- Cesarman E, Nador RG, Aozasa K, Delsol G, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am J Pathol. 1996;149(1):53–7. [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435(7038):108–14. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- Chuck S, Grant RM, Katongole-Mbidde E, Conant M, Ganem D. Frequent presence of a novel herpesvirus genome in lesions of human immunodeficiency virus-negative Kaposi’s sarcoma. J Infect Dis. 1996;173(1):248–51. doi: 10.1093/infdis/173.1.248. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Charlotte F, Mansour G, Maloum K, Hummel M, Stein H. HHV-8-associated T-cell lymphoma in a lymph node with concurrent peritoneal effusion in an HIV-positive man. Am J Surg Pathol. 2005;29(5):647–52. doi: 10.1097/01.pas.0000157937.01624.1d. [DOI] [PubMed] [Google Scholar]
- Curreli F, Cerimele F, Muralidhar S, Rosenthal LJ, Cesarman E, Friedman-Kien AE, Flore O. Transcriptional downregulation of ORF50/Rta by methotrexate inhibits the switch of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 from latency to lytic replication. J Virol. 2002;76(10):5208–19. doi: 10.1128/JVI.76.10.5208-5219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan DJ, Patel AH, Subak-Sharpe JH. PREPs: herpes simplex virus type 1-specific particles produced by infected cells when viral DNA replication is blocked. J Virol. 1995;69(8):4924–32. doi: 10.1128/jvi.69.8.4924-4932.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol Biol Rev. 2003;67(2):175–212. doi: 10.1128/MMBR.67.2.175-212.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96(8):4546–51. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein DA, Penn ML, Korin YD, Scripture-Adams DD, Zack JA, Kreisberg JF, Roederer M, Sherman MP, Chin PS, Goldsmith MA. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15(4):671–82. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Markham P, Kao V, Barillari G, Fiorelli V, Gendelman R, Raffeld M, Zon G, Gallo RC. Block of AIDS-Kaposi’s sarcoma (KS) cell growth, angiogenesis, and lesion formation in nude mice by antisense oligonucleotide targeting basic fibroblast growth factor. A novel strategy for the therapy of KS. J Clin Invest. 1994;94(5):1736–46. doi: 10.1172/JCI117521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo RC. AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243(4888):223–6. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Sgadari C, Barillari G, Sirianni MC, Sturzl M, Monini P. Biology of Kaposi’s sarcoma. Eur J Cancer. 2001;37(10):1251–69. doi: 10.1016/s0959-8049(01)00121-6. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Sturzl M. Kaposi’s sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998;9(1):63–83. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Faumont N, Durand-Panteix S, Schlee M, Gromminger S, Schuhmacher M, Holzel M, Laux G, Mailhammer R, Rosenwald A, Staudt LM, Bornkamm GW, Feuillard J. c-Myc and Rel/NF-kappaB are the two master transcriptional systems activated in the latency III program of Epstein-Barr virus-immortalized B cells. J Virol. 2009;83(10):5014–27. doi: 10.1128/JVI.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli V, Gendelman R, Samaniego F, Markham PD, Ensoli B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi’s sarcoma spindle cells. J Clin Invest. 1995;95(4):1723–34. doi: 10.1172/JCI117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambassi G, Semeraro R, Suma V, Sebastio A, Incalzi RA. Aggressive behavior of classical Kaposi’s sarcoma and coexistence with angiosarcoma. J Gerontol A Biol Sci Med Sci. 2005;60 (4):520–3. doi: 10.1093/gerona/60.4.520. [DOI] [PubMed] [Google Scholar]
- Ganem D. KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu Rev Pathol. 2006;1:273–96. doi: 10.1146/annurev.pathol.1.110304.100133. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1(12):1320–2. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- Grivel JC, Santoro F, Chen S, Faga G, Malnati MS, Ito Y, Margolis L, Lusso P. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J Virol. 2003;77(15):8280–9. doi: 10.1128/JVI.77.15.8280-8289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann C, Ganem D. Effects of NFkappaB activation on KSHV latency and lytic reactivation are complex and context-dependent. Virology. 2008;375(1):94–102. doi: 10.1016/j.virol.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199(7):993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gunther T, Grundhoff A. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog. 2010;6(6):e1000935. doi: 10.1371/journal.ppat.1000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WJ, Jr, Bagasra O, Sosa CE, Bobroski LE, Baum M, Wen XL, Cabral L, Byrne GE, Pomerantz RJ, Wood C. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi’s sarcoma patients. J Infect Dis. 1996;174(5):1101–5. doi: 10.1093/infdis/174.5.1101. [DOI] [PubMed] [Google Scholar]
- Herndier B, Ganem D. The biology of Kaposi’s sarcoma. Cancer Treat Res. 2001;104:89–126. doi: 10.1007/978-1-4615-1601-9_4. [DOI] [PubMed] [Google Scholar]
- Inoue N, Winter J, Lal RB, Offermann MK, Koyano S. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J Virol. 2003;77(14):8147–52. doi: 10.1128/JVI.77.14.8147-8152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Lieberman PM. Cell cycle control of Kaposi’s sarcoma-associated herpesvirus latency transcription by CTCF-cohesin interactions. J Virol. 2009;83(12):6199–210. doi: 10.1128/JVI.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JF, Yonemoto W, Greene WC. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J Exp Med. 2006;203(4):865–70. doi: 10.1084/jem.20051856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M, Bechtel J, Venetsanakos E, Roy AM, Abbey N, Herndier B, McMahon M, Ganem D. De novo infection and serial transmission of Kaposi’s sarcoma-associated herpesvirus in cultured endothelial cells. J Virol. 2002;76(5):2440–8. doi: 10.1128/jvi.76.5.2440-2448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechapt-Zalcman E, Challine D, Delfau-Larue MH, Haioun C, Desvaux D, Gaulard P. Association of primary pleural effusion lymphoma of T-cell origin and human herpesvirus 8 in a human immunodeficiency virus-seronegative man. Arch Pathol Lab Med. 2001;125(9):1246–8. doi: 10.5858/2001-125-1246-AOPPEL. [DOI] [PubMed] [Google Scholar]
- Lospalluti M, Mastrolonardo M, Loconsole F, Conte A, Rantuccio F. Classical Kaposi’s sarcoma: a survey of 163 cases observed in Bari, south Italy. Dermatology. 1995;191(2):104–8. doi: 10.1159/000246525. [DOI] [PubMed] [Google Scholar]
- Martin D, Gutkind JS. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene. 2008;27(Suppl 2):S31–42. doi: 10.1038/onc.2009.351. [DOI] [PubMed] [Google Scholar]
- Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Pleural and peritoneal lymphoma among people with AIDS in the United States. J Acquir Immune Defic Syndr. 2002;29(4):418–21. doi: 10.1097/00126334-200204010-00014. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Arvanitakis L, Rafii S, Moore MA, Posnett DN, Knowles DM, Asch AS. Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med. 1996;183(5):2385–90. doi: 10.1084/jem.183.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nador RG, Cesarman E, Knowles DM, Said JW. Herpes-like DNA sequences in a body-cavity-based lymphoma in an HIV-negative patient. N Engl J Med. 1995;333(14):943. doi: 10.1056/NEJM199510053331417. [DOI] [PubMed] [Google Scholar]
- Penn ML, Grivel JC, Schramm B, Goldsmith MA, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci U S A. 1999;96(2):663–8. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol. 2008;82(10):4793–806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72(6):5182–8. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2 (3):342–6. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- Roush KS, Domiati-Saad RK, Margraf LR, Krisher K, Scheuermann RH, Rogers BB, Dawson DB. Prevalence and cellular reservoir of latent human herpesvirus 6 in tonsillar lymphoid tissue. Am J Clin Pathol. 2001;116(5):648–54. doi: 10.1309/Y2HH-B1CK-0F5L-U7B8. [DOI] [PubMed] [Google Scholar]
- Said JW, Shintaku IP, Asou H, deVos S, Baker J, Hanson G, Cesarman E, Nador R, Koeffler HP. Herpesvirus 8 inclusions in primary effusion lymphoma: report of a unique case with T-cell phenotype. Arch Pathol Lab Med. 1999;123(3):257–60. doi: 10.5858/1999-123-0257-HIIPEL. [DOI] [PubMed] [Google Scholar]
- Sirianni MC, Vincenzi L, Topino S, Scala E, Angeloni A, Gonnella R, Uccini S, Faggioni A. Human herpesvirus 8 DNA sequences in CD8+ T cells. J Infect Dis. 1997;176(2):541. doi: 10.1086/514072. [DOI] [PubMed] [Google Scholar]
- Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 2008;27(4):654–66. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA, Munz C. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4 (2):e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1(1):75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Toth Z, Maglinte DT, Lee SH, Lee HR, Wong LY, Brulois KF, Lee S, Buckley JD, Laird PW, Marquez VE, Jung JU. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog. 2010;6(7):e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, O’Hearn P, Kimball L, Chandran B, Corey L. Activation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75(3):1378–86. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, O’Hearn PM. Use of the red fluorescent protein as a marker of Kaposi’s sarcoma-associated herpesvirus lytic gene expression. Virology. 2004;325(2):225–40. doi: 10.1016/j.virol.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Wu W, Vieira J, Fiore N, Banerjee P, Sieburg M, Rochford R, Harrington W, Jr, Feuer G. KSHV/HHV-8 infection of human hematopoietic progenitor (CD34+) cells: persistence of infection during hematopoiesis in vitro and in vivo. Blood. 2006;108(1):141–51. doi: 10.1182/blood-2005-04-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Zhang YJ, Deng JH, Wang XP, Pan HY, Hettler E, Gao SJ. Efficient infection by a recombinant Kaposi’s sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J Virol. 2002;76(12):6185–96. doi: 10.1128/JVI.76.12.6185-6196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tonsillar cells were stimulated with monomeric soluble CD40L (sCD40L), IL-10, IL-4 (A) or in combination of CD40L (10 μg/ml) and IL-4/IL-10 (B) at various concentrations as indicated for 24 hours before virus infection (MOI=0.7). At 48 hours post-infection, cells were stained with anti-CD19 and subjected to flow cytometry. KSHV infectivity in tonsillar B cells was not affected with/without cytokine stimulation when viruses were infected at lower MOI (0.1 or 0.04) (data not shown).
Infected tonsillar cells were induced by valproate at 300 μM for 5 days as described in Figure 3. Cells were subjected to FACS analysis for infectivity and RFP expression in CD8+ (top), CD4+ (middle), and CD19+ (bottom).
The number of surviving infected lymphocytes in tonsillar cultures. The number of infected cells were calculated by multiplying total number of cells in a given culture at the indicated days post-infection with percentage of specific lymphocyte subsets (CD4+ or CD8+ T cells and CD19 B cells). Experiments were performed on 2 individual tonsils. Percentage of lymphocyte subsets is given on top of each histogram.