Abstract
Cancer development is often associated with increased fibroblast proliferation and extensive fibrosis; however, the role of fibroblasts during carcinogenesis remains largely unknown. Using the 7,12-dimethylbenz-(a)anthracene and 12-O-tetradecanoylphorbol-13-acetate–induced two-stage skin carcinogenesis model, we demonstrated here that there was a massive accumulation and proliferation of fibroblasts in the skin shortly after application of carcinogen. Selective abatement of these cells during the promotion stage drastically decreased incidence and progression of papillomas. This correlated well with reduced macrophage infiltration and impaired cytokine storm in the affected skin. 12-O-tetradecanoylphorbol-13-acetate stimulated skin fibroblasts, secreting high levels of monocyte chemotactic protein-1, and neutralization of this chemokine eliminated almost completely the fibroblast-induced chemotaxis of macrophages. These results strongly suggest that fibroblasts promote skin tumor development by producing monocyte chemotactic protein-1 and maintaining chronic inflammation.
It has been noted putatively that distinct cellular changes in the microenvironment accompany tumor formation.1,2 Increased fibroblast proliferation is often observed adjacent to cancer cells. For example, cancers of lung, liver, and breast are often associated with fibroblast activation, proliferation, and collagen deposition.3–5 Fibroblasts may influence cancer development in different ways. Several factors secreted by fibroblasts, such as epidermal growth factor, hepatocyte growth factor, and interleukin-6 (IL-6), can stimulate cancer cell proliferation and enhance their invasive properties.6,7 Others, such as vascular endothelial growth factor, stromal cell-derived factor-1, and matrix metalloproteinases, can promote angiogenesis.8–10 Fibroblast-derived cytokine IL-1 and chemokine (C-X-C motif) ligand 14 have been shown to play vital roles as immune modulators.11,12 Most of these studies, however, came from tumor transplantation experiments, which focus mainly on the role of fibroblasts during the growth of transplanted tumor cells. The physiological role of fibroblasts and the corresponding molecular mechanisms during chemical carcinogenesis is largely unknown.
The 7,12-dimethylbenz-(a)anthracene/12-O-tetradecanoylphorbol-13-acetate (DMBA/TPA)–induced two-stage skin carcinogenesis serves as a well-characterized in vivo mouse model for inflammation-induced epithelial neoplasia, and it mimics the multistage nature of human cancer development.13 A single application of DMBA induces DNA mutations in epidermal cells and causes tumor initiation. Promotion requires repeated exposure of the skin to TPA, which boosts tumor development via induction of chronic inflammation.14 Considering the fact that most of human neoplasias are of epithelial origin, and 20% of the cancers are clearly associated with chronic inflammation,15–17 it is interesting to analyze the role of fibroblasts during skin carcinogenesis with the well established DMBA/TPA model.
For this purpose, FSP-TK transgenic mice were used, in which the expression of herpes simplex virus–derived thymidine kinase (TK) was expressed under the control of the promoter of a fibroblast-specific gene fibroblast specific protein-1 (FSP1).18–20 With activation of the “suicide” gene TK, the phosphorylated products of ganciclovir (GCV)21 are able to deplete the proliferating FSP1+ cells in vivo selectively. In the current study, we showed for the first time that depletion of proliferating fibroblasts during the tumor promotion stage decreased the incidence and number of papillomas as well as their malignant conversion rate. Furthermore, fibroblasts influenced skin carcinogenesis by direct production of MCP-1 and up-regulation of local inflammation.
Materials and Methods
Mice
BALB/c mice were purchased from Weitonglihua Corporation (Beijing, China). FSP-TK transgenic mice and wild-type control littermates were obtained from Dr. Eric G. Neilson's lab. All mice were bred under specific pathogen-free conditions in the animal facilities at the Institute of Biophysics, Chinese Academy of Sciences. All animal studies were performed with sex- and age-matched mice after being approved by the Institutional Laboratory Animal Care and Use Committee.
Skin Carcinogenesis and GCV Treatment
Groups of FSP-TK mice and control littermates were subjected to a single topical application of 50 μg DMBA (Sigma), and 1 week later, 4 μg TPA (Sigma) twice a week for 14 weeks. To deplete proliferating fibroblasts, 0.5 mg GCV (HuBeiKeYi Pharmaceutic Corporation) dissolved in acetone was given 0.5 hours after TPA administration. Mice were divided into the following groups: (1) FSP-TK mice treated with DMBA, TPA, and GCV in acetone; (2) FSP-TK mice treated with DMBA, TPA, and acetone alone; (3) control littermates treated with DMBA, TPA, and GCV in acetone; and (4) control littermates treated with DMBA, TPA, and acetone alone.
After shaving a 2-cm2 area of the dorsal skin, DMBA, TPA, or GCV that was dissolved in 100 μl acetone was administered by a micropipette. The area was regularly shaved and tumors of ≥1 mm in diameter, which had been present for at least 2 weeks, were assessed twice per week. Papillomas were evaluated as typically well demarcated, symmetrical, pedunculated, or dome-shaped papules without erosion or ulceration. Carcinomas were scored as poorly demarcated, asymmetrical, nonpedunculated, or dome-shaped papules with erosion or ulceration. The malignancy of all visually detected carcinomas was verified by histological analysis as described previously.22 The paraffin sections of tumor tissues were stained and analyzed in a double-blinded fashion, and the discrimination between invasive carcinomas and non-neoplastic lesions was performed according to the following major criteria: invasion of the underlying dermis and subcutaneous layer, presence of horn pearls, and atypical cells.
Hyperplasia and Immunohistochemistry
Preparation of cryostat or paraffin tissue sections and immunohistochemistry were done as described previously.23 The thickness of the epidermis (in micrometers) was measured with an image system (Photoshop; Adobe Systems Inc., San Jose, CA) and calculated as following: the actual thickness of epidermis = on-screen measurements of epidermis/magnification (10 fields per section). For FSP1 staining, paraffin sections were incubated with rabbit anti-FSP1 polyclonal antibodies (a gift from Dr. Eric G. Neilson), then incubated with biotinylated secondary antibody followed by streptavidin-peroxidase. The peroxidase activity was detected with diaminobenzidine (DAB; Sigma) and the sections were counterstained with hematoxylin. For proliferating cell nuclear antigen (PCNA), α smooth muscle actin (α-SMA), F4/80 and CD11b detection, paraffin or frozen sections were incubated with anti-PCNA (Sigma), anti-α-SMA (Abcam), anti-F4/80 (eBioscience, San Diego, CA), and anti-CD11b (BD Pharmingen) monoclonal antibodies respectively. Then biotinylated secondary antibody and Rhodamine-labeled streptavidin were used. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma). For FSP1 and Ki-67 double labeling, paraffin sections were incubated with a mixture of rabbit anti-FSP1 and mouse anti-Ki-67 (BD Pharmingen) antibodies followed by the mixture of Rhodamine-labeled goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)–labeled horse anti-mouse IgG. For FSP1 and α-SMA double labeling, paraffin sections were incubated with a mixture of rabbit anti-FSP1 and mouse anti-α-SMA antibodies followed by the mixture of FITC-labeled goat anti-rabbit IgG and Rhodamine-labeled horse anti-mouse IgG. For cytokeratin (CK) and PCNA double labeling, paraffin sections were incubated with a mixture of rabbit anti-CK (Abcam) and mouse anti-PCNA antibodies followed by the mixture of FITC-labeled goat anti-rabbit IgG and Rhodamine-labeled horse anti-mouse IgG. Sections were evaluated under the microscope (DP71, Olympus) for bright-field and fluorescence microscopy.
Cytokine Determination
For detection of cytokines in skin tissues, dorsally shaved TPA-treated skins were homogenized in ice-cold TE buffer. Homogenates were centrifuged at 12,000Xg for 15 minutes. The supernatant was collected and the levels of IL-6, MCP-1, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-10, and IL-12p70 were assayed by a Mouse Inflammation Cytometric Bead Array (CBA) Kit (BD Pharmingen). The data were analyzed using the CBA software. The relative amount of a target cytokine in one sample = the concentration analyzed by CBA/the weight of the sample. IL-4 and IL-17 were detected by enzyme-linked immunoabsorbent assay with paired antibodies (R&D Systems). For detection of cytokines secreted by fibroblasts, mice skin fibroblasts were plated into 96 multiwell plates at 1 × 104 cells/well with 0.2 ml of Dulbecco's modified Eagle's medium (DMEM) and cultured for 24 hours. When cells reached confluence, they were incubated with 10 nmol/L TPA or without TPA as control for 12 and 24 hours after replacement of the culture medium with serum-free DMEM. Cytokine levels in the supernatant were measured with the CBA kit.
Chemotaxis Assay
Primary fibroblasts were obtained from the back skin of mice as previously described.18 Fibroblasts were seeded in 24 multiwell plates (1 × 105 cells/well) in serum-free DMEM and were treated with or without 10 nmol/L TPA for 24 hours. Then the fibroblast-conditioned medium was collected. Macrophages of FSP-TK mice or control littermates were collected by peritoneal lavage24 and were labeled with carboxyfluorescein succinimidyl ester (CFSE). Macrophage chemotactic activity was assayed using 24-well transwell chambers fitted with 5-μm-pore polycarbonate membranes (Corning Costar). Five × 104 CFSE-labeled peritoneal macrophages in serum-free DMEM were seeded in the upper chamber. The lower chamber contained each of the following: DMEM, DMEM-containing TPA, fibroblast-conditioned medium, fibroblast-conditioned medium containing TPA, fibroblast-conditioned media containing TPA and anti-MCP-1 neutralizing antibody (5 μg/ml; eBioscience), anti-IL-6 neutralizing antibody (2 μg/ml; BD Pharmingen), or isotype IgG. Six hours later, cells that remained at the upper surface of the membrane were removed using a swab; photos of cells that migrated to the lower membrane surface were taken with a microscope digital camera (DP71; Olympus). The number of cells migrating through the filter was counted and plotted as the number of cells per high power field (HPF, ×200).
Statistical Analysis
Data were analyzed using two-tailed unpaired Student's t-test. Means ± SD were presented. P < 0.05 was considered statistically significant.
Results
Skin Carcinogenesis Promoted Proliferation of Local FSP1+ Fibroblasts
DMBA/TPA-induced two-stage carcinogenesis induces not only DNA mutations in epidermal cells but also a permanent local chronic inflammation, such as the infiltration of leukocytes and dendritic cells.14 However, it is still not clear whether fibroblasts are involved in this process. The dynamic changes of fibroblasts in the skin of BALB/c mice before and at different times after the DMBA/TPA application were examined. As shown in Figure 1A and Supplemental Figure S1 (http://ajp.amjpathol.org), FSP1+ fibroblasts were mainly nonproliferating cells, scattering under the basal layer proliferating keratinocytes in normal skin. However, DMBA/TPA treatment led to proliferation and a rapid increase of these cells. The mean number of FSP1+ fibroblasts in the affected skin was more than that in untreated skin (31.3 vs. 8.3, per HPF, ×400) after three TPA treatments (twice a week, for 3 weeks) which increased further after six applications of TPA (66.7 ± 15.5, per HPF). Interestingly, almost all of the proliferating fibroblasts were FSP1 positive, which did not express α-SMA, a marker for myofibroblasts (Figure 1B). Although FSP1+ cells were scattered under the basal layer, α-SMA+ cells were distributed mainly around the blood vessels (Figure 1C, and see Supplemental Figure S2, http://ajp.amjpathol.org). A similar increase of proliferating FSP1+ fibroblasts had been observed when FSP-TK mice were analyzed (data not shown). The increased numbers and the early proliferation of FSP1+ fibroblasts in response to DMBA/TPA treatment indicate that they may play an important part in local inflammation and tumor development.
Figure 1.
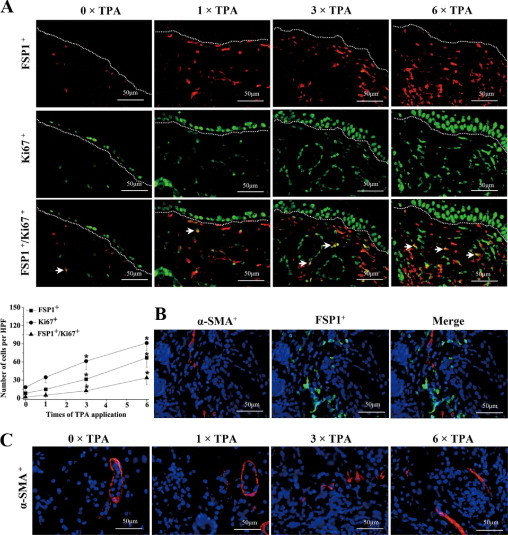
Skin carcinogenesis promoted proliferation of local FSP1+ fibroblasts. A: Groups of BALB/c mice were treated with DMBA and then with TPA, twice a week, as described in Materials and Methods. Twenty-four hours after 0, 1, 3, or 6 times of TPA treatment, skin sections were stained with anti-FSP1 for fibroblasts and with anti-Ki-67 for cell proliferation. Arrows indicate proliferating fibroblasts that are double positive for both FSP1 and Ki-67. Each group contained four mice, and representative immunofluorescent images are shown. Epidermis and dermis boundary is marked by dotted line. The mean numbers of FSP1+ cells, Ki-67+ cells, and FSP1+/Ki-67+ cells, per HPF (×400) are shown at the bottom. *P < 0.05 between the DMBA/TPA-treated group and the untreated group. B: BALB/c mice were treated with DMBA and TPA as above. Twenty-four hours after three times of TPA treatment, skin sections were double-stained with anti-α-SMA (red) and anti-FSP1 (green). The merged image shows that FSP1+ cells hardly express α-SMA. C: Skin sections after 0, 1, 3, or 6 times of TPA treatment were stained with anti-α-SMA and representative immunofluorescent images are shown.
Abatement of Proliferating Fibroblasts Reduced Skin Tumor Development
The proliferating FSP1+ fibroblasts in the skin were selectively depleted during the promotion stage by applying GCV locally. FSP-TK mice and wild-type control littermates were treated with DMBA/TPA and then either with GCV in acetone or acetone alone as control, for 15 weeks as illustrated in Figure 2A. As anticipated, the number of FSP1+ fibroblasts in the skin decreased dramatically in FSP-TK mice after GCV treatment for 3 weeks, while this did not occur in control littermates (Figure 2B). Importantly, the abatement of proliferating fibroblasts led to a significant delay of papilloma development in FSP-TK mice (Figure 2C). Papilloma appeared 8 weeks after DMBA application without GCV treatment and, at week 15, all mice had developed at least one papilloma. However, in GCV-treated group, the first papilloma appeared at week 10, and only 50% of the mice had developed papilloma at week 15. Furthermore, the average number of papilloma/mouse was significantly fewer at this time point in those mice whose proliferating fibroblasts had been abated (1.3 ± 0.7 vs. 4.1 ± 1.1) (Figure 2D). A typical result is shown in Figure 2E.
Figure 2.
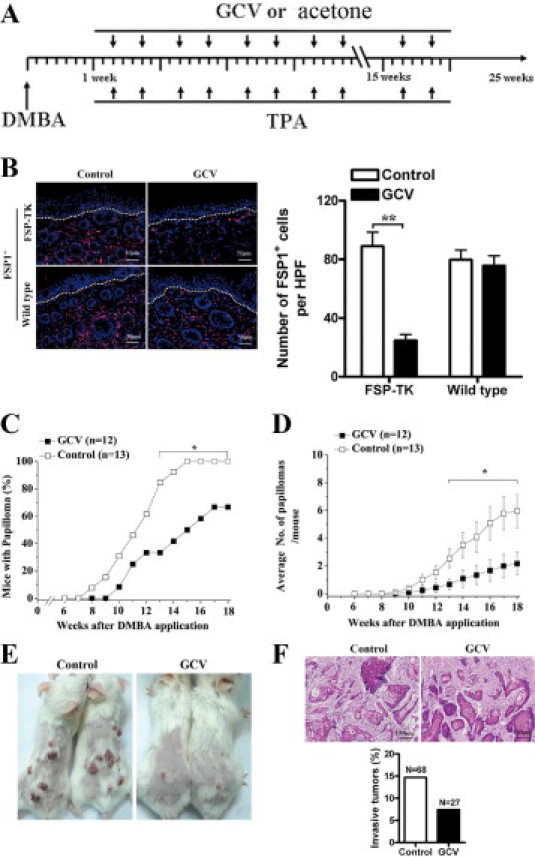
Abatement of proliferating fibroblasts reduced skin tumor development. A: Groups of FSP-TK mice and control littermates were treated at first with DMBA/TPA and then with either GCV in acetone to deplete proliferating fibroblasts or acetone alone as control for 15 weeks as illustrated. B: Groups of FSP-TK mice and control littermates were treated with DMBA/TPA and either acetone alone as control or GCV in acetone. After 3 weeks of GCV treatment, skin sections were stained with anti-FSP1 for fibroblasts (red). As counterstaining, DAPI was used for cell nucleus (blue). Epidermis and dermis boundary is marked by dotted line. The mean numbers of FSP1+ cells per HPF (×200) are shown on the right. *P < 0.05; **P < 0.01. C: Groups of FSP-TK mice were treated as illustrated above, percentages of mice with papilloma as well as (D) average numbers of papillomas per mouse were recorded at different times after the application of DMBA/TPA, *P < 0.05. E: Representative pictures of papillomas developed on the dorsal skin of two pairs of control and GCV-treated FSP-TK mice at week 15. F: Tumors arising in GCV-treated FSP-TK mice exhibited lower incidence of malignant conversion. Top, H&E section of invasive squamous cell carcinomas. Tumors were evaluated as described in Materials and Methods. Bottom, percentages of invasive squamous cell carcinomas in the indicated number of papillomas (N) in control and GCV-treated FSP-TK mice at week 25.
Whereas most tumors were benign papillomas, a fraction of tumors had progressed to carcinomas with clear evidence of local invasion by week 25. As assayed by histological analysis, malignant tumors were squamous cell carcinomas and no apparent morphological differences in tumor structure had been detected between the control and GCV-treated groups (Figure 2F). However, the invasive tumors were rarely found after the abatement of proliferating fibroblasts in FSP-TK mice. In the control group, 14.7% of papillomas developed into invasive squamous cell carcinomas, whereas 7.4% of papillomas in GCV-treated group developed into invasive squamous cell carcinomas (Figure 2F). In wild-type control mice, the incidence, average number, and malignant conversion rate of papillomas with GCV treatment were similar to the group without GCV treatment (see Supplemental Figure S3, http://ajp.amjpathol.org), indicating that the delay of skin tumor development in GCV-treated FSP-TK mice was merely due to the abatement of proliferating fibroblasts rather than the direct effect or indirect effect of GCV. The fact that the abatement of FSP1+ cells at the promotion stage confers mice a significant protection against skin carcinogenesis strongly suggests that proliferating fibroblasts elicit a tumor-promoting function in the DMBA/TPA model.
Epithelial Cell Proliferation and Local Inflammation Were Reduced in the Absence of Proliferating Fibroblasts
Histological examination of papillomas in FSP-TK mice with or without GCV treatment was conducted at week 15 after DMBA/TPA application. As shown in Figure 3A, besides the reduced epidermal thickness, there were less FSP1+ and PCNA+ cells in GCV-treated papillomas than in control papillomas. Actually this difference was observed even at week 3 after DMBA/TPA application. Inflammation-induced epidermal incrassation was significantly impaired in GCV-treated mice (40.8 ± 11.7 μm) compared with that in control mice (96.1 ± 18.1 μm) after receiving six treatments (Figure 3B). The proliferation potential of epidermal cells is another marker of the TPA-induced inflammatory reaction in the skin. Immunohistochemistry analysis by PCNA/CK double staining revealed that there were fewer proliferating keratinocytes in the skin after the abatement of proliferating fibroblasts (Figure 3C).
Figure 3.
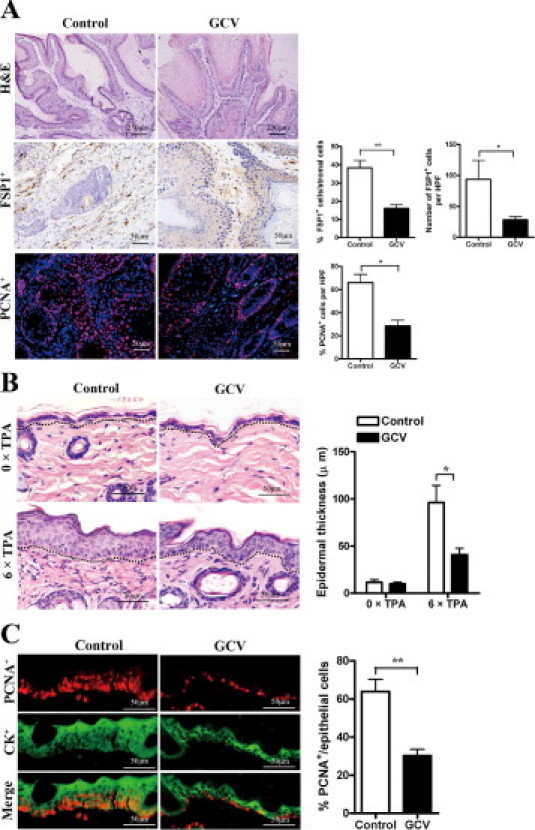
Epithelial cell proliferation and local inflammation were reduced in the absence of proliferating fibroblasts. A: Groups of FSP-TK mice were treated with DMBA/TPA and GCV as above. At week 15, papillomas were sectioned and stained with H&E, anti-FSP1 (brown), and anti-PCNA (red). Percentages of FSP1+ cells in the stroma and the mean numbers of FSP1+ and PCNA+ cells per HPF (×200) are shown on the right. *P < 0.05; **P < 0.01. B: Groups of FSP-TK mice were treated with DMBA/TPA and either with GCV or with acetone as control for 3 weeks (6 × TPA). Skin sections were obtained 24 hours after the last application of TPA and analyzed by H&E-staining (left) and epidermal thickness (right). Epidermis and dermis boundaries are marked by a dotted line. *P < 0.05. C: Groups of FSP-TK mice were treated with DMBA/TPA and GCV as above for 3 weeks. Skin sections were double-stained with anti-PCNA (red) and anti-CK (green). Three mice per group were analyzed, and representative results from indicated groups are shown. The mean percentages of PCNA+ cells in CK+ epithelial cells are shown. **P < 0.01.
Cytokines and chemokines are important mediators of inflammation. To investigate the effect of fibroblast depletion on the local inflammation, we quantified a series of proinflammatory cytokines and chemokines in both the TPA-treated skin tissues with and without GCV treatment. The level of IL-10 and IL-12p70 were below the detection threshold in both groups of mice. There were no significant differences in the levels of IFN-γ, IL-17, and IL-4 between the two groups. However, the concentration of MCP-1, IL-6, and TNF-α decreased significantly with GCV treatment for 3 weeks (Figure 4A). These results indicate the importance of fibroblasts for maintaining the chronic inflammation.
Figure 4.
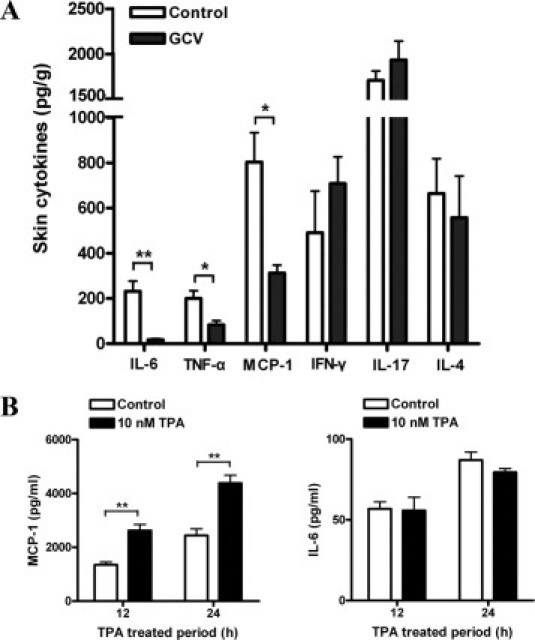
TPA directly stimulated the production of MCP-1 by skin fibroblasts. A: Groups of FSP-TK mice (n = 3) were treated with DMBA/TPA and GCV or acetone for 3 weeks. Twenty-four hours after the last application of TPA, total protein was extracted from skin treated with GCV or acetone. Protein levels of IL-6, TNF-α, MCP-1, IFN-γ, IL-17, and IL-4 were detected. *P < 0.05; **P < 0.01. B: TPA stimulated MCP-1 production by skin fibroblasts. Fibroblasts (1 × 104) isolated from FSP-TK mice were cultured in the absence or presence of 10 nmol/L TPA for the indicated times in serum-free medium, and the concentrations of MCP-1 and IL-6 in the supernatant were measured. Each value represents the mean (±SD) of triplicate measurements. **P < 0.01.
TPA Directly Stimulated the Production of MCP-1 by Skin Fibroblasts
To investigate how fibroblasts influence skin inflammation, we examined the direct effect of TPA on the production of the key proinflammatory cytokines by skin fibroblasts in vitro. The amounts of TNF-α, IL-10, IFN-γ, IL-12p70, IL-4, and IL-17 secreted by skin fibroblasts were below the detection limit either with or without TPA stimulation. And the level of IL-6 secreted by fibroblasts was not influenced significantly by TPA (Figure 4B). Interestingly, skin fibroblasts produced large amounts of MCP-1 and after addition of 10 nmol/L TPA; the secretion of MCP-1 was significantly increased from 1345.3 ± 189.9 to 2622.2 ± 224.1 pg/ml at 12 hours, and from 2443.8 ± 424.6 to 4381.7 ± 297.7 pg/ml at 24 hours (Figure 4B). Similar results were obtained with skin fibroblasts isolated from wild-type control mice (data not shown). Therefore, the secretion of MCP-1 by fibroblasts may play an important role in DMBA/TPA-induced papilloma development, and the decreased level of MCP-1 in skin tissues may result from the abatement of proliferating fibroblasts.
Proliferating Fibroblasts Maintained Macrophage Infiltration During Inflammation
MCP-1 has been reported to be an important chemokine for the recruitment of macrophages.25 We wonder whether the local macrophage accumulation was influenced by the depletion of proliferating fibroblasts. The macrophage infiltration has been subsequently evaluated at different time points both in the presence and absence of proliferating fibroblasts in the DMBA/TPA-treated skin. Indeed, in both papillomas and skin tissues 3 weeks after the exposure of DMBA/TPA, a drastic reduction of macrophage numbers was observed in GCV-treated FSP-TK mice (Figure 5). The difference existed for at least 15 weeks (data not shown). The finding suggests that fibroblasts contribute to the maintaining of chronic inflammation, at least partly, via recruitment of macrophages to the inflammatory tissue.
Figure 5.
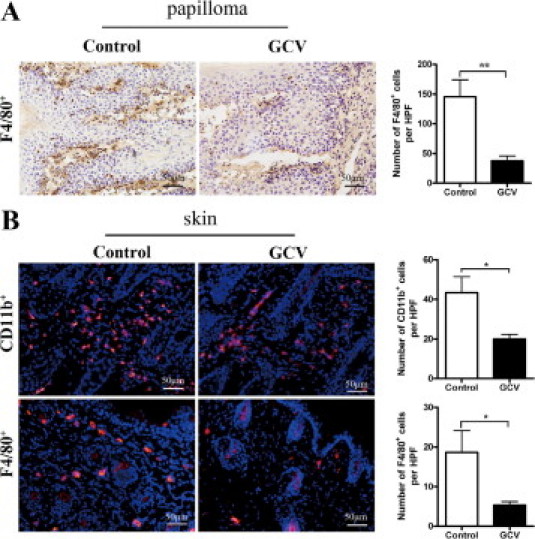
Proliferating fibroblasts maintained macrophage infiltration during inflammation. A: Groups of FSP-TK mice were treated with DMBA/TPA and either with GCV or with acetone. At week 15 after DMBA application, papilloma sections were stained with anti-F4/80 (brown). B: Groups of FSP-TK mice were treated with DMBA/TPA and either with GCV or with acetone for 3 weeks (6 × TPA). Twenty-four hours after the last application of TPA, skin sections were stained with anti-CD11b (red) and anti-F4/80 (red) to detect macrophage infiltration. The mean numbers of CD11b+ and F4/80+ cells per HPF (×200) are shown. *P < 0.05; **P < 0.01.
Skin Fibroblasts Could Recruit Macrophages via Secretion of MCP-1
To confirm that MCP-1 secreted by TPA-treated fibroblasts leads to macrophage recruitment, a transwell assay was performed. Hardly any macrophages migrated toward DMEM (Figure 6A) and TPA (Figure 6B). However, the motility of macrophages to fibroblasts was significantly increased by the TPA treatment of fibroblasts (Figure 6C-D). Furthermore, the increased migration of macrophages could be blocked by neutralization of MCP-1 (Figure 6E), but not IL-6 activity in the culture (Figure 6F). And the mean numbers of macrophages migrated through the filter are shown in Figure 6G.
Figure 6.
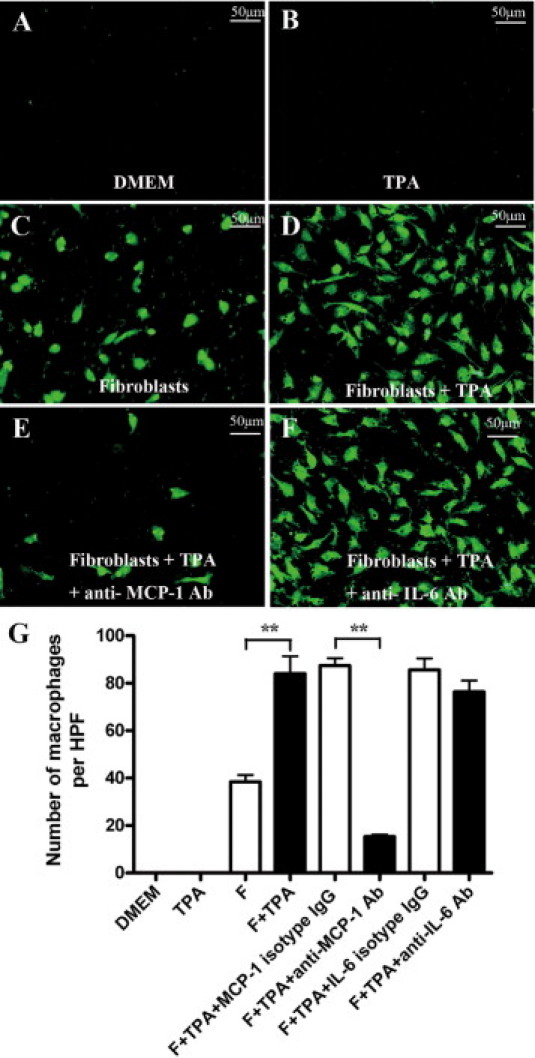
Skin fibroblasts could recruit macrophages via secretion of MCP-1. Chemotactic activity was analyzed to evaluate the role of fibroblasts on the migration of macrophages as described in Materials and Methods. CFSE-labeled macrophages migrated toward DMEM (A), TPA (B), fibroblasts (C), TPA-treated fibroblasts (D), TPA and anti-MCP-1 antibody (Ab)-treated fibroblasts (E), or TPA- and anti-IL-6 Ab-treated fibroblasts (F) are shown. And the mean numbers of cells migrated through the filter per HPF (×200) are shown (G). Each value represents the mean (±SD) of triplicate measurements. **P < 0.01.
Together, these results suggested that fibroblasts enhance inflammatory reaction by production of MCP-1 and promoting macrophage recruitment, which in turn promote cancer development.
Discussion
In this study, we have demonstrated that fibroblasts promote the early stages of DMBA/TPA-induced two-stage skin carcinogenesis by sustaining MCP-1 associated inflammation. DMBA/TPA treatment induced the proliferation of fibroblasts in the skin. The inflammatory reaction was impaired after the selective depletion of proliferating fibroblasts in the skin in FSP-TK transgenic mice. This led to reduced local MCP-1 concentration and impaired macrophage infiltration. As a result of down-regulated inflammation, the incidence and malignant conversion rate of DMBA/TPA-induced papillomas were reduced. However, the direct relationship between MCP-1 derived from FSP1+ fibroblasts and skin carcinogenesis still need further investigation, eg, by conditional knockout of MCP-1 in FSP1+ cells.
The role of fibroblasts in tumors has been investigated intensively recently. Most of the studies have demonstrated that fibroblasts-derived matrix metalloproteinases26 and cytokines such as vascular endothelial growth factor,8 stromal cell–derived factor-1,9 and IL-6,7 enhanced epithelial tumor growth and progression by using tumor transplantation models. However, whether the results are unique for transplanted tumors is not clear. This study is the first report in which abatement of proliferating fibroblasts in vivo was achieved to study the role of fibroblasts in chemical-induced skin carcinogenesis.
Recent studies have revealed the heterogeneity of fibroblasts in different tumor types and tissues, and both α-SMA and FSP1 are usually used to identify fibroblasts.27 Interestingly, in TPA-treated skin tissues, α-SMA+ cells are mainly located around the blood vessels in the skin (Figure 1C and see Supplemental Figure S2, http://ajp.amjpathol.org) and these cells are likely to be vascular smooth muscle cells or pericytes around the vascular walls.28 We double-stained the skin tissue with α-SMA and FSP1 and found that there were seldom α-SMA and FSP1 double positive cells in the TPA-treated skin (Figure 1B), which is similar with the former discovery in the tumor,29 indicating that TPA mainly simulated the proliferation of a unique population of FSP1+ fibroblasts that are regarded as the most important class of fibroblasts present in many tissues.20,30 FSP1 usually serves as a sensitive and specific marker for fibroblasts.31 Recently, using FSP1 as the promoter in Cre-loxp system, Trimboli et al32 described that Pten in stromal fibroblasts suppresses mammary epithelial tumors. And in normal mice, it has been proved that FSP1 is specific for fibroblasts by genetic and protein criteria.18 Also, we have found that no expression of FSP1 in F4/80+ macrophages and CD31+ endothelial cells in TPA-treated mouse skin tissues (see Supplemental Figure S4, http://ajp.amjpathol.org). Therefore, during our experiments, FSP1 was used as the marker for fibroblasts in TPA-treated skin.
Tumor development is a multistep process. In the DMBA/TPA-induced skin carcinogenesis model, tumor development undergoes different stages–initiation, promotion, and progression. Here, for the first time, it was demonstrated that the proliferating fibroblasts accelerated tumor development during the promotion stage. The model was also well suited to analyze the “FSP1+ fibroblasts”-dependency at other stages. And we found that tumor development was not obviously influenced by GCV treatment during the initiation stage, in which fibroblasts largely were not activated (data not shown). However, the role of fibroblasts during progression stage still needs further investigation.
It has been reported that TPA induces substantial cellular changes when applied to mouse epidermis, such as the infiltration of leukocytes and dendritic cells.33 However, whether fibroblasts participate in TPA-induced hyperplasia or not has not been studied. Here we found that fibroblasts could be activated and proliferate to the repeated applications of TPA. FSP1+ fibroblasts increased significantly and were maintained at a relatively high level throughout the promotion stage (Figure 1). Remarkably, FSP1 is also a prototypical marker for cells after epithelial-mesenchymal transition (EMT) during cancer development and fibrogenesis.34 During tissue repair and inflammatory injury, most epithelial cells undergoing type 2 EMT express FSP1 early in transition to fibroblasts.34 By double-staining of E-cadherin and FSP1, a few FSP1+ epithelial cells were indeed detected after repeated applications of TPA (data not shown). Because no evidence showed cancer development when GCV was applied to deplete proliferating fibroblasts in the promotion stage, type 2 EMT may be one source of FSP1+ fibroblasts in this process.35 The results suggest that fibroblasts contribute to the generation and maintenance of an inflammatory microenvironment.
Fibroblasts may maintain local inflammation via secretion of MCP-1, subsequently promoting papilloma development. Previous studies have indicated that activated fibroblasts produce high levels of cytokines including IL-1, IL-6, IL-8, IL-10, and MCP-1,27,36 and primary fibroblasts isolated from breast carcinomas secrete high levels of IL-6 and MCP-1.37 In this study, we found that levels of IL-6, TNF-α, and MCP-1 decreased after the abatement of proliferating fibroblasts (Figure 4A). It has been reported that mice deficient in TNF-α or MCP-1 are resistant to skin carcinogenesis.38,39 However, there are no significant differences in DMBA/TPA-induced papillomas between IL-6+/+ and IL-6−/− C57/BL6 mice.40 In the current study, TPA stimulated MCP-1 secretion by skin fibroblasts in vitro more effectively, rather than TNF-α and IL-6 (Figure 4B), suggesting that the secretion of MCP-1 by fibroblast might play a role in skin tumor development. Therefore, the activation of an inflammatory tumor microenvironment may depend at least partly on fibroblasts. In agreement with this finding, Mueller et al41 recently reported that stromal fibroblasts could generate an inflammatory microenvironment to promote colorectal liver metastases. In addition, recently Erez et al42 demonstrated that cancer-associated fibroblasts mediate tumor-enhancing inflammation in a nuclear factor-κB–dependent manner.
MCP-1 is one of the key factors involved in the infiltration of inflammatory cells. Its expression has been observed in multiple inflammatory diseases,43–45 and in many types of tumors.46 It has been found that tumor stromal cells can express a high level of MCP-1 in prostate carcinomas,47 ovarian cancers,48 and invasive cervix carcinomas.49 MCP-1 is important in inflammation-associated cancer because of a chemoattractant effect on monocytes and macrophages.25 Here, we found that the migration of macrophages in response to TPA-treated fibroblasts increased and inhibition of MCP-1 by neutralizing antibodies reduced the migration of macrophages in vitro (Figure 6). These results suggest that MCP-1 promotes skin tumor development mainly by recruitment of macrophages and elevation of inflammation, which is consistent with reduced papilloma development in MCP-1−/− mice.39 It should be noted that MCP-1 can be secreted by different cell types, such as fibroblasts,50 macrophages,51,52 endothelial cells,53 and epithelial cells.54 In our experiments, the decreased level of MCP-1 in the skin on depletion of FSP1+ cells and the enhanced ability to produce MCP-1 after TPA stimulation by fibroblasts indicate that MCP-1 secreted by fibroblasts is sufficient to maintain DMBA/TPA-induced skin inflammation. Whether MCP-1 secreted by other cell types also plays a role certainly needs further investigation.
In conclusion, our results demonstrate that fibroblasts directly participate in skin carcinogenesis, at least partly, by producing MCP-1 and maintaining local inflammation. This provides direct evidence and a novel explanation for how fibroblasts influence skin carcinogenesis. Fibroblast engagement may provide a promising strategy for treatment of inflammation-associated cancer.
Acknowledgments
We thank Dr. Eric G. Neilson for generously providing us with FSP-TK mice and anti-FSP1 rabbit polyclonal antibody; Dr. Thomas Blankenstein for his continuous help; and Jun Wan, Zibing Wang, Zhiguang Li, Xueqiang Zhao, Yu Lu, and Xiaoyan Chang for technical assistance and discussion.
Footnotes
Supported by the National Natural Science Foundation of China (81030049, 30700287 and 31071261) and the Ministry of Science and Technology of China (2009CB918901).
J.Z and L.C. contributed equally to this work.
None of the authors disclosed any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2010.11.017.
Supplementary data
Skin carcinogenesis promoted proliferation of local FSP1+ fibroblasts. Groups of BALB/c mice were treated with DMBA and then with TPA, twice a week, as described in Materials and Methods. Twenty-four hours after 0, 1, 3, or 6 times of TPA treatment, skin sections were stained with anti-FSP1 for fibroblasts and with anti-PCNA for cell proliferation. Arrows indicate proliferating fibroblasts that are double positive for both FSP1 and PCNA. Epidermis and dermis boundaries are marked by a dotted line. Each group contained four mice, and representative immunofluorescent images are shown.
α-SMA + cells distributed mainly around the blood vessels in TPA-treated mouse skin tissues. FSP-TK mice (n = 4) were treated with DMBA and then with TPA as described in Materials and Methods. Twenty-four hours after three times of TPA treatment, skin frozen sections were double-stained with anti-CD31 (red) and anti-α-SMA (green). Representative immunofluorescent images are shown.
Tumor development in wild type littermates with GCV treatment was similar to those without GCV treatment. Groups of wild type control littermates were treated with DMBA/TPA and then with either GCV in acetone (■, n = 12) or acetone alone (□, n = 12) for 15 weeks as illustrated in Figure 2A. Shown are percentages of mice with papillomas (A) and average numbers of papillomas per mouse (B). C: Histopathological examinations were done on the indicated numbers (N) of papillomas from each group. Invasive squamous cell carcinomas were evaluated as described in Materials and Methods. Shown are percentages of invasive squamous cell carcinomas in the indicated number of papillomas (N) in control littermates at week 25.
No expression of FSP1 in F4/80+ macrophages and CD31+ endothelial cells in TPA-treated mouse skin tissues. FSP-TK mice (n = 4) were treated with DMBA and then with TPA as described in Materials and Methods. Twenty-four hours after three times of TPA treatment, skin frozen sections were double-stained with anti-F4/80 for macrophages (red) and anti-FSP1 for fibroblasts (green) (A) or with anti-CD31 for endothelial cells (red) and anti-FSP1 for fibroblasts (green) (B). Representative immunofluorescent images are shown.
References
- 1.Ronnov-Jessen L., Petersen O.W., Bissell M.J. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 2.van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988;50:159–196. doi: 10.1016/s0065-230x(08)60437-6. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P., Goldstraw P., Yamada K., Nicholson A.G., Wells A.U., Hansell D.M., Dubois R.M., Ladas G. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg. 2003;125:1321–1327. doi: 10.1016/s0022-5223(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 4.Farazi P.A., DePinho R.A. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 5.Sappino A.P., Skalli O., Jackson B., Schurch W., Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmick N.A., Neilson E.G., Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studebaker A.W., Storci G., Werbeck J.L., Sansone P., Sasser A.K., Tavolari S., Huang T., Chan M.W., Marini F.C., Rosol T.J., Bonafe M., Hall B.M. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68:9087–9095. doi: 10.1158/0008-5472.CAN-08-0400. [DOI] [PubMed] [Google Scholar]
- 8.Fukumura D., Xavier R., Sugiura T., Chen Y., Park E.C., Lu N., Selig M., Nielsen G., Taksir T., Jain R.K., Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 9.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Lederle W., Hartenstein B., Meides A., Kunzelmann H., Werb Z., Angel P., Mueller M.M. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31:1175–1184. doi: 10.1093/carcin/bgp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apte R.N., Dotan S., Elkabets M., White M.R., Reich E., Carmi Y., Song X., Dvozkin T., Krelin Y., Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 12.Augsten M., Hagglof C., Olsson E., Stolz C., Tsagozis P., Levchenko T., Frederick M.J., Borg A., Micke P., Egevad L., Ostman A. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci USA. 2009;106:3414–3419. doi: 10.1073/pnas.0813144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp C.J. Multistep skin cancer in mice as a model to study the evolution of cancer cells. Semin Cancer Biol. 2005;15:460–473. doi: 10.1016/j.semcancer.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 15.Miller S.J., Lavker R.M., Sun T.T. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756:25–52. doi: 10.1016/j.bbcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Zaenker K.S. Infection, inflammation and neoplasia: an interdisciplinary challenge. Contrib Microbiol. 2006;13:232–239. doi: 10.1159/000092977. [DOI] [PubMed] [Google Scholar]
- 17.Hanada T., Kobayashi T., Chinen T., Saeki K., Takaki H., Koga K., Minoda Y., Sanada T., Yoshioka T., Mimata H., Kato S., Yoshimura A. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med. 2006;203:1391–1397. doi: 10.1084/jem.20060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwano M., Fischer A., Okada H., Plieth D., Xue C., Danoff T.M., Neilson E.G. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther. 2001;3:149–159. doi: 10.1006/mthe.2000.0251. [DOI] [PubMed] [Google Scholar]
- 19.Okada H., Inoue T., Kanno Y., Kobayashi T., Watanabe Y., Ban S., Neilson E.G., Suzuki H. Selective depletion of fibroblasts preserves morphology and the functional integrity of peritoneum in transgenic mice with peritoneal fibrosing syndrome. Kidney Int. 2003;64:1722–1732. doi: 10.1046/j.1523-1755.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 20.Okada H., Danoff T.M., Fischer A., Lopez-Guisa J.M., Strutz F., Neilson E.G. Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. Am J Physiol. 1998;275:F306-–314. doi: 10.1152/ajprenal.1998.275.2.F306. [DOI] [PubMed] [Google Scholar]
- 21.Salomon B., Maury S., Loubiere L., Caruso M., Onclercq R., Klatzmann D. A truncated herpes simplex virus thymidine kinase phosphorylates thymidine and nucleoside analogs and does not cause sterility in transgenic mice. Mol Cell Biol. 1995;15:5322–5328. doi: 10.1128/mcb.15.10.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeran S.M., Vaid M., Punathil T., Katiyar S.K. Dietary grape seed proanthocyanidins inhibit 12-O-tetradecanoyl phorbol-13-acetate-caused skin tumor promotion in 7,12-dimethylbenz[a]anthracene-initiated mouse skin, which is associated with the inhibition of inflammatory responses. Carcinogenesis. 2009;30:520–528. doi: 10.1093/carcin/bgp019. [DOI] [PubMed] [Google Scholar]
- 23.Xiao M., Wang C., Zhang J., Li Z., Zhao X., Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69:2010–2017. doi: 10.1158/0008-5472.CAN-08-3479. [DOI] [PubMed] [Google Scholar]
- 24.Steinhauser M.L., Kunkel S.L., Hogaboam C.M., Evanoff H., Strieter R.M., Lukacs N.W. Macrophage/fibroblast coculture induces macrophage inflammatory protein-1alpha production mediated by intercellular adhesion molecule-1 and oxygen radicals. J Leukoc Biol. 1998;64:636–641. doi: 10.1002/jlb.64.5.636. [DOI] [PubMed] [Google Scholar]
- 25.Allavena P., Sica A., Solinas G., Porta C., Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Taniwaki K., Fukamachi H., Komori K., Ohtake Y., Nonaka T., Sakamoto T., Shiomi T., Okada Y., Itoh T., Itohara S., Seiki M., Yana I. Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice. Cancer Res. 2007;67:4311–4319. doi: 10.1158/0008-5472.CAN-06-4761. [DOI] [PubMed] [Google Scholar]
- 27.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 28.Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto H., Mundel T.M., Kieran M.W., Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 30.Strutz F., Okada H., Lo C.W., Danoff T., Carone R.L., Tomaszewski J.E., Neilson E.G. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Hir M., Kaissling B. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;68:2400. doi: 10.1111/j.1523-1755.2005.00704_1.x. author reply 2400–2401. [DOI] [PubMed] [Google Scholar]
- 32.Trimboli A.J., Cantemir-Stone C.Z., Li F., Wallace J.A., Merchant A., Creasap N., Thompson J.C., Caserta E., Wang H., Chong J.L., Naidu S., Wei G., Sharma S.M., Stephens J.A., Fernandez S.A., Gurcan M.N., Weinstein M.B., Barsky S.H., Yee L., Rosol T.J., Stromberg P.C., Robinson M.L., Pepin F., Hallett M., Park M., Ostrowski M.C., Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenback F., Sellakumar A. Squamous metaplasia and respiratory tumors induced by intratracheal installations of 7,12-dimethylbenz(a)-anthracene in Syrian golden hamsters. Eur J Cancer. 1974;10:483–486. doi: 10.1016/0014-2964(74)90070-x. [DOI] [PubMed] [Google Scholar]
- 34.Zeisberg M., Neilson E.G. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostman A., Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Silzle T., Randolph G.J., Kreutz M., Kunz-Schughart L.A. The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int J Cancer. 2004;108:173–180. doi: 10.1002/ijc.11542. [DOI] [PubMed] [Google Scholar]
- 37.Silzle T., Kreutz M., Dobler M.A., Brockhoff G., Knuechel R., Kunz-Schughart L.A. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol. 2003;33:1311–1320. doi: 10.1002/eji.200323057. [DOI] [PubMed] [Google Scholar]
- 38.Suganuma M., Okabe S., Marino M.W., Sakai A., Sueoka E., Fujiki H. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- 39.Moore R.J., Owens D.M., Stamp G., Arnott C., Burke F., East N., Holdsworth H., Turner L., Rollins B., Pasparakis M., Kollias G., Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 40.Suganuma M., Okabe S., Kurusu M., Iida N., Ohshima S., Saeki Y., Kishimoto T., Fujiki H. Discrete roles of cytokines: TNF-alpha, IL-1, IL-6 in tumor promotion and cell transformation. Int J Oncol. 2002;20:131–136. [PubMed] [Google Scholar]
- 41.Mueller L., Goumas F.A., Affeldt M., Sandtner S., Gehling U.M., Brilloff S., Walter J., Karnatz N., Lamszus K., Rogiers X., Broering D.C. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007;171:1608–1618. doi: 10.2353/ajpath.2007.060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erez N, Truitt M, Olson P, Hanahan D: Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB–dependent manner. Cancer Cell 17:135–147 [DOI] [PubMed]
- 43.Taylor P.C., Peters A.M., Paleolog E., Chapman P.T., Elliott M.J., McCloskey R., Feldmann M., Maini R.N. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 44.Van Coillie E., Van Damme J., Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 45.Tucci M., Quatraro C., Frassanito M.A., Silvestris F. Deregulated expression of monocyte chemoattractant protein-1 (MCP-1) in arterial hypertension: role in endothelial inflammation and atheromasia. J Hypertens. 2006;24:1307–1318. doi: 10.1097/01.hjh.0000234111.31239.c3. [DOI] [PubMed] [Google Scholar]
- 46.O'Hayre M., Salanga C.L., Handel T.M., Allen S.J. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 47.Mazzucchelli L., Loetscher P., Kappeler A., Uguccioni M., Baggiolini M., Laissue J.A., Mueller C. Monocyte chemoattractant protein-1 gene expression in prostatic hyperplasia and prostate adenocarcinoma. Am J Pathol. 1996;149:501–509. [PMC free article] [PubMed] [Google Scholar]
- 48.Negus R.P., Stamp G.W., Relf M.G., Burke F., Malik S.T., Bernasconi S., Allavena P., Sozzani S., Mantovani A., Balkwill F.R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95:2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleine-Lowinski K., Gillitzer R., Kuhne-Heid R., Rosl F. Monocyte-chemo-attractant-protein-1 (MCP-1)-gene expression in cervical intra-epithelial neoplasias and cervical carcinomas. Int J Cancer. 1999;82:6–11. doi: 10.1002/(sici)1097-0215(19990702)82:1<6::aid-ijc2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Cochran B.H., Reffel A.C., Stiles C.D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura T., Robinson E.A., Tanaka S., Appella E., Leonard E.J. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989;142:1956–1962. [PubMed] [Google Scholar]
- 52.Yoshimura T., Yuhki N., Moore S.K., Appella E., Lerman M.I., Leonard E.J. Human monocyte chemoattractant protein-1 (MCP-1): Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 53.Cushing S.D., Berliner J.A., Valente A.J., Territo M.C., Navab M., Parhami F., Gerrity R., Schwartz C.J., Fogelman A.M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Standiford T.J., Kunkel S.L., Phan S.H., Rollins B.J., Strieter R.M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–9918. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Skin carcinogenesis promoted proliferation of local FSP1+ fibroblasts. Groups of BALB/c mice were treated with DMBA and then with TPA, twice a week, as described in Materials and Methods. Twenty-four hours after 0, 1, 3, or 6 times of TPA treatment, skin sections were stained with anti-FSP1 for fibroblasts and with anti-PCNA for cell proliferation. Arrows indicate proliferating fibroblasts that are double positive for both FSP1 and PCNA. Epidermis and dermis boundaries are marked by a dotted line. Each group contained four mice, and representative immunofluorescent images are shown.
α-SMA + cells distributed mainly around the blood vessels in TPA-treated mouse skin tissues. FSP-TK mice (n = 4) were treated with DMBA and then with TPA as described in Materials and Methods. Twenty-four hours after three times of TPA treatment, skin frozen sections were double-stained with anti-CD31 (red) and anti-α-SMA (green). Representative immunofluorescent images are shown.
Tumor development in wild type littermates with GCV treatment was similar to those without GCV treatment. Groups of wild type control littermates were treated with DMBA/TPA and then with either GCV in acetone (■, n = 12) or acetone alone (□, n = 12) for 15 weeks as illustrated in Figure 2A. Shown are percentages of mice with papillomas (A) and average numbers of papillomas per mouse (B). C: Histopathological examinations were done on the indicated numbers (N) of papillomas from each group. Invasive squamous cell carcinomas were evaluated as described in Materials and Methods. Shown are percentages of invasive squamous cell carcinomas in the indicated number of papillomas (N) in control littermates at week 25.
No expression of FSP1 in F4/80+ macrophages and CD31+ endothelial cells in TPA-treated mouse skin tissues. FSP-TK mice (n = 4) were treated with DMBA and then with TPA as described in Materials and Methods. Twenty-four hours after three times of TPA treatment, skin frozen sections were double-stained with anti-F4/80 for macrophages (red) and anti-FSP1 for fibroblasts (green) (A) or with anti-CD31 for endothelial cells (red) and anti-FSP1 for fibroblasts (green) (B). Representative immunofluorescent images are shown.


