Abstract
Limbs have a proximodistal axis that usually is not apparent early in development, a striking example of epigenesis. The proximodistal axis was the subject of experimental and theoretical study before any molecular genetic understanding emerged. As developmental genetic studies in Drosophila advanced, the descriptive polar coordinate model of the 1970s evolved into an understanding of how preexisting developmental compartments interact to express signaling molecules, including Hedgehog, Wingless, and Decapentaplegic, and how these define a proximodistal axis as limbs appear.
Progress in understanding of the mechanisms of developmental patterning was one of the significant achievements of 20th-century biology. As Ashburner has put it, if developmental biology is now a virtual fusion of embryology and genetics, the catalyst for this change very clearly came from the camp of the geneticists (Ashburner 1993). This article is a personal perspective of how molecular genetic studies in Drosophila melanogaster began to address proximodistal axis formation in the leg, describing the beginnings of progress in this field. The current view of limb formation in Drosophila has been well reviewed elsewhere (Morata 2001).
The proximodistal axis is fundamental to limb development; a limb would be unrecognizable without one, and perhaps would not be a limb at all. The proximodistal axis is also interesting in that it is usually not apparent in early development, a reminder that new structures arise during development that are not obviously predetermined in the embryo, the process referred to as “epigenesis” (Maienschein 2008). How proximodistal axes behave was analyzed by methods of classical developmental biology, and the findings were summarized in the 1970s (French et al. 1976). Molecular genetic approaches then began to uncover the molecular mechanisms involved (Campbell and Tomlinson 1995).
POLAR COORDINATE MODEL
Prior to molecular studies of developmental mechanisms, the classic embryological approach was to use surgery, explants, and grafting approaches as assays for developmental potential. The polar coordinate model was formulated to summarize results obtained from the manipulation of diverse limbs, including Drosophila imaginal discs, regenerating limbs of hemi-metabolous insects such a cockroaches, and amphibians (French et al. 1976) (Figure 1A). These studies described not only surgical and grafting experiments in which partial or complete regeneration of limbs was observed, but also experiments that led to pattern duplications, and even duplication or triplication of entire limbs. Related changes in limb patterning were also seen following exposure of vertebrate limbs to retinoic acid. It seemed remarkable that a common set of rules could summarize regulation in all these systems, especially in the 1970s when the extent of genetic relatedness between distinct organisms was not yet known. The wide applicability of the polar coordinate model raised the possibility that proximodistal patterning in multiple organisms might share common molecular features. While the extent to which this is true remains uncertain, the idea that such common mechanisms might exist helped renew interest in developmental biology and its molecular basis.
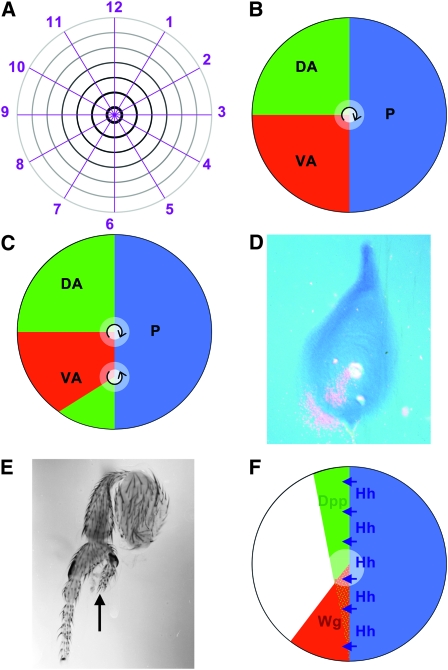
Figure 1.—
(A) The polar coordinate model represented the surface of limbs and appendages collapsed onto a two-dimensional disc, as is actually the case for Drosophila imaginal discs before they evert during pupation (French et al. 1976). Cells are thought to have circumferential information, represented by numbers 1–12 as in a clockface, and proximodistal information radiating form the distal center (black) to the proximal periphery (gray). Circumferential regulation is thought to occur by intercalation by the shortest route. Thus, removing less than half the values, e.g., 2–6, permits regeneration of the missing values, but removing more than half the values, e.g., 2–9, results in a duplication of the remaining values 10–1. Missing distal values are generated wherever the entire clockface 1–12 is present, such as in the center of the normal limb (French et al. 1976). (B) The boundary model posited non-autonomous signals emanating from at least three zones, such as the ventral-anterior (red), dorsal-anterior (green), and posterior (blue). Cells would measure circumferential location, and polarity (arrow) by comparing the red, green, and blue signals. Peak levels of all three signals define the distal point (white), which could be the source of a proximodistal morphogen (Meinhardt 1983). (C) In the boundary model, respecification of patches of cells could promote limb duplications and triplications of various polarities. C shows how respecification of a patch of ventral-anterior cells as dorsal-anterior could lead to a second proximodistal axis with reverse polarity. Respecified patches in other locations could give rise to other defects, depending on the number of points at which all three zones intersect: smaller respecified patches that intersect the anterior-posterior boundary on both sides could give rise to triplications; patches closer to the normal distal tip could alter circumferential patterns without much proximodistal effect. These possibilities are discussed in much more detail by Meinhardt (1983), although he used respecification of posterior cells as the example. Here the example is ventral-anterior because of the correspondence with wingless. (D) Wingless expression in a third instar leg imaginal disc, detected using in situ hybridization with a radioactive probe complementary to the wingless transcript, the main method available in the 1980s. Autoradiography of the dried, hybridized tissue reveals signal as silver grains, shown in pink superimposed over a phase-contrast image of the tissue. Third instar wg expression defines a ventral-anterior domain similar to that hypothesized in the boundary model (Baker 1988b). (E) Foreleg dissected from a hypomorphic wg mutant genotype (wgCX3/wgCX4) (Baker 1988b). The duplicated proximodistal axis (arrow) has reversed polarity and the line of mirror symmetry, which extends basally to the femur, runs through ventral-anterior pattern elements, exactly as the Boundary Model predicted for patchy loss of ventral-anterior identity (Figure 1C). Legs from wg mutants show a range of pattern defects that could be explained by defects at different locations within the ventral-anterior domain (Meinhardt 1983; Baker 1988b). The effect on gene transcription of wgCX3 and wgP, two alleles with similar phenotypes and breakpoints at the 3′ end of the wg gene (Baker 1987), have not been studied, but other studies of mutual antagonism between wg and Dpp expression suggests that ventral cells losing wg would adopt dorsal fate and express Dpp (Brook and Cohen 1996). (F) Unlike Hh expression, which is stably conferred by inheritance of the posterior compartment selector gene from embryogenesis, expression of Dpp and wg is induced close to the posterior compartment by Hh (Basler and Struhl 1994). Expression of wg is limited to ventral cells where it limits the expression and function of Dpp. High levels of Wg and Dpp signaling in the center of the leg disc confer distal fates on cells in this location. The most anterior cells (unlabeled) remain proximal because their low exposure to Hh indirectly reduces levels of Wg and Dpp signaling (Basler and Struhl 1994). Although Wg and Dpp signaling must collaborate in the expression of distal genes (Kim et al. 1996; Lecuit and Cohen 1997), they antagonize one another in dorsal and ventral patterning (Struhl and Basler 1993; Brook and Cohen 1996).
At its core, the polar coordinate model (Figure 1A) suggested that cells in limbs and appendages each had a way to measure their position in two independent axes, one circumferential and one proximodistal, and that the regulation seen in all systems could be summarized by two rules for interpreting this positional information (French et al. 1976). The first rule was that intercalation to replace missing structures occurred by the shortest route around the limb circumference (i.e., from dorsal to ventral and back). Although intercalation often replaces the missing parts, intercalation may result in duplicated patterns if the shortest route passes the other way around the circular “clockface.” Indeed, the finding that regulation can duplicate circumferential features of the limb is the main reason to propose circular positional information. The second rule is the complete circle rule for distalization, which states that missing distal structures can be replaced only when a complete set of circumferential values is obtained. This rule was later refined further (Bryant et al. 1981). If multiple complete circumferential values are present, such as happens following certain patterns of intercalation, multiple distal regions are specified, resulting in duplicated limbs, each with their own proximodistal axis (French et al. 1976) (Figure 1A).
At the time the molecular basis of positional information was known in none of the systems described by the polar coordinate model, which did not itself make explicit molecular predictions. It could easily be imagined that a gradient morphogen might be involved in the proximal-distal axis, but harder to envisage around the limb circumference. A circular gradient would have its high point next to its low point, which seemed molecularly unfeasible. Sequences of discrete states, like rainbow colors, could also generate circumferential or proximodistal positional values. Such “state” models beg questions about how gaps in positional values could be sensed and lead to regulation, and how change could occur during evolution. Whatever the basis of these axes, the complete circle rule for distalization implied some link between the two.
BOUNDARY MODEL
Later, Meinhardt (1983) proposed another way of looking at the same experimental observations, trying to relate them to the emerging genetic insights into Drosophila development. In the boundary model, Meinhardt (1983) imagined that the leg imaginal disc was divided into at least three zones, like the segments of an orange (Figure 1B). If each of the three zones produced a non-autonomous signal, then in principle every cell would be able to tell its circumferential location from the relative levels of the three signals. Intriguingly, cells would also be able to deduce their chirality, i.e., which way around the limb was clockwise. Meinhardt (1983) further proposed that the close juxtaposition of the three domains, which would normally occur only at the center of the disc, generated a distal identity in response to high levels of all three signals and that this initiated the proximodistal axis by, for example, defining the source of a proximodistal morphogen. There could be more than three zones, and they could vary in size or shape, but the three-zone model was the simplest that could be envisaged. Two domains that were well known experimentally were the anterior and posterior compartments defined by clonal analysis and by the posterior function of the engrailed gene. There was also evidence that the ventral portion of the anterior compartment had some special properties, so that the example of posterior, ventral-anterior, and anterior-dorsal domains was used to illustrate the model (Figure 1B). Meinhardt (1983) explained how this model could account for the same experimental data as the polar coordinate model.
Interesting experimental details from many prior studies were marshaled in support of the boundary model. One example concerns Bateson's rules. The biologist William Bateson, who had already given the field of genetics its name, made a particular study of anatomical aberrations that could be found in the museums and curiosity cabinets of the Victorian world, believing that such aberrations would provide insight into the origins of morphology. In addition to defining and drawing attention to homeosis, Bateson also listed rules that seemed to apply to all the cases of extra limbs that he examined (examples of extra limbs can now readily be seen by entering “five-legged frog,” or some such phrase, into your favorite Internet search engine). Bateson's rules are that, whenever a limb is duplicated, the extra limb is a mirror image of the normal limb and that, whenever a limb is triplicated, the three limbs occupy a straight line, with the middle limb a mirror image of the normal limb and the more distant ectopic limb of normal chirality (Meinhardt 1983).
In the boundary model, duplications and triplications arise when the three domains are opposed ectopically, generating ectopic distal sites and therefore additional proximodistal axes. For example, Figure 1C shows how respecification of a part of the ventral-anterior domain as dorsal-anterior would lead to a duplication. Importantly, respecification might not be a complicated event molecularly: if the dorsal-anterior domain was a default state, it could result from a simple failure of local gene expression within one of the other domains. The facility with which the boundary model both explains Bateson's rules and parsimoniously suggests a basis for supernumerary limbs through a loss of part of the normal pattern was one of its attractive features.
The polar coordinate model remained the more widely known. The boundary model made more concrete predictions, however, by replacing the abstract circumferential clockface concept with specific domains, thereby relating the limb field to the earlier embryonic epidermis, emphasizing non-autonomous interactions at the boundaries between the domains as the fundamental basis of patterning in both axes and suggesting a specific mechanism connecting the circumferential and proximodistal axes.
ROLE OF WINGLESS IN LEG DEVELOPMENT
Some of the first molecular data came from the segmentation gene wingless, the Drosophila prototype of the Wnt family of signaling proteins. Since wingless encoded a secreted protein and wg mutations acted cell non-autonomously, wingless was a prime candidate to encode a signaling molecule (Cabrera et al. 1987; Rijsewijk et al. 1987; Baker 1988a). It was in leg development that a correspondence between wg expression and function and the boundary model appeared (Figure 1, D and E) (Baker 1988b).
The wg gene was named on the basis of the homeotic transformation of wings (and halteres) to thorax in the viable wg1 allele (Sharma and Chopra 1976). Null alleles fell into the segment polarity class of segmentation mutants (Nusslein-Volhard and Wieschaus 1980). Legs and other adult appendages were affected when wg function was reduced in the second larval instar in hypomorphic genotypes such as wgP/wgts and wgCX3/null [both wgP and wgCX3 are rearrangements affecting a 3′ regulatory region (Figure 1E)] (Baker 1988a,b). The effects of wg mutations were in no way comparable to those of a mutation such as engrailed, whose straightforward cell-autonomous homeotic effect on the posterior compartment predicted its expression in posterior cells (Garcia-Bellido and Santamaria 1972; Morata and Lawrence 1975; Kornberg et al. 1985). Instead, variable leg defects were seen in wg phenotypes that included loss of circumferential pattern, duplications of many parts of the circumferential axis, growth defects, loss of distal structures, or duplication of all or part of the proximal distal axis (Baker 1988a). This mix of wg phenotypes seemed complex and possibly hard to interpret developmentally.
Once wg expression was discovered in a ventral-anterior wedge of the leg imaginal disc (Figure 1D); however, the boundary model immediately suggested how wg loss of function might give rise to all these phenotypes (Figure 1C). In the boundary model, loss of ventral-anterior signaling predicted a distinct spectrum of defects depending on where expression was lost, which could include both the loss of ventral elements and duplications centered on the ventral-anterior domain, which could in turn regenerate a new proximodistal axis and duplicate the limb (see Figure 1C for more details). Remarkably, the spectrum of wg leg phenotypes corresponded with these predictions (e.g., Figure 1E) (Baker 1988b). This correspondence seemed too striking to be coincidental.
One concern about these findings related to the timing of proximodistal axis specification. Studies with a temperature-sensitive allele of wg indicated that leg patterning depended on wg function before the third larval instar, whereas interrupting wg in the third instar had little morphological effect (Baker 1988a). The grafting and surgery experiments, the results of which served as the basis for the polar coordinate and boundary models, made use of third instar tissues (French et al. 1976). How could these findings be compatible? Recent studies suggest that imaginal disc regulation is preceded by a regression to an earlier developmental state as a response to injury, so that operations performed on third instar tissues probably trigger genes and patterning mechanisms that act in the second instar of normal development (Smith-Bolton et al. 2009).
DECAPENTAPLEGIC AND WINGLESS TOGETHER DEFINE THE PROXIMODISTAL AXIS
Confirming the role of wg, and further understanding the genetic basis of the proximodistal axis, required several years, the development of reagents to express wg and other genes ectopically, markers for distal cell fates within the leg disc, and an appreciation for the role of other secreted signaling molecules. Most importantly, the distalless (Dll) and aristaless (al) genes were found to encode homeodomain proteins expressed in distal cells, which could be markers for distal fate determination in imaginal discs. Decapentaplegic (Dpp), a member of the TGF-β superfamily of signals, was also required for distal limb development (Spencer et al. 1982; Padgett et al. 1987). Once it was possible to express wg or Dpp ubiquitously, two groups of researchers demonstrated that overexpressing Dpp or Wg induced Dll and al wherever they overlapped. Thus, it seemed that overlapping Dpp and Wg signaling defined distal cell fates, which normally occurred in the center of the leg disc, corresponding to the general idea of the boundary model (Campbell et al. 1993; Diaz-Benjumea et al. 1994).
COMPARTMENTS AND SEQUENTIAL ROLES FOR HEDGEHOG, DPP, AND WG
In contrast to the expression of wg, which corresponded to a zone already highlighted in the boundary model, the manner in which Wg and Dpp come to overlap at the center of the leg disk differed from anything envisioned beforehand (Campbell and Tomlinson 1995) (Figure 1F). Studies of Hedgehog (Hh), another important and conserved secreted signaling molecule (Lee et al. 1992; Ingham and McMahon 2001), made a key contribution. Hh is secreted in the posterior compartment and required to maintain Wg and Dpp expression in the neighboring anterior compartment (Figure 1F) (Basler and Struhl 1994). Dpp is expressed in a stripe of both dorsal and ventral cells near to the boundary with the posterior compartment (Masucci et al. 1990; Posakony et al. 1990). Dpp and Wg expression overlap in all ventral cells along the compartment boundary, but both the expression level and function of Dpp are suppressed by Wg signaling, so that levels sufficient for distal development are confined to a small central region by this rather than solely by the expression pattern (Figure 1F) (Campbell et al. 1993). Such mutual antagonism between Dpp and Wg pathways acts at many levels and is a recurrent feature often shared by their homologs in the Wnt and TGF-β families (Brook and Cohen 1996; Zeng et al. 2008). Thus, although three signals do interact to define distal, Dpp does not have a dorsal-anterior distribution, and Hh does not collaborate with Wg and Dpp to form a tripartite distal cue, but instead acts first to restrict the others (Campbell and Tomlinson 1995). How the distinction is made between cells that express Dpp or Wg in response to Hh, and how Hh maintains a stripe of Dpp expression but a wedge of Wg expression, still remain little understood.
NO NEED FOR A PROXIMODISTAL MORPHOGEN?
As distal transcription of genes such as Dll in response to Wg and Dpp signaling becomes better characterized, the idea of a distal source of a proximodistal morphogen has been superceded. It is now thought that proximodistal information is conferred by Wg and Dpp directly, with the most distal fates defined by high levels of both Dpp and Wg signals (Lecuit and Cohen 1997; Estella et al. 2008). This idea was proposed in studies of the vestigial gene, which is required for proximodistal outgrowth of wing tissue from the body and is expressed in a central region of the wing imaginal disc in response to both Dpp and Wg (Kim et al. 1996). In addition to Dll and al, other parts of the proximodistal leg axis are characterized by expression of other transcription factors (Abu-Shaar and Mann 1998). These might depend on intermediate levels of Wg and Dpp signaling although this is not entirely straightforward, given that the sources of Wg and Dpp are asymmetric in the disc.
In a sense, the two axes of the leg are defined by a different logic at the promoters of target genes. Promoters where Wg and Dpp signaling cooperate in transcription are active at particular proximodistal locations; promoters where Wg and Dpp signaling have antagonistic effects are active at particular locations around the circumference. Because of the latter functions, Wg and Dpp each individually contribute to ventral and dorsal leg features, respectively, in addition to proximodistal patterning (Morata and Lawrence 1977; Baker 1988b; Couso et al. 1993; Struhl and Basler 1993; Held et al. 1994; Kwon et al. 2004; Svendsen et al. 2009). The idea of a dedicated proximodistal morphogen no longer seems necessary to explain patterning, since the proximodistal axis itself, once considered a new feature of limb or appendage development, in fact exists latently within the embryonic epidermis or in principle in any other planar tissue with two axes of positional information.
The fact that no proximodistal morphogen is essential to make an axis does not preclude evolution from using one; in fact, there is a graded signal that acts only within the most distal and evolutionarily most ancient portion of the leg, the tarsus. Tarsus patterning depends on a gradient of EGF receptor signaling emanating from the most distal cells, and this depends on the activation of Dll (Campbell 2002; Galindo et al. 2002). In another example, the remodeled organizer at the future wing boundary seems to employ Wingless as a distal morphogen (Zecca et al. 1996; Neumann and Cohen 1997). There may be proximodistal morphogens in vertebrate limb buds (Tabin and Wolpert 2007). There are also aspects of limb development that are less well understood, such as cellular polarity or the coordination of growth in the limb axis, in which morphogens might be important.
DIFFERENT GENES FOR DIFFERENT LIMBS
Although the Drosophila leg is a useful starting point for understanding proximodistal axes, other Drosophila appendages differ, as do limbs from other organisms. In dorsal imaginal discs such as wing, haltere, and eye, the expression of wg is similar in the second instar, but becomes quite different by the third instar (Baker 1988b; Couso et al. 1993; Williams et al. 1993). This reorganization is now known to be related to the establishment of a Notch (N)-dependent signaling center at the future wing margin that expands the wing primordium through a wing-specific transcription factor gene, vestigial (see Morata 2001 and Zecca and Struhl 2010 for more details).
Before wg expression was described in the second instar wing disc (Couso et al. 1993; Williams et al. 1993), the different third instar pattern was a concern, since many thought of wing and leg discs as parallel, homologous structures that should have homologous axes. In fact, homology of dorsal and ventral discs had been challenged for decades by some of the foremost authorities on insect development, but with surprisingly little impact on popular opinion. Donald Anderson had described the origins of the wing and haltere discs as outgrowths of the dorsal leg discs in studies of the Queensland fruitfly, Dacus tryoni, and in doing so presented a critique of the prevailing view of independent origins, tracing this to uncritical observations published in 1900 (Anderson 1963). V. B. Wigglesworth, apparently independently, argued on the basis of comparative anatomy that insect wings evolved from the limb base (Wigglesworth 1973). In addition, clonal analysis by Wieschaus and Gehring (1976), found that wing and leg discs shared common progenitor cells in D. melanogaster. By the mid-1980s, developmental genetics was also raising challenges to the notion of homologous dorsal and ventral discs because the traditional view that wing and haltere discs invaginated with the tracheal pits was contradicted by the finding that tracheal pits form at segment boundaries, while imaginal discs have to originate at parasegment boundaries (Struhl 1984; Martinez-Arias and Lawrence 1985). There were thus legitimate reasons to question the homology of wing and leg discs, even before the budding of wing discs off the leg disc after their common ventral invagination was finally observed directly in D. melanogaster (Cohen 1990). Nevertheless, it was easier to accept the role of wg in the proximodistal axis once it was known that the wing disc passed through a stage of expression similar to the leg disc (Couso et al. 1993; Williams et al. 1993).
Comparing Drosophila and vertebrate limb development is not straightforward. In vertebrates, for example, Hox gene clusters play important roles in limb patterning that seem unrelated to anything in Drosophila (Zakany and Duboule 2007). One similarity is that both Drosophila legs and vertebrate limbs seem to depend on an Sp class of transcription factors, which in Drosophila bring the Dll gene under regulation of Dpp and Wg (Estella and Mann 2010). Another similarity is the conserved role of particular homodomain proteins in distinguishing limbs from a nearby body wall (Morata 2001). The role of the apical ectodermal ridge in vertebrate limbs more resembles the modified distal organizer of a dorsal Drosophila wing (Morata 2001; Tabin and Wolpert 2007). Most relevant to this article, however, is the fact that, whereas the Wnt, TGF-β, and Hh signaling families are important in vertebrate limbs, as in almost every aspect of development, it is difficult to conclude that they are used homologously to define their proximodistal axis.
ARE THERE GENERAL MECHANISMS FOR LIMB FORMATION?
Is it surprising that limbs that behaved similarly in studies using classical embryological approaches differ in their molecular construction? Perhaps there is some genetic “deep homology” behind these common properties. Perhaps the common feature is the interaction of compartments (or other cell populations) in defining new signaling centers. Perhaps the similarity is in gene network topology whereby signals that, when in opposition, define distinct circumferential values and define a new proximodistal axis through target genes at which they collaborate and reinforce. It is intriguing to wonder whether Wg and Dpp could be replaced by other molecules in a similar network, such as N and the receptor tyrosine kinase-MAPK pathways, which also often interact both antagonistically and collaboratively (Sundaram 2005).
CROSS TALK BETWEEN MODELS AND MOLECULAR GENETICS OF LIMB FORMATION
It certainly was an exciting and unexpected experience for me to personally contribute to this field and to uncover a piece of data such as the leg-disc expression pattern of wg that unexpectedly linked several regions of the jigsaw puzzle that has been limb patterning. Having cloned wg in Peter Lawrence's lab in Cambridge, UK (Baker 1987), I was fortunate enough to find a postdoc for myself in Gerry Rubin's lab at the University of California, Berkeley, where on a regular basis Jim Fristrom's lab was mass-isolating third instar imaginal discs (Eugene et al. 1979), which could easily be used for in situ hybridization experiments. Serendipitously, a young Ph.D. student at Berkeley had been given the difficult task of connecting the proximodistal limb axis to the patterning of the embryonic epidermis in his 1987 qualifying exam, a subject on which the polar coordinate model was silent, so the boundary model articles were under much local discussion at exactly this time. Mark Fortini later lamented that he was unable to unveil the wg expression pattern and mutant phenotype in his qualifying proposal, but he did manage to pass nevertheless. Because the boundary model was already worked out, connections between wg expression and wg phenotypes and their implications for limb formation were exceptionally rapid, which made the science exciting.
How else did the models contribute to understanding limb development? The leg expression of wg and hh may have corresponded to the boundary model in particular, but the expression of Dpp was not as envisaged, the way in which hh interacts with wg and dpp was unexpected, and the proximodistal axis was understood in terms of response to Wg and Dpp signaling rather than to a proximodistal morphogen that they induce (Campbell and Tomlinson 1995; Lecuit and Cohen 1997; Estella et al. 2008). The dorsal imaginal discs, and perhaps the vertebrate limbs, also differ in many molecular respects. Some would therefore argue that most studies of the Drosophila limb would have proceeded, or did proceed, regardless of any models, or that such preexisting models may even have distracted researchers from forward experimental progress. It is straightforward and increasingly common today to describe the molecular basis of limb development without reference to the polar coordinate or boundary models.
Both models were attempts to interpret and synthesize experimental data, not just abstract theories. The polar coordinate model defined the general questions; the boundary model made mechanistic predictions specific enough to be falsifiable, as some were. Both models raised the profile of limb research by suggesting its general significance. In the case of my research, the imaginal disc expression pattern of wg would have been of interest regardless, but I doubt that the wg leg-disc expression pattern would have led as directly to examination and interpretation of the wg leg phenotype without the knowledge of the boundary model, which therefore had this positive effect. The influence on experimental studies of Dpp or Hh may be less direct, although its relevance was acknowledged as the central role of Dpp and Wg in distal development became established (Campbell and Tomlinson 1995). The boundary model also signified an appreciation for the potential of developmental boundaries as organizers of new features. Whereas it was certainly appreciated beforehand that neighboring Drosophila compartments had to interact if the tissue pattern was to be continuous across such boundaries—as is the case when compartment boundaries do not correspond to anatomical boundaries—and also that segment and compartment boundaries might have special roles in patterning, such as sources or sinks for hypothesized gradients (Crick and Lawrence 1975; Lawrence 1981), the idea that the interaction between compartments or other domains was the primary source of patterning signals and even new axes was not widespread, and the possibility that compartment boundaries were actually barriers to the spread of information received more attention (Warner and Lawrence 1982; Weir and Lo 1984). In retrospect, one can see similarities in embryological concepts of interactions between different cells, such as in the work of Horstadius (Ernst 1997), but at the time it was not easy in practice to associate compartment boundaries, in particular, with cell interactions or with generating novel organizers.
Acknowledgments
I thank Gerard Campbell, Stephen Cohen, Mark Fortini, Andrew Tomlinson, and an anonymous reviewer for helpful comments. The author's laboratory is supported by grants from the National Institutes of Health.
References
- Abu-Shaar, M., and R. S. Mann, 1998. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development 125 3821–3830. [DOI] [PubMed] [Google Scholar]
- Anderson, D. T., 1963. The embryology of Dacus tryoni. 2. Development of imaginal discs. J. Embryol. Exp. Morphol. 11 339–351. [PubMed] [Google Scholar]
- Ashburner, M. A., 1993. Epilogue, pp. 1493–1506 in The Development of Drosophila melanogaster, edited by M. Bate and A. Martinez-Arias. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Baker, N. E., 1987. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. Eur. Mol. Biol. Org. J. 6 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, N. E., 1988. a Embryonic and imaginal requirements for wingless, a segment polarity gene in Drosophila. Dev. Biol. 125 96–108. [DOI] [PubMed] [Google Scholar]
- Baker, N. E., 1988. b Transcription of the segment polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development 102 489–497. [DOI] [PubMed] [Google Scholar]
- Basler, K., and G. Struhl, 1994. Compartment boundaries and the control of Drosophila limb patterning by hedgehog protein. Nature 368 208–214. [DOI] [PubMed] [Google Scholar]
- Brook, W. J., and S. M. Cohen, 1996. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science 273 1373–1377. [DOI] [PubMed] [Google Scholar]
- Bryant, S. V., V. French and P. J. Bryant, 1981. Distal regeneration and symmetry. Science 212 993–1002. [DOI] [PubMed] [Google Scholar]
- Cabrera, C. V., M. C. Alonso, P. Johnston, R. G. Phillips and P. A. Lawrence, 1987. Phenocopies induced with antisense RNA identify the wingless gene. Cell 50 659–663. [DOI] [PubMed] [Google Scholar]
- Campbell, G., 2002. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature 418 781–785. [DOI] [PubMed] [Google Scholar]
- Campbell, G., and A. Tomlinson, 1995. Initiation of the proximodistal axis in insect legs. Development 121 619–628. [DOI] [PubMed] [Google Scholar]
- Campbell, G., T. Weaver and A. Tomlinson, 1993. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell 74 1113–1123. [DOI] [PubMed] [Google Scholar]
- Cohen, S. M., 1990. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature 343 173–177. [DOI] [PubMed] [Google Scholar]
- Couso, J. P., M. Bate and A. Martinez-Arias, 1993. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science 259 484–489. [DOI] [PubMed] [Google Scholar]
- Crick, F. H., and P. A. Lawrence, 1975. Compartments and polyclones in insect development. Science 189 340–347. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea, F. J., B. Cohen and S. M. Cohen, 1994. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature 372 175–179. [DOI] [PubMed] [Google Scholar]
- Ernst, S., 1997. A century of sea urchin development. Am. Zool. 37 250–259. [Google Scholar]
- Estella, C., and R. S. Mann, 2010. Non-redundant selector and growth-promoting functions of two sister genes, buttonhead and Sp1, in Drosophila leg development. PLoS Genet. 6 e1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella, C., D. J. McKay and R. S. Mann, 2008. Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev. Cell 14 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugene, O. E., M. A. Yund and J. W. Fristrom, 1979. Preparative isolation and short-term culture of imaginal discs of Drosophila melanogaster. Tiss. Cult. Assoc. Manual 5 1055–1062. [Google Scholar]
- French, V., P. J. Bryant and S. V. Bryant, 1976. Pattern regulation in epimorphic fields. Science 193 969–981. [DOI] [PubMed] [Google Scholar]
- Galindo, M. I., S. A. Bishop, S. Greig and J. P. Couso, 2002. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science 297 256–259. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido, A., and P. Santamaria, 1972. Developmental analysis of the wing disc in the mutant engrailed of Drosophila melanogaster. Genetics 72 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham, P. W., and A. P. McMahon, 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15 3059–3087. [DOI] [PubMed] [Google Scholar]
- Held, L. I. J., M. A. Heup, J. M. Sappington and S. D. Peters, 1994. Interactions of decapentaplegic, wingless, and Distalless in the Drosophila leg. Rouxs Arch. Dev. Biol. 203 310–319. [DOI] [PubMed] [Google Scholar]
- Ingham, P. W., and A. P. McMahon, 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15 3059–3087. [DOI] [PubMed] [Google Scholar]
- Kim, J., A. Sebring, J. J. Esch, M. E. Kraus, M. Vorwerk et al., 1996. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382 133–138. [DOI] [PubMed] [Google Scholar]
- Kornberg, T., I. Siden, P. O'Farrell and M. Simon, 1985. The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell 40 45–53. [DOI] [PubMed] [Google Scholar]
- Kwon, C., R. Hays, J. Fetting and T. V. Orenic, 2004. Opposing inputs by Hedgehog and Brinker define a stripe of hairy expression in the Drosophila leg imaginal disc. Development 131 2681–2692. [DOI] [PubMed] [Google Scholar]
- Lawrence, P. A., 1981. The cellular basis of segmentation in insects. Cell 26 3–10. [DOI] [PubMed] [Google Scholar]
- Lecuit, T., and S. M. Cohen, 1997. Proximal-distal axis formation in the Drosophila leg. Nature 388 139–145. [DOI] [PubMed] [Google Scholar]
- Lee, J. J., D. P. von Kessler, S. Parks and P. A. Beachy, 1992. Secretion and localized transcription suggest a role in positional signalling for products of the segmentation gene hedgehog. Cell 71 33–50. [DOI] [PubMed] [Google Scholar]
- Maienschein, J., 2008. Epigenesis and preformationism, in The Stanford Encyclopedia of Philosophy, Fall 2008 Edition, edited by E. N. Zalta. http://plato.stanford.edu/archives/fall2008/entries/epigenesis/
- Martinez-Arias, A., and P. A. Lawrence, 1985. Parasegments and compartments in the Drosophila embryo. Nature 313 639–642. [DOI] [PubMed] [Google Scholar]
- Masucci, J. D., R. J. Miltenberger and F. M. Hoffmann, 1990. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal disks is regulated by 3′ cis -regulatory elements. Genes Dev. 4 2011–2023. [DOI] [PubMed] [Google Scholar]
- Meinhardt, H., 1983. Cell determination boundaries as organizing regions for secondary embryonic fields. Dev. Biol. 96 375–385. [DOI] [PubMed] [Google Scholar]
- Morata, G., 2001. How Drosophila appendages develop. Nat. Rev. Mol. Cell Biol. 2 89–97. [DOI] [PubMed] [Google Scholar]
- Morata, G., and P. A. Lawrence, 1975. Control of compartment development by the engrailed gene in Drosophila. Nature 255 614–617. [DOI] [PubMed] [Google Scholar]
- Morata, G., and P. A. Lawrence, 1977. The development of wingless, a homeotic mutation of Drosophila. Dev. Biol. 56 227–240. [DOI] [PubMed] [Google Scholar]
- Neumann, C. J., and S. M. Cohen, 1997. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124 871–880. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard, C., and E. Wieschaus, 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287 795–801. [DOI] [PubMed] [Google Scholar]
- Padgett, R. W., R. D. St Johnston and W. M. Gelbart, 1987. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature 325 81–84. [DOI] [PubMed] [Google Scholar]
- Posakony, L. G., L. A. Raftery and W. M. Gelbart, 1990. Wing formation in Drosophila melanogaster requires decapentaplegic gene function along the anterior-posterior compartment boundary. Mech. Dev. 33 69–82. [DOI] [PubMed] [Google Scholar]
- Rijsewijk, F., M. Schuermann, E. Wagenaar, P. Parren, D. Weigel et al., 1987. The Drosophila homolog of the mouse mammary tumor virus int-1 is identical to the segment polarity gene wingless. Cell 50 649–657. [DOI] [PubMed] [Google Scholar]
- Sharma, R. P., and V. L. Chopra, 1976. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev. Biol. 48 461–465. [DOI] [PubMed] [Google Scholar]
- Smith-Bolton, R. K., M. I. Worley, H. Kanda and I. K. Hariharan, 2009. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev. Cell 16 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, F. A., F. M. Hoffmann and W. M. Gelbart, 1982. Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28 451–461. [DOI] [PubMed] [Google Scholar]
- Struhl, G., 1984. Splitting the bithorax complex of Drosophila. Nature 308 454–457. [Google Scholar]
- Struhl, G., and K. Basler, 1993. Organizing activity of wingless protein in Drosophila. Cell 72 527–540. [DOI] [PubMed] [Google Scholar]
- Sundaram, M. V., 2005. The love-hate relationship between Ras and Notch. Genes Dev. 19 1825–1839. [DOI] [PubMed] [Google Scholar]
- Svendsen, P. C., A. Formaz-Preston, S. M. Leal and W. J. Brook, 2009. The Tbx20 homologs midline and H15 specify ventral fate in the Drosophila melanogaster leg. Development 136 2689–2693. [DOI] [PubMed] [Google Scholar]
- Tabin, C., and L. Wolpert, 2007. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 21 1433–1442. [DOI] [PubMed] [Google Scholar]
- Warner, A. E., and P. A. Lawrence, 1982. Permeability of gap junctions at the segmental border in insect epidermis. Cell 28 243–252. [DOI] [PubMed] [Google Scholar]
- Weir, M. P., and C. W. Lo, 1984. Gap-junctional communication compartments in the Drosophila wing imaginal disk. Dev. Biol. 102 130–146. [DOI] [PubMed] [Google Scholar]
- Wieschaus, E., and W. Gehring, 1976. Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev. Biol. 50 249–263. [DOI] [PubMed] [Google Scholar]
- Wigglesworth, V. B., 1973. Evolution of insect wings and flight. Nature 246 127–129. [Google Scholar]
- Williams, J. A., S. W. Paddock and S. B. Carroll, 1993. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117 571–584. [DOI] [PubMed] [Google Scholar]
- Zakany, J., and D. Duboule, 2007. The role of Hox genes during vertebrate limb development. Curr. Opin. Genet. Dev. 17 359–366. [DOI] [PubMed] [Google Scholar]
- Zecca, M., and G. Struhl, 2010. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 8 e1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca, M., K. Basler and G. Struhl, 1996. Direct and long-range action of a wingless morphogen gradient. Cell 87 833–844. [DOI] [PubMed] [Google Scholar]
- Zeng, Y. A., M. Rahnama, S. Wang, W. Lee and E. M. Verheyen, 2008. Inhibition of Drosophila Wg signaling involves competition between Mad and Armadillo/beta-catenin for dTcf binding. PLoS One 3 e3893. [DOI] [PMC free article] [PubMed] [Google Scholar]