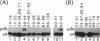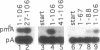Abstract
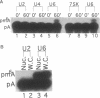
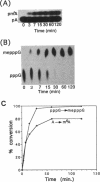
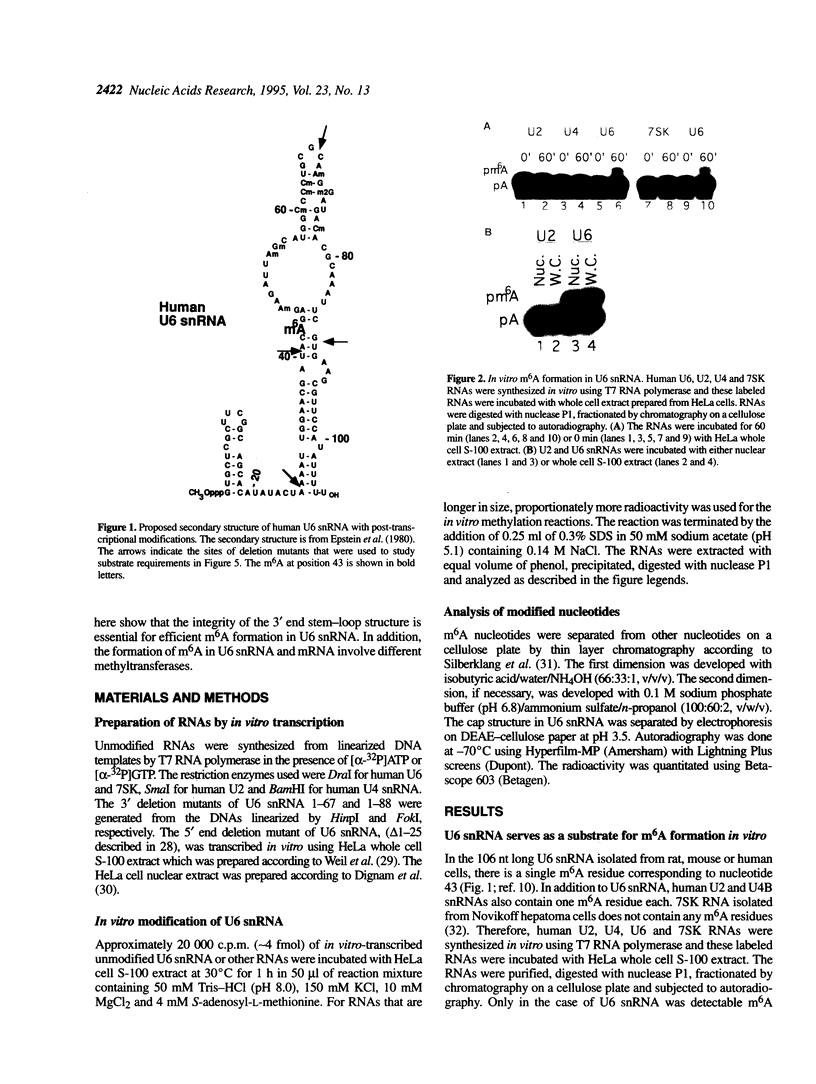
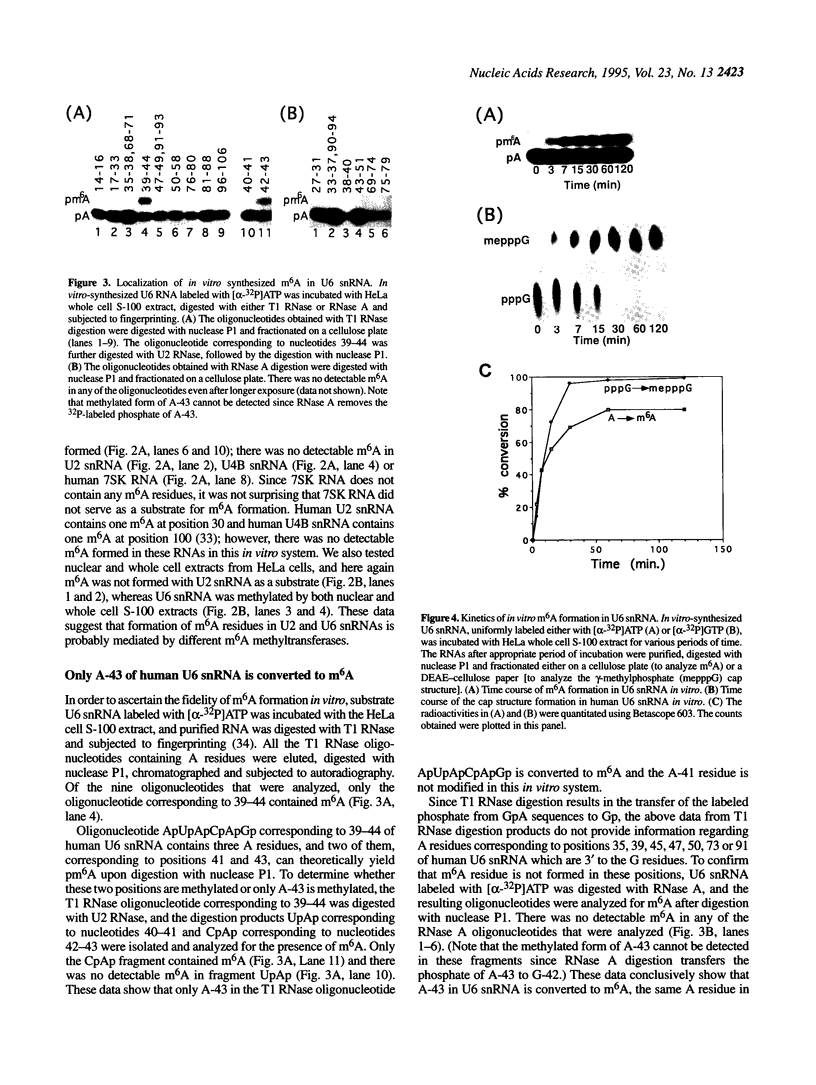
Human U6 small nuclear RNA (U6 snRNA), an abundant snRNA required for splicing of pre-mRNAs, contains several post-transcriptional modifications including a single m6A (N-6-methyladenosine) at position 43. This A-43 residue is critical for the function of U6 snRNA in splicing of pre-mRNAs. Yeast and plant U6 snRNAs also contain m6A in the corresponding position showing that this modification is evolutionarily conserved. In this study, we show that upon incubation of an unmodified U6 RNA with HeLa cell extract, A-43 residue in human U6 snRNA was rapidly converted to m6A-43. This conversion was detectable as early as 3 min after incubation and was nearly complete in 60 min; no other A residue in U6 snRNA was converted to m6A. Deletion studies showed that the stem-loop structure near the 5' end of U6 snRNA is dispensable for m6A formation; however, the integrity of the 3' stem-loop was necessary for efficient m6A formation. These data show that a short stretch of primary sequence flanking the methylation site is not sufficient for U6 m6A methyltransferase recognition and the enzyme probably recognizes secondary and/or tertiary structural features in U6 snRNA. The enzyme that catalyzes m6A formation in U6 snRNA appears to be distinct from the prolactin mRNA methyltransferase which is also present in HeLa nuclear extracts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Robberson B. L. U1, U2, and U4/U6 small nuclear ribonucleoproteins are required for in vitro splicing but not polyadenylation. Cell. 1986 Aug 29;46(5):691–696. doi: 10.1016/0092-8674(86)90344-2. [DOI] [PubMed] [Google Scholar]
- Black D. L., Steitz J. A. Pre-mRNA splicing in vitro requires intact U4/U6 small nuclear ribonucleoprotein. Cell. 1986 Aug 29;46(5):697–704. doi: 10.1016/0092-8674(86)90345-4. [DOI] [PubMed] [Google Scholar]
- Bokar J. A., Rath-Shambaugh M. E., Ludwiczak R., Narayan P., Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem. 1994 Jul 1;269(26):17697–17704. [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1988 Jul 21;334(6179):213–218. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Nucleotide sequences from the low molecular weight ribosomal RNA of Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):337–353. doi: 10.1016/s0022-2836(67)80109-8. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Albers R. J., Coward J. K., Rottman F. M. Effect of undermethylation on mRNA cytoplasmic appearance and half-life. Mol Cell Biol. 1984 Mar;4(3):538–543. doi: 10.1128/mcb.4.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979 Jun 25;6(8):2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Csepany T., Lin A., Baldick C. J., Jr, Beemon K. Sequence specificity of mRNA N6-adenosine methyltransferase. J Biol Chem. 1990 Nov 25;265(33):20117–20122. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations: studies at the monomer level. Biochemistry. 1974 Sep 24;13(20):4143–4158. doi: 10.1021/bi00717a013. [DOI] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Henning D., Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980 Sep 25;255(18):8901–8906. [PubMed] [Google Scholar]
- Finkel D., Groner Y. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology. 1983 Dec;131(2):409–425. doi: 10.1016/0042-6822(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Gu J., Reddy R. Compilation of small RNA sequences. Nucleic Acids Res. 1994 Sep;22(17):3481–3482. doi: 10.1093/nar/22.17.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Busch R. K., Singh R., Reddy R. Characterization of U6 small nuclear RNA cap-specific antibodies. Identification of gamma-monomethyl-GTP cap structure in 7SK and several other human small RNAs. J Biol Chem. 1990 Nov 5;265(31):19137–19142. [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. U4 and U6 RNAs coexist in a single small nuclear ribonucleoprotein particle. Nucleic Acids Res. 1984 Apr 11;12(7):3283–3293. doi: 10.1093/nar/12.7.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandels-Lewis S., Séraphin B. Involvement of U6 snRNA in 5' splice site selection. Science. 1993 Dec 24;262(5142):2035–2039. doi: 10.1126/science.8266100. [DOI] [PubMed] [Google Scholar]
- Lesser C. F., Guthrie C. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science. 1993 Dec 24;262(5142):1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Cyclic 2',3'-phosphates and nontemplated nucleotides at the 3' end of spliceosomal U6 small nuclear RNA's. Science. 1992 Jan 17;255(5042):327–330. doi: 10.1126/science.1549778. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bordonné R., Guthrie C. Multiple roles for U6 snRNA in the splicing pathway. Genes Dev. 1990 Dec;4(12B):2264–2277. doi: 10.1101/gad.4.12b.2264. [DOI] [PubMed] [Google Scholar]
- Narayan P., Ludwiczak R. L., Goodwin E. C., Rottman F. M. Context effects on N6-adenosine methylation sites in prolactin mRNA. Nucleic Acids Res. 1994 Feb 11;22(3):419–426. doi: 10.1093/nar/22.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Rottman F. M. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science. 1988 Nov 25;242(4882):1159–1162. doi: 10.1126/science.3187541. [DOI] [PubMed] [Google Scholar]
- Narayan P., Rottman F. M. Methylation of mRNA. Adv Enzymol Relat Areas Mol Biol. 1992;65:255–285. doi: 10.1002/9780470123119.ch7. [DOI] [PubMed] [Google Scholar]
- Patton J. R., Jacobson M. R., Pederson T. Pseudouridine formation in U2 small nuclear RNA. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3324–3328. doi: 10.1073/pnas.91.8.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Das G., Harless M., Wright D. The capped U6 small nuclear RNA is transcribed by RNA polymerase III. J Biol Chem. 1987 Jan 5;262(1):75–81. [PubMed] [Google Scholar]
- Reddy R., Henning D., Subrahmanyam C. S., Busch H. Primary and secondary structure of 7-3 (K) RNA of Novikoff hepatoma. J Biol Chem. 1984 Oct 10;259(19):12265–12270. [PubMed] [Google Scholar]
- Sawa H., Abelson J. Evidence for a base-pairing interaction between U6 small nuclear RNA and 5' splice site during the splicing reaction in yeast. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11269–11273. doi: 10.1073/pnas.89.23.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H., Shimura Y. Association of U6 snRNA with the 5'-splice site region of pre-mRNA in the spliceosome. Genes Dev. 1992 Feb;6(2):244–254. doi: 10.1101/gad.6.2.244. [DOI] [PubMed] [Google Scholar]
- Shibata H., Ro-Choi T. S., Reddy R., Choi Y. C., Henning D., Busch H. The primary nucleotide sequence of nuclear U-2 ribonucleic acid. The 5'-terminal portion of the molecule. J Biol Chem. 1975 May 25;250(10):3909–3920. [PubMed] [Google Scholar]
- Shimba S., Reddy R. Purification of human U6 small nuclear RNA capping enzyme. Evidence for a common capping enzyme for gamma-monomethyl-capped small RNAs. J Biol Chem. 1994 Apr 29;269(17):12419–12423. [PubMed] [Google Scholar]
- Shumyatsky G., Wright D., Reddy R. Methylphosphate cap structure increases the stability of 7SK, B2 and U6 small RNAs in Xenopus oocytes. Nucleic Acids Res. 1993 Oct 11;21(20):4756–4761. doi: 10.1093/nar/21.20.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Singh R., Gupta S., Reddy R. Capping of mammalian U6 small nuclear RNA in vitro is directed by a conserved stem-loop and AUAUAC sequence: conversion of a noncapped RNA into a capped RNA. Mol Cell Biol. 1990 Mar;10(3):939–946. doi: 10.1128/mcb.10.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Reddy R. Gamma-monomethyl phosphate: a cap structure in spliceosomal U6 small nuclear RNA. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8280–8283. doi: 10.1073/pnas.86.21.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J., Forne T., Jeanteur P., Cathala G., Brunel C. Mammalian U6 small nuclear RNA undergoes 3' end modifications within the spliceosome. Mol Cell Biol. 1993 Mar;13(3):1641–1650. doi: 10.1128/mcb.13.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck M. T. Partial purification of a 6-methyladenine mRNA methyltransferase which modifies internal adenine residues. Biochem J. 1992 Nov 15;288(Pt 1):233–240. doi: 10.1042/bj2880233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., McGuigan C., Mattaj I. W. Domains of U4 and U6 snRNAs required for snRNP assembly and splicing complementation in Xenopus oocytes. EMBO J. 1990 Oct;9(10):3397–3404. doi: 10.1002/j.1460-2075.1990.tb07541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman D. A., Steitz J. A. Interactions of small nuclear RNA's with precursor messenger RNA during in vitro splicing. Science. 1992 Sep 25;257(5078):1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. 5'-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976 Jan 27;15(2):397–401. doi: 10.1021/bi00647a024. [DOI] [PubMed] [Google Scholar]