Abstract
Over one million prostate biopsies are performed in the U.S. every year. A failure to find cancer is not definitive in a significant percentage of patients due to the presence of equivocal structures or continuing clinical suspicion. We have identified gene expression changes in stroma that can detect tumor nearby. We compared gene expression profiles of 13 biopsies containing stroma near tumor and 15 biopsies from volunteers without prostate cancer. About 3800 significant expression changes were found and thereafter filtered using independent expression profiles to eliminate possible age-related genes and genes expressed at detectable levels in tumor cells. A stroma-specific classifier for nearby tumor was constructed based on 114 candidate genes and tested on 364 independent samples, including 243 tumor-bearing samples and 121 non-tumor samples (normal biopsies, normal autopsies, remote stroma, as well as stroma within a few millimeters of tumor). The classifier predicted the tumor status of patients using tumor-free samples with an average accuracy of 97% (sensitivity = 98% and specificity = 88%) whereas classifiers trained with sets of 100 randomly generated genes had no diagnostic value. These results indicate that the prostate cancer microenvironment exhibits reproducible changes useful for categorizing the presence of tumor in patients when a prostate sample is derived from near the tumor but does not contain any recognizable tumor.
Keywords: prostate cancer, adjacent stroma, cancer microenvironment, diagnosis, linear regression model, diagnostic profile, microarray
INTRODUCTION
There are over one million prostate biopsy procedures carried out in the U.S. every year (1). Over 60% are read as negative (2-4). However even the best current methods, including transrectal ultrasound (TRUS) procedures, may miss up to 30% of clinically significant prostate cancers (5). Indeed, about, 20-30% of patients that are negative on initial biopsy are re-biopsied in ~3 to ~12 months (~190,000 patients owing to the presence of prostatic intraepithelial neoplasia (PIN), high-grade prostatic intraepithelial neoplasia (HGPIN), atypical small acinar proliferation (ASAP) or other grounds for clinical suspicion of the presence of tumor (2-4, 6, 7). Many repeat biopsies are found to be adenocarcinoma. For example, 16-23% of HGPIN and up to 59% of ASAP cases prove to be adenocarcinoma upon repeat biopsy (2-4, 8). Patients deferred to repeat biopsy, receive little treatment or guidance during the interim – a period when tumors may continue to progress. Therefore, there is a need for methods that resolve false negative and equivocal cases.
Equivocal and negative biopsies are, by definition, deficient in diagnostic tumor but contain ample stroma. Moreover, stroma near tumor may contain changes in gene expression that are not found in non-tumor samples, which could be the basis for a clinical test. Epithelial cells of prostate cancer infiltrate and propagate in a microenvironment consisting largely of myofibroblast cells as well as inflammatory cells and other supporting cells and structures. It has long been appreciated that this mesenchymal component is not passive but responds to signals from the tumor component and, in turn, alters tumor properties, some of which are essential for tumor growth and progression (9, 10). Indeed, studies of prostate cancer were among the first to demonstrate an important role of the stroma in cancer progression. Mouse model studies showed that survival and growth of immortalized nontumorigenic human prostate epithelial cells as renal subcapsular xenografts required stroma from tumor-bearing prostate (10). Numerous studies have subsequently demonstrated large numbers of gene expression changes at the RNA level specific to the tumor microenvironment of prostate cancer (e.g., (11-18)). Similarly, a variety of protein expression changes have been associated with the microenvironment of prostate cancer. For example, reactive stroma, which is believed to occur in a subset of aggressive tumors, has been shown to correlate with changes in a variety of proteins including FGF2, CTGF, Vimentin, ACTA, COL1A, and Tenascin, some of which have been attributed to epithelial-derived TGFβ (12, 19).
Here, we investigate whether RNA expression changes may be identified that are sufficiently reliable to distinguish normal stroma from stroma near tumor. We have previously developed linear regression method for the identification of cell-type specific expression of RNA from array data of prostate tumor samples (20). The method was validated using immunohistochemistry and using quantitative PCR applied to LCM samples of tumor, stroma, and epithelia of benign prostate hyperplasia for 28 genes involving over 400 measurements (20). Here we have extended this approach to identify differentially expressed genes between normal volunteer prostate biopsy samples versus stroma from near tumors. Over a thousand gene expression changes were observed. A subset of stroma-specific genes was used to derive a classifier of 114 genes which accurately identifies tumor or nontumor status of a large number of independent test cases. The classifier may be useful in the diagnosis of stroma-rich biopsies from patients with equivocal pathology.
MATERIAL AND METHODS
Prostate Cancer Patients Samples and Expression Analysis
Datasets 1 and 2 (Table 1) are based on post-prostatectomy frozen tissue samples obtained by informed consent using IRB-approved and HIPPA-compliant protocols. All tissues, except where noted, were collected at surgery and escorted to pathology for expedited review, dissection, and snap freezing in liquid nitrogen. In addition Dataset 1 contains 27 prostate biopsy specimens obtained as fresh snap-frozen biopsy cores from 18 normal prostates. These samples were obtained from the control untreated subjects of a clinical trial to evaluate the role of Difluoromethylornithine (DFMO) to decrease the prostate size of normal men. Eighteen of these were collected before the treatment period and nine were collected after the treatment period had ended (21). Finally, 13 samples of normal prostate tissue were obtained from the rapid autopsy program of the Sun Health Research Institute (Sun City, AZ) and were frozen within 6 hours of demise.
Table 1.
Datasets used in the study1.
| Data | Platform | Subj. Num. | Array Num. | Array: Tumor/Nontumor/Normal | Reference |
|---|---|---|---|---|---|
| 1 Training + Test | P=87 | 108 | 68/40/0 | ||
| U133Plus2 | B=18 | 27 | 0/0/27 | GSE17951 | |
| A=13 | 13 | 0/0/13 | |||
| 2 | U133A | P=82 | 136 | 65/71/0 | GSE08218 |
| 3 | U133A | P=79 | 79 | 79/0/0 | Unpublished, see (22)2 GSE25136 |
| 4 | U133A | P=44 | 57 | 44/13/0 | E-TABM-26(24)3 |
P = Samples from prostate cancer patients. B = Biopsies from normal donors. A = Prostate donated by rapid autopsy. Datasets 1 and 2 were collected from five participating institutions in San Diego County, CA. Demographic, pathology, and clinical values are individually recorded in shadow charts and maintained in the UCI SPECS consortium database.
Data Set 3 was provided by William L. Gerald (Stephenson et al., ref. 22).
url for the source for down loading Data Set 4.
RNA for expression analysis was prepared directly from frozen tissue following dissection of OCT (optimum cutting temperature compound) blocks with the aid of a cryostat. For expression analysis 50 micrograms (10 micrograms for biopsy tissue) of total RNA samples were processed for hybridization to Affymetrix GeneChips. Expression analysis for all samples for Dataset 1 were assessed using the U133 Plus 2.0 platform, while for Dataset 2 the U133A platform was used. The data has been deposited in the Gene Expression Omnibus (GEO) database with accession numbers GSE17951 (Dataset 1) and GSE8218 (Dataset 2). For Datasets 1 and 2, the distributions for the four principal cell types (tumor epithelial cells, stroma cells, epithelial cells of BPH, and epithelial cells of dilated cystic glands) were estimated by three pathologists (Dataset 1) or four (Dataset 2), whose estimates were averaged as described (20).
Datasets 3 and 4 were independently developed and used as test sets (Table 1). Dataset 3 consists of a series of 79 samples (22, 23) while Dataset 4 (24) is composed of 57 samples from 44 patients, including 13 samples of stroma near tumor and 44 tumor-bearing samples. Expression analysis of the Datasets was determined using the U133A platform.
Manual Microdissection
71 of the tumor-bearing samples of Dataset 2 were manually microdissected to obtain tumor-adjacent stroma which was used for validation of the Diagnostic Classifier. For manual microdissection, the tumor-bearing tissue was embedded in an OCT block then mounted in a cryostat. Frozen sections were stained using hematoxylin and eosin (H and E) to visualize the location of the tumor. A border between tumor and adjacent stroma was marked on the glass slide using a Pilot Ultrafine Point Pen which was used as a guide to locate the border on the OCT-block surface. Then the OCT-embedded block was etched with a single straight cut with a scalpel (~ 1 mm deep) to divide the embedded tissue into a tumor zone and tumor-adjacent stroma. Subsequent cryosections produced two halves at the site of the etched cut and were separately used for H&E staining and examined to confirm their composition. Multiple subsequent frozen sections of the tumor-adjacent stroma half were then pooled and used for RNA preparation and microarray hybridization. A final frozen section was used for H&E staining and examined to confirm that the tumor-adjacent stroma remained free of tumor cells.
Statistical tools implemented in R
The U133 Plus 2.0 platform used for Dataset 1 has about 55,000 probe sets whereas the U133A used for Datasets 2, 3 and 4, contains 22,000 probe sets. Normalization was carried out across multiple datasets using the ~22,000 probe sets in common to all Datasets. First, Dataset 1 was quantile-normalized using the function ‘normalizeQuantiles’ of LIMMA routine (25). Datasets 2 - 4 were then quantile-normalized by referencing normalized Dataset 1 using a modified function ‘REFnormalizeQuantiles’ which was coded by ZJ and is available at the SPECS website (26).
The LIMMA package from Bioconductor was used to detect differentially expressed genes.
Prediction Analysis of Microarray (PAM (27)), implemented in R, was used to develop an expression-based classifier from the training sets and then applied to the test sets without further change.
A multiple linear regression (MLR) model was used to fit gene expression data, and known percent cell-type composition for four cell types to estimate expression coefficients for each cell component (see Supplement for details). Percent cell-type distributions were estimated by three (Dataset 1) or four (Dataset 2) pathologists and exhibited an overall agreement of 4.3% standard deviation for the four estimated cell types. The resulting significantly differentially expressed genes for the comparison of normal prostate biopsies to tumor-bearing prostate tissue were used for development of the diagnostic classifier.
RESULTS
Identification of stroma-derived genes and development of the diagnostic classifier
We hypothesized that stroma within and directly adjacent to prostate cancer epithelial cells exhibits significant RNA expression changes compared to normal prostate stroma. To test this, we developed a three step strategy. First, we identified genes that are differentially expressed between tumor-adjacent stroma and normal stroma. Second, these differences were filtered by removing the age-related genes and removing the genes that are also expressed in tumor cells in order to create a stroma-specific set of differentially expressed genes. Finally, owing to the limiting number of normal biopsies, we repeated steps (1) and (2) using a permutation procedure which greatly enhanced the extraction of information in the normal biopsies. In step (1) Affymetrix gene expression data was acquired from normal frozen biopsies from each of 15 subjects that were judged to be free of cancer by histological examination of the six cores of the volunteer biopsies (21). Data from 13 of these samples (with two held in reserve as explained later) were compared to the gene expression data for 13 tumor-bearing patient cases from Dataset 1 selected with tumor cell content (T) greater than 0% but less than 10% tumor cell content (the average stroma content is ~80%). These criteria ensured that the majority of stroma tissues included from the cancer-positive patients was close to tumor, while T < 10% ensured that the impact from tumor cells is minimal to allow capture of altered expression signals from stroma cells rather than tumor cells. Using a moderated t-test implemented in the LIMMA package of R (25), this comparison yielded 3888 significant expression changes between these two groups with a p value < 0.05. We used a relatively relaxed p value cutoff for the first-step of feature selection to allow more genes to enter subsequent screening steps. The 3888 probe sets were composed of a nearly equal number of up- and down-regulated genes.
There was a substantial difference in age between the normal stroma group (average age = 51.9 years) and the near-tumor stroma group (average age = 60.6 years). In step (2), we compared the overall gene expression of the 13 normal stroma samples used for training versus 13 normal prostate specimens obtained by rapid autopsy (Materials and Methods) with an average age of 82. The comparison revealed 8898 significant expression changes (p value < 0.05). 1678 of these probe sets were also detected in the comparison of normal stroma samples to stroma near tumor. After eliminating all of these potential aging-related genes, the remaining 2210 probe sets consisted of nearly equal numbers of up- and down- regulated genes.
It remained likely that some differential expression in this comparison included expression changes specific to the residual tumor cells or epithelium cells in some samples, rather than changes between two types of stromal cells. To reduce the possibility that epithelial-cell derived expression changes might influence subsequent results, we removed genes that appeared to be expressed in tumor at 10% or more of the expression in stroma. However, even “pure” tumor samples are contaminated with stroma thereby risking the elimination of genes expressed only in stroma. So, identification of genes expressed in tumor was achieved using multiple linear regression (MLR) analysis (described in Materials and Methods and Supplement). The percent cell composition of 108 samples from 87 patients in Dataset 1 intentionally encompassing a wide range of tissue percentages was determined by a panel of three pathologists (20). The distribution is shown in Figure 1(a). Model diagnostics showed that the fitted model for genes significantly expressed in tumor or stroma accounted for > 70% of the total variation (i.e., the variation of error, e in Equation 1, was < 30% of the total variation), indicating a plausible modeling scheme.
Figure 1.
Histogram of tumor percentage for Datasets 1 – 4. The tumor percentage data of (a) and (b) were provided by SPECS pathologists, while the tumor percentage data of (c) and (d) were estimated by CellPred program (29). The stars in (a) mark the tumor percentages of the misclassified tumor-bearing cases in Dataset 1, which CellPred indicates may actually be non-tumor samples.
Of 2210 probe sets, derived above, we obtained 160 probe sets that were predominantly expressed in stroma cells and also show differential expression between near-tumor stroma and normal stroma. The average expression of these 160 probe sets was estimated to be more than twofold greater than the average of all genes expressed in stroma, which is a consequence for the filtering steps for robustness, and also favors good sensitivity.
Finally in step (3), a permutation analysis was performed. The above procedure for the generation of differentially expressed genes between 13 of the 15 normal stroma biopsies and the 13 biopsies of stroma near tumor was repeated using a different selection of 13 biopsy samples from 15, until all 105 possible combinations of 13 normal biopsy samples drawn from 15 (, where is the number of combinations of m elements chosen from a total of n elements) was complete. After filtering for genes associated with aging (discussed earlier), a total of 339 probe sets that were differentially expressed between stroma near tumor and normal stroma were generated by the 105-fold gene selection procedure (the frequency of selection is summarized in Figure S1). Thus, the permutation increased the basis set by 339/160 or over 2-fold. 146 probe sets with at least 50 occurrences in the 105-fold permutation were selected for classifier construction (listed in Table 3).
Table 3.
146 diagnostic probe sets with incidence number greater than 50 for 105-fold gene selection procedure.
| Probe set | Gene symbol | Gene title | LogFC# |
|---|---|---|---|
| 213764_s_at | MFAP5 | microfibrillar associated protein 5 | -1.73 |
| 209758_s_at | MFAP5 | microfibrillar associated protein 5 | -1.48 |
| 213765_at | MFAP5 | microfibrillar associated protein 5 | -1.36 |
| 210280_at | MPZ | myelin protein zero (Charcot-Marie-Tooth neuropathy 1B) | -1.20 |
| 210198_s_at | PLP1 | proteolipid protein 1 (Pelizaeus-Merzbacher disease, spastic paraplegia 2, uncomplicated) | -1.18 |
| 215104_at | NRIP2 | nuclear receptor interacting protein 2 | -0.94 |
| 213847_at | PRPH | peripherin | -0.93 |
| 214767_s_at | HSPB6 | heat shock protein, alpha-crystallin-related, B6 | -0.88 |
| 209843_s_at | SOX10 | SRY (sex determining region Y)-box 10 | -0.61 |
| 209686_at | S100B | S100 calcium binding protein B | -0.94 |
| 209915_s_at | NRXN1 | neurexin 1 | -0.80 |
| 214023_x_at | TUBB2B | tubulin, beta 2B | -0.75 |
| 214954_at | SUSD5 | sushi domain containing 5 | -0.98 |
| 204584_at | L1CAM | L1 cell adhesion molecule | -1.20 |
| 204777_s_at | MAL | mal, T-cell differentiation protein | -0.99 |
| 205132_at | ACTC1 | actin, alpha, cardiac muscle 1 | -0.99 |
| 203151_at | MAP1A | microtubule-associated protein 1A | -0.69 |
| 210869_s_at | MCAM | melanoma cell adhesion molecule | -0.71 |
| 204627_s_at | ITGB3 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) | -0.82 |
| 209086_x_at | MCAM | melanoma cell adhesion molecule | -0.61 |
| 219314_s_at | ZNF219 | zinc finger protein 219 | -0.51 |
| 221204_s_at | CRTAC1 | cartilage acidic protein 1 | -0.56 |
| 212886_at | CCDC69 | coiled-coil domain containing 69 | -0.59 |
| 210814_at | TRPC3 | transient receptor potential cation channel, subfamily C, member 3 | -0.75 |
| 212793_at | DAAM2 | dishevelled associated activator of morphogenesis 2 | -0.56 |
| 212565_at | STK38L | serine/threonine kinase 38 like | -0.58 |
| 214606_at | TSPAN2 | tetraspanin 2 | -0.54 |
| 336_at | TBXA2R | thromboxane A2 receptor | -0.65 |
| 218660_at | DYSF | dysferlin, limb girdle muscular dystrophy 2B (autosomal recessive) | -0.55 |
| 214434_at | HSPA12A | heat shock 70kDa protein 12A | -0.57 |
| 212274_at | LPIN1 | lipin 1 | -0.48 |
| 206874_s_at | --- | --- | -0.44 |
| 203939_at | NT5E | 5'-nucleotidase, ecto (CD73) | -0.49 |
| 205954_at | RXRG | retinoid X receptor, gamma | -0.53 |
| 219909_at | MMP28 | matrix metallopeptidase 28 | -0.54 |
| 206425_s_at | TRPC3 | transient receptor potential cation channel, subfamily C, member 3 | -0.57 |
| 205433_at | BCHE | butyrylcholinesterase | -0.93 |
| 35846_at | THRA | thyroid hormone receptor, alpha (erythroblastic leukemia viral (v-erb-a) oncogene homolog, avian) | -0.46 |
| 204736_s_at | CSPG4 | chondroitin sulfate proteoglycan 4 | -0.55 |
| 202806_at | DBN1 | drebrin 1 | -0.43 |
| 212097_at | CAV1 | caveolin 1, caveolae protein, 22kDa | -0.38 |
| 201841_s_at | HSPB1 | heat shock 27kDa protein 1 | -0.44 |
| 206382_s_at | BDNF | brain-derived neurotrophic factor | -0.62 |
| 219091_s_at | MMRN2 | multimerin 2 | -0.44 |
| 205076_s_at | MTMR11 | myotubularin related protein 11 | -0.57 |
| 204159_at | CDKN2C | cyclin-dependent kinase inhibitor 2C (p18, inhibits CDK4) | -0.46 |
| 212992_at | AHNAK2 | AHNAK nucleoprotein 2 | -0.60 |
| 206024_at | HPD | 4-hydroxyphenylpyruvate dioxygenase | -0.57 |
| 218094_s_at | DBNDD2 /// SYS1-DBNDD2 | dysbindin (dystrobrevin binding protein 1) domain containing 2 /// SYS1-DBNDD2 | -0.41 |
| 211276_at | TCEAL2 | transcription elongation factor A (SII)-like 2 | -0.52 |
| 209191_at | TUBB6 | tubulin, beta 6 | -0.51 |
| 213675_at | --- | CDNA FLJ25106 fis, clone CBR01467 | -0.44 |
| 211340_s_at | MCAM | melanoma cell adhesion molecule | -0.46 |
| 210632_s_at | SGCA | sarcoglycan, alpha (50kDa dystrophin-associated glycoprotein) | -0.58 |
| 218651_s_at | LARP6 | La ribonucleoprotein domain family, member 6 | -0.34 |
| 207876_s_at | FLNC | filamin C, gamma (actin binding protein 280) | -0.45 |
| 218877_s_at | TRMT11 | tRNA methyltransferase 11 homolog (S. cerevisiae) | +0.44 |
| 219416_at | SCARA3 | scavenger receptor class A, member 3 | -0.57 |
| 209981_at | CSDC2 | cold shock domain containing C2, RNA binding | -0.56 |
| 214212_x_at | FERMT2 | fermitin family homolog 2 (Drosophila) | -0.42 |
| 207554_x_at | TBXA2R | thromboxane A2 receptor | -0.44 |
| 205231_s_at | EPM2A | epilepsy, progressive myoclonus type 2A, Lafora disease (laforin) | -0.42 |
| 215306_at | --- | MRNA; cDNA DKFZp586N2020 (from clone DKFZp586N2020) | -0.48 |
| 218435_at | DNAJC15 | DnaJ (Hsp40) homolog, subfamily C, member 15 | -0.49 |
| 203597_s_at | WBP4 | WW domain binding protein 4 (formin binding protein 21) | -0.34 |
| 205303_at | KCNJ8 | potassium inwardly-rectifying channel, subfamily J, member 8 | -0.42 |
| 201389_at | ITGA5 | integrin, alpha 5 (fibronectin receptor, alpha polypeptide) | -0.50 |
| 204940_at | PLN | phospholamban | -0.49 |
| 220765_s_at | LIMS2 | LIM and senescent cell antigen-like domains 2 | -0.41 |
| 203299_s_at | AP1S2 | adaptor-related protein complex 1, sigma 2 subunit | -0.41 |
| 201344_at | UBE2D2 | ubiquitin-conjugating enzyme E2D 2 (UBC4/5 homolog, yeast) | -0.38 |
| 218648_at | CRTC3 | CREB regulated transcription coactivator 3 | -0.33 |
| 204939_s_at | PLN | phospholamban | -0.45 |
| 201431_s_at | DPYSL3 | dihydropyrimidinase-like 3 | -0.40 |
| 215534_at | --- | MRNA; cDNA DKFZp586C1923 (from clone DKFZp586C1923) | -0.46 |
| 209169_at | GPM6B | glycoprotein M6B | -0.34 |
| 209651_at | TGFB1I1 | transforming growth factor beta 1 induced transcript 1 | -0.42 |
| 218711_s_at | SDPR | serum deprivation response (phosphatidylserine binding protein) | +0.41 |
| 212358_at | CLIP3 | CAP-GLY domain containing linker protein 3 | -0.47 |
| 218691_s_at | PDLIM4 | PDZ and LIM domain 4 | -0.42 |
| 218266_s_at | FREQ | frequenin homolog (Drosophila) | -0.46 |
| 210319_x_at | MSX2 | msh homeobox 2 | +0.45 |
| 218545_at | CCDC91 | coiled-coil domain containing 91 | -0.31 |
| 44702_at | SYDE1 | synapse defective 1, Rho GTPase, homolog 1 (C. elegans) | -0.38 |
| 221014_s_at | RAB33B | RAB33B, member RAS oncogene family | -0.38 |
| 221246_x_at | TNS1 | tensin 1 | -0.27 |
| 208789_at | PTRF | polymerase I and transcript release factor | -0.42 |
| 220722_s_at | SLC5A7 | solute carrier family 5 (choline transporter), member 7 | -0.41 |
| 209087_x_at | MCAM | melanoma cell adhesion molecule | -0.40 |
| 221667_s_at | HSPB8 | heat shock 22kDa protein 8 | -0.40 |
| 205561_at | KCTD17 | potassium channel tetramerisation domain containing 17 | -0.32 |
| 213808_at | --- | Clone 23688 mRNA sequence | -0.43 |
| 202565_s_at | SVIL | supervillin | -0.36 |
| 211964_at | COL4A2 | collagen, type IV, alpha 2 | -0.39 |
| 219563_at | C14orf139 | chromosome 14 open reading frame 139 | -0.38 |
| 214122_at | PDLIM7 | PDZ and LIM domain 7 (enigma) | -0.30 |
| 212589_at | RRAS2 | related RAS viral (r-ras) oncogene homolog 2 | -0.29 |
| 205973_at | FEZ1 | fasciculation and elongation protein zeta 1 (zygin I) | -0.35 |
| 218818_at | FHL3 | four and a half LIM domains 3 | -0.36 |
| 212120_at | RHOQ | ras homolog gene family, member Q | -0.31 |
| 219073_s_at | OSBPL10 | oxysterol binding protein-like 10 | -0.37 |
| 221480_at | HNRNPD | heterogeneous nuclear ribonucleoprotein D (AU-rich element RNA binding protein 1, 37kDa) | -0.36 |
| 207071_s_at | ACO1 | aconitase 1, soluble | -0.27 |
| 211717_at | ANKRD40 | ankyrin repeat domain 40 | -0.28 |
| 201313_at | ENO2 | enolase 2 (gamma, neuronal) | -0.36 |
| 204628_s_at | ITGB3 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) | -0.31 |
| 204303_s_at | KIAA0427 | KIAA0427 | -0.35 |
| 214439_x_at | BIN1 | bridging integrator 1 | -0.29 |
| 209015_s_at | DNAJB6 | DnaJ (Hsp40) homolog, subfamily B, member 6 | -0.29 |
| 213547_at | CAND2 | cullin-associated and neddylation-dissociated 2 (putative) | -0.31 |
| 204058_at | ME1 | malic enzyme 1, NADP(+)-dependent, cytosolic | -0.34 |
| 219902_at | BHMT2 | betaine-homocysteine methyltransferase 2 | -0.33 |
| 214306_at | OPA1 | optic atrophy 1 (autosomal dominant) | -0.27 |
| 210201_x_at | BIN1 | bridging integrator 1 | -0.29 |
| 212509_s_at | MXRA7 | matrix-remodelling associated 7 | -0.27 |
| 213231_at | DMWD | dystrophia myotonica, WD repeat containing | -0.30 |
| 201843_s_at | EFEMP1 | EGF-containing fibulin-like extracellular matrix protein 1 | -0.32 |
| 206289_at | HOXA4 | homeobox A4 | -0.29 |
| 203501_at | PGCP | plasma glutamate carboxypeptidase | -0.30 |
| 216894_x_at | CDKN1C | cyclin-dependent kinase inhibitor 1C (p57, Kip2) | -0.27 |
| 216500_at | --- | HL14 gene encoding beta-galactoside-binding lectin, 3' end, clone 2 | -0.29 |
| 220050_at | C9orf9 | chromosome 9 open reading frame 9 | -0.32 |
| 209362_at | MED21 | mediator complex subunit 21 | -0.26 |
| 202931_x_at | BIN1 | bridging integrator 1 | -0.27 |
| 213480_at | VAMP4 | vesicle-associated membrane protein 4 | -0.24 |
| 205611_at | TNFSF12 | tumor necrosis factor (ligand) superfamily, member 12 | -0.29 |
| 204365_s_at | REEP1 | receptor accessory protein 1 | -0.29 |
| 203389_at | KIF3C | kinesin family member 3C | -0.26 |
| 205368_at | FAM131B | family with sequence similarity 131, member B | -0.27 |
| 217066_s_at | DMPK | dystrophia myotonica-protein kinase | -0.29 |
| 212457_at | TFE3 | transcription factor binding to IGHM enhancer 3 | -0.25 |
| 200685_at | SFRS11 | splicing factor, arginine/serine-rich 11 | -0.16 |
| 200788_s_at | PEA15 | phosphoprotein enriched in astrocytes 15 | -0.22 |
| 202522_at | PITPNB | phosphatidylinositol transfer protein, beta | -0.16 |
| 208869_s_at | GABARAPL1 | GABA(A) receptor-associated protein like 1 | -0.19 |
| 209524_at | HDGFRP3 | hepatoma-derived growth factor, related protein 3 | -0.14 |
| 211347_at | CDC14B | CDC14 cell division cycle 14 homolog B (S. cerevisiae) | -0.21 |
| 211677_x_at | CADM3 | cell adhesion molecule 3 | -0.21 |
| 212610_at | PTPN11 | protein tyrosine phosphatase, non-receptor type 11 (Noonan syndrome 1) | -0.23 |
| 212848_s_at | C9orf3 | chromosome 9 open reading frame 3 | -0.27 |
| 214643_x_at | BIN1 | bridging integrator 1 | -0.23 |
| 217820_s_at | ENAH | enabled homolog (Drosophila) | -0.19 |
| 218597_s_at | CISD1 | CDGSH iron sulfur domain 1 | -0.18 |
| 221502_at | KPNA3 | karyopherin alpha 3 (importin alpha 4) | -0.20 |
| 222221_x_at | EHD1 | EH-domain containing 1 | -0.20 |
| 32625_at | NPR1 | natriuretic peptide receptor A/guanylate cyclase A (atrionatriuretic peptide receptor A) | -0.22 |
logFC is the logarithm Fold Change as tumorous stroma being compared to normal stroma. +/- represents up-/down- regulated expression level in tumorous stroma.
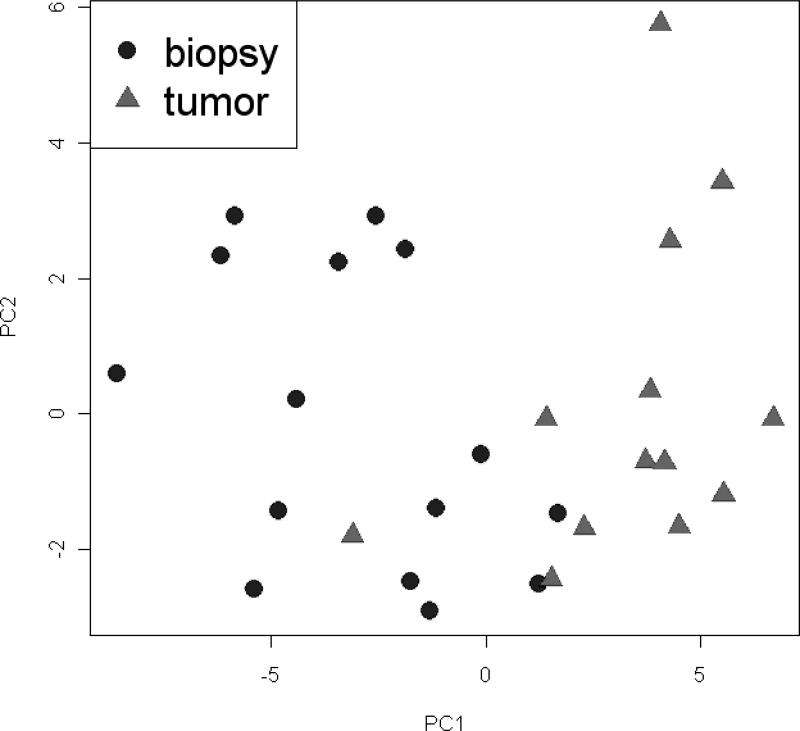
Prediction Analysis for Microarrays (PAM) (28) was used to build a diagnostic classifier. The training set (Table 2, line 1) included all the 15 normal biopsies and the initial 13 samples of stroma near tumor. Of the 146 PAM-input probe sets, 131 probe sets – corresponding to 114 genes - were retained following the 10-fold cross validation procedure of PAM (Prediction Analysis of Microarrays (28)) leading to a prediction accuracy of 96% (Table 2). Figure S2 presents a “heatmap” of the relative expression of the 131 probe sets among all training samples. The separation of normal and near-tumor stroma samples of the training set by the classifier is illustrated by the two distinct populations shown in Figure 2.
Table 2.
Operating characteristics (OC) for training and testing.
| Dataset | Sample Number | Accuracy % | ||
|---|---|---|---|---|
| 1 | Training set | 1 | 28 (15 + 13) | 96.4 |
| Test set | ||||
| Tumor | ||||
| 2 | Tumor-bearing | 1 | 551 | 96.4 |
| 3 | Tumor-bearing | 2 | 65 | 100 |
| 4 | Tumor-bearing | 3 | 79 | 100 |
| 5 | Tumor-bearing | 4 | 44 | 100 |
| Normal | ||||
| 6 | Biopsies (1) | 1 | 7 | 100 |
| 7 | Biopsies (2) | 1 | 5 | 60 |
| 8 | Rapid autopsies | 1 | 13 | 92.3 |
| Microdissected | ||||
| 9 | Stroma adjacent to tumor | 2 | 71 | 97.1 |
| 10 | Stroma adjacent to tumor | 4 | 13 | 100 |
| 11 | Stroma close to tumor | 1 | 12 | 75 |
| 12 | Stroma > 15 mm from tumor | 1 | 28 | 35.7 |
55 test samples is less than the potential 68 of Table 1 owing to the use of 15 samples for training (line 1).
Figure 2.
Plot of the Principal Component Analysis of training cases using the 131 probe-set Diagnostic Classifier.
Testing with Independent Datasets
The 131-probe set classifier was then tested on 243 samples that had not been used for training, and that all contained tumor, though usually very little tumor (Table 2, lines 2 to 5). Almost all the 243 samples were recognized as being from cancer patients with high average accuracy ~99% (see Table S1 for derived operating characteristics). Only two cases were misclassified. In Figure 1(a) the two misclassified test are marked with “*”. Although these samples are ostensibly given tumor percentages of 20% and 25% by pathologists, they are predicted to possibly contain little or no tumor using the CellPred program which estimates the tissue components using an in silico multiple-variate linear regression model (29). It is possible that these two exceptions were archived incorrectly and are not from patients with cancer or are from a very distant location relative to the tumor.
We examined whether the PAM classification results correlated with cell composition (Figure 1). For the test cases of Datasets 1 and 2 these values are known from the pathologists estimates while for Datasets 3 and 4 (Figure 1(c) and 1(d) respectively) these tumor cell contents were estimated using the CellPRed program (29). Examining the tumor cell percentages in all the samples in Figure 1, it is clear that the PAM classification is successful on independent test samples with a broad range of tumor epithelial cells including samples with just a few percent of epithelial cells. These observations argue that the classifier is accurate in the categorization of prostate cancer cases independent of the presence or amount of the tumor epithelial component.
The classifier was then tested using specimens composed of normal prostate stroma and epithelium. Twelve biopsies from the DMFO study, all of them different from the 15 samples used earlier for training, were separated into two groups. In group 1 were seven second biopsies from the same participants whose first biopsy samples were included in the training set, taken 12 months later. These were accurately (100%) identified as nontumor (Table 2, line 6). In group 2 were five biopsy samples not from subjects previously used for training. Two out of these 5 biopsy samples were categorized as being from cancer patients (Table 2 line 7). When the histories for these volunteers were investigated it was found that both donors had consistently exhibited elevated PSA levels of 6.1 and 8 ng/ml, (normal values < 3 ng/ml) respectively although no tumor was observed in either of two sets of sextant biopsies obtained from these volunteers. The volunteers also had a history of prostate cancer in the family. All other donors of the normal biopsy volunteers exhibited normal PSA values. The IRB-approved protocol precluded following up further to establish that these patients had cancer that had been missed in the biopsies.
The classifier was then tested on 13 specimens obtained by rapid autopsy of individuals dying of unrelated causes (Table 2, line 8). Twelve out of 13 of these samples, 92% accuracy, were classified as nontumor. Histological examination of all embedded tissue of the one “misclassified” case revealed multiple foci of small “latent” tumors.
In summary, 25 nominally normal samples were classified as being from donors without prostate cancer or were classified in accordance with abnormal features that were subsequently uncovered. These results provide further support for the ability of the classifier to discriminate among normal and abnormal prostate tissue in the absence of histological recognizable tumor cells in the samples studied.
Validation by Manual Microdissection, Random Classifiers and the Published Literature
We sought to validate the classifier by developing histological confirmed samples of stroma adjacent to tumor. An etching procedure was used to prepare 71 samples of tumor-adjacent stroma from patient tissues of Dataset 2, and 13 samples from Dataset 4. An additional 12 samples from Dataset 1 were obtained from OCT blocks entirely by manual microdissection, i.e. without etching but leaving a margin of tissue between tumor and stroma, followed by histologically examined by frozen section analysis of the OCT surface and bottom side of the pieces, to insure the absence of tumor. These 12 manually excised pieces are termed “close stroma” (~ 3 mm). The expression values for all 96 samples were used to test the 131 probe set classifier using the PAM procedure. The accuracy in classifying that the samples were from patients with tumor was 97% for the 71 adjacent stroma samples from dataset 2, 100% for 13 adjacent stroma samples from dataset 4, and 75% for the 12 “close” stroma samples from dataset 1 (Table 2, lines 9-11). This is an overall accuracy of 95% for the 96 independent samples.
Five of the 96 samples appeared “misclassified” as normal. Three of these misclassifications were among the 12 “close” stroma samples in dataset 1. These 12 samples were obtained by manual excision and therefore some of the samples may not have been as near to tumor as the samples obtained by the etching method. Therefore, we examined how far the expression changes characteristic of tumor stroma may extend away from the tumor; We obtained 28 samples greater than 15 mm from any known tumor and generally from the contralateral lobe (Table 2, line 12). Only ten of the 28 samples (36%) were categorized as tumor-associated stroma. Using the Fisher Exact Test, the distribution for the 28 “remote” samples was significantly different from the 12 stroma samples from “close” to tumor of the same patient tissues (p value = 0.038). This result, as well as the observation of a gradient of classification frequency values from 98%, 75%, and 36% for samples adjacent, close, and >15 mm from tumor, suggests that the expression changes recognized by the classifier decline with increasing distance of stroma from tumor. Such observation bears on the likely mechanism for the production of differential gene expression in tumor adjacent stroma which is generally believed to involve the influence of “paracrine” factors emanating from tumor foci (10, 30, 31).
We found that the normal samples and rapid autopsy samples can be easily distinguished from samples containing tumor using many of the individual genes (e.g., heatmap, Figure S3). However, the differences that allow near stroma to be distinguished from control stroma are more subtle and vary between patients, requiring a classifier based on a number of genes.
Further validation included a comparison with 100 random classifiers generated by arbitrarily sampling 131 probe sets for each classifier. The results (Table S1 and Supplement) showed that these random classifiers had no diagnostic value, further indicating that the results obtained with the 131-probe set classifier cannot be attributed to chance.
Finally we sought to validate that representative genes were in fact preferentially expressed in stroma by PCR. In addition, to test the translational relevance, we utilized independent cases from a formalin-fixed and paraffin-embedded (FFPE) clinical collection. Gene expression was assessed by a modified quantitative PCR procedure (Materials and Methods). In a limited survey, four genes were found to have reliably preserved short amplicons. Blocks of sixty three tumor cases were examined and tumor and stroma regions in H & E sections were demarcated by a pathologist (DAM). Punches were removed from adjacent unstained sections and used for PCR for 63 tumor portions and 38 stroma portions. For all four genes, highly significant preferential expression in stroma was observed (Table S4). These results for independent cases and by an independent method further support the preferential expression of these genes in tumor stroma and further argue that the classifier may be adapted to clinical biopsies preserved in FFPE, the standard method of archiving patient biopsies.
Finally, we also reviewed two recent studies describing expression analysis results for subclasses of the stroma of prostate cancer (16, 17), which showed consistent findings (see Supplement). In particular the 339 probe sets (Affymetrix arrays) we identified map to 557 genes on Agilent arrays which have been used for deriving profiles for “reactive” stroma, a special case of adjacent stroma associated with poor outcome disease (17). A total of 31 genes or probe sets appeared to be concordant (in terms of gene identity and the direction of expression alteration) between the 339 probe sets (Affymetrix arrays) we identified in this study and the 557 mapped genes (Agilent arrays) in the “reactive” stroma study (17) with P value = 0.0001 (Table S2). The formation of this stroma in prostate cancer has been associated with poor prognosis, suggesting that given that reactive stroma has been associated with poor prognosis (32), it is possible that some diagnostic markers in stroma could also be of prognostic interest.
DISCUSSION
We compared the expression profiles of 15 normal biopsy samples and 13 tumor-adjacent stroma samples from prostatectomies using a permutation strategy to enhance detection of significant differences. About 3800 significant gene expression changes were observed, which were then filtered to exclude genes known to be expressed at similar levels in epithelial tumor cells and to remove genes that change with age. Prostate glands from the rapid autopsy series with an average age of 84 years exhibited a markedly increased heterogeneity of gland shapes with stroma containing increased fibroblast and myofibroblast-like cells. The top ranked 146 probe sets remaining after applying these filters were used for the ten-fold cross-validation procedure of PAM using the same 28 samples used for the initial training. The PAM procedure led to a 131 probe set classifier, which had a training accuracy of 96%. We then tested the classifier on a number of independent expression microarray Datasets of tumor-bearing tissue including data from 110 samples generated by us (Table 2, Datasets 1 & 2) and data from 123 samples generated elsewhere (Table 2, Datasets 3 & 4). These samples were classified as being from cancer patients with an overall accuracy of 98%, a value compares favorably with the diagnostic accuracy of PSA-based methods of ~70% (33). Only two samples recorded as containing tumor cells were misclassified. Upon further investigation of these two samples using CellPred, a method to determine the tumor percentage of samples based solely on their expression profile (29), these samples were predicted to have little or no tumor, although they had been booked as having over 20% tumor, indicating their assignment as tumor may have been a bookkeeping error. Similarly, we generated data from 25 samples of normal prostate, which were recognized as non-tumor with an accuracy of 92%. Only three samples were “misclassified”. Two of these samples were biopsies donated by men with abnormally high PSA levels and a family history of prostate cancer, although no tumor was recognized in any of the sextant biopsies taken at the beginning and end of the study period for which these volunteers were controls. In addition, one sample derived from the rapid autopsy donors was potentially “misclassified” as non-cancerous. Examination of multiple blocks of the glands taken from both lobes and all zones revealed tumor foci in the misclassified case. Thus, the “misclassifications” correlate well with the unusual clinical and pathological features of the cases. In summary, the handful of misclassifications of tumor and normal each had evidence that they had been mislabeled before the test, potentially raising the actual sensitivity and specificity for classifying these samples to 100%.
Finally, for validation we used 153 samples from datasets 1 and 4 to prepare “pure” stroma adjacent, close, and far (>15 mm) from known tumor foci. These datasets were able to detect the presence of tumor in the prostate with a decreasing accuracy of 98%, 75% and 36%, respectively. The observation of a gradual reduction in the sensitivity of the classifier as the distance increases bears on the likely mechanism for the production of differential gene expression in tumor adjacent stroma which is generally believed to involve the influence of “paracrine” factors emanating from tumor foci (10, 30, 31). Indeed the tumor microenvironment is likely the source of factors that are required for tumor formation by the epithelial component (10). The amount of diffusible paracrine factors of this complex interaction mechanism likely declines with separation of target cells from the secreting cells. Indeed a simple radial dilution model would predict a decline of effects of tumor-derived factors by at least the square of the distance of target stroma cells from a tumor focus. Based on this simple model, the decrease in the frequency of categorization stroma taken from over 15 mm from a known tumor focus to 36% suggests a 50% recognition distance of ~ 13 mm in fresh frozen tissue. In view of the modest average fold-change of the 131 probe sets of the classifier (Table 3) the distance at which “presence-of-tumor” is recognized suggests a surprisingly large range of “influence” of tumor over steady state gene expression changes in nearby stroma. Systematic studies of differential expression as a function of known distances will be required to confirm and refine this inference.
The classifier developed here used highly selective methods to enrich for mesodermal and ectodermal derivatives compared to endoderm/epithelial derivatives. Computer assisted gene enrichment analysis classification using DAVID (34) identified a number of statistically significant gene enrichment categories. The 10 most significant are summarized in Table S3. Numerous genes associated with expression in nerve and muscle are apparent, such as the nine genes of the actin cytoskeleton enrichment category, and in the disease mutation category including MPZ (Charcot-Maire-Tooth neuropathy 1b), optic atrophy 1, EPM2a (Lafora Disease), BDGF, PLN (phospholamban), SGCA (dystophin-associated glycoprotein), and EFEMP. Biochemical associations include genes related to the TGFβ pathway (SMAD3, TGFIT, ID4, CKDN1C/p57), the Wnt pathway (FZD7, SMAD3, DAAM1 and WISP2) and interacting genes (PCH12, PCDH7, CDH19). These pathways are associated with tumor-stroma paracrine interactions (16, 17, 32, 35, 36). Given that reactive stroma has been associated with poor prognosis (32), it is possible that some of the 131 diagnostic markers identified in stroma could also be of prognostic interest. Nevertheless, we have not ruled that classifier developed here can distinguish other prostate conditions such as acute and chronic inflammation of the prostate and, therefore, stroma near these lesions may conceivably be misdiagnosed. Additional work with samples containing such lesions could identify genes that distinguish inflammation from cancer.
Our preclinical results suggest practical applications. Assessment of suspicious initial biopsies for expression of the classifier genes were identified here by microarray but could also potentially by any number of other gene quantification methods, including those available for assessment of RNA in FFPE samples. Such quantitation may have use in defining “presence-of-tumor” based solely on the detection of changes in the microenvironment near a focus of tumor by quantitative criteria similar to those used here. Such a method would be applicable to cases with an initial negative biopsies that would otherwise be referred for re-biopsy owing to the presence of ASAP or PIN. The determination of “presence of tumor” may strengthen guidance for neoadjuvant therapy or prevention therapy or an accelerated scheduling of re-biopsy. Finally, because stroma facilitates tumor growth (10) the expression changes that occur in stroma indicating the presence-of-tumor might be targets for therapeutic intervention that could leave normal stroma relatively unaffected.
Supplementary Material
Acknowledgment
Samples of Dataset 1 deposited in GEO (GSE17951) have been used with average cell distributions based in part on readings by David Tarin, M.D, and Linda Wasserman, M.D., Ph.D. We thank Dr. Eileen Adamson for her effort in proofreading the manuscript. This research was supported by the National Institute of Health SPECS Consortium grant U01 CA1148102 and NCI Early Detection Research Network (EDRN) Consortium grant U01 CA152738 and the UCI Faculty Career Development Award to ZJ.
Footnotes
Disclosures: M. McClelland and D. Mercola are cofounders and W. Lernhardt is CEO of Proveri Inc. which is engaged in translational development of aspects of the subject matter.
REFERENCES
- 1.Marks LS, Bostwick DG. Prostate Cancer Specificity of PCA3 Gene Testing: Examples from Clinical Practice. Rev Urol. 2008;10(3):175–81. [PMC free article] [PubMed] [Google Scholar]
- 2.O'Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55(4):553–9. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 3.Che M, Sakr W, Grignon D. Pathologic features the urologist should expect on a prostate biopsy. Urol Oncol. 2003;21(2):153–61. doi: 10.1016/s1078-1439(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 4.Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70(6):1131–5. doi: 10.1016/j.urology.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 5.Andriole GL, Bullock TL, Belani JS, et al. Is there a better way to biopsy the prostate? Prospects for a novel transrectal systematic biopsy approach. Urology. 2007;70(6 Suppl):22–6. doi: 10.1016/j.urology.2007.06.1128. [DOI] [PubMed] [Google Scholar]
- 6.Mian BM, Naya Y, Okihara K, Vakar-Lopez F, Troncoso P, Babaian RJ. Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology. 2002;60(5):836–40. doi: 10.1016/s0090-4295(02)01950-7. [DOI] [PubMed] [Google Scholar]
- 7.Leite KR, Camara-Lopes LH, Cury J, Dall'oglio MF, Sanudo A, Srougi M. Prostate cancer detection at rebiopsy after an initial benign diagnosis: results using sextant extended prostate biopsy. Clinics. 2008;63(3):339–42. doi: 10.1590/S1807-59322008000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin MM, Jeyaganth S, Fahmy N, et al. Subsequent prostate cancer detection in patients with prostatic intraepithelial neoplasia or atypical small acinar proliferation. Can Urol Assoc J. 2007;1(3):245–9. doi: 10.5489/cuaj.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70(9-10):473–85. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107(1):1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 11.Ernst T, Hergenhahn M, Kenzelmann M, et al. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am J Pathol. 2002;160(6):2169–80. doi: 10.1016/S0002-9440(10)61165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–23. [PubMed] [Google Scholar]
- 13.Chandran UR, Dhir R, Ma C, Michalopoulos G, Becich M, Gilbertson J. Differences in gene expression in prostate cancer, normal appearing prostate tissue adjacent to cancer and prostate tissue from cancer free organ donors. BMC Cancer. 2005;5(1):45. doi: 10.1186/1471-2407-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SZ, Dong JH, Li K, Zhang Y, Zhu J. Detection of AFPmRNA and melanoma antigen gene-1mRNA as markers of disseminated hepatocellular carcinoma cells in blood. Hepatobiliary Pancreat Dis Int. 2005;4(2):227–33. [PubMed] [Google Scholar]
- 15.Verona EV, Elkahloun AG, Yang J, Bandyopadhyay A, Yeh IT, Sun LZ. Transforming growth factor-beta signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 2007;67(12):5737–46. doi: 10.1158/0008-5472.CAN-07-0444. [DOI] [PubMed] [Google Scholar]
- 16.Richardson AM, Woodson K, Wang Y, et al. Global expression analysis of prostate cancer-associated stroma and epithelia. Diagn Mol Pathol. 2007;16(4):189–97. doi: 10.1097/PDM.0b013e3180de20ac. [DOI] [PubMed] [Google Scholar]
- 17.Dakhova O, Ozen M, Creighton CJ, et al. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15(12):3979–89. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heul-Nieuwenhuijsen L, Dits N, Van Ijcken W, de Lange D, Jenster G. The FOXF2 pathway in the human prostate stroma. Prostate. 2009 doi: 10.1002/pros.20996. [DOI] [PubMed] [Google Scholar]
- 19.Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65(19):8887–95. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- 20.Stuart RO, Wachsman William, Berry Charles C., Arden Karen, Goodison Steven, Klacansky Igor, McClelland Michael, Wang-Rodriquez Jessica, Wasserman Linda, Sawyers Ann, Yipeng Wang, Kalcheva Iveata, Tarin David, Mercola Dan. In silico dissection of cell-type associated patterns of gene expression in prostate cancer. Proceeding of the National Academy of Sciences USA. 2004;101:615–20. doi: 10.1073/pnas.2536479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simoneau AR, Gerner EW, Nagle R, et al. The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2008;17(2):292–9. doi: 10.1158/1055-9965.EPI-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephenson AJ, Smith A, Kattan MW, et al. Integration of gene expression profiling and clinical variables to predict prostate carcinoma recurrence after radical prostatectomy. Cancer. 2005;104(2):290–8. doi: 10.1002/cncr.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Goodison S. Optimizing molecular signatures for predicting prostate cancer recurrence. Prostate. 2009;69(10):1119–27. doi: 10.1002/pros.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Ramachandran S, Ali Seyed M, et al. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006;66(8):4011–9. doi: 10.1158/0008-5472.CAN-05-3055. [data available at http://www.ebi.ac.uk/arrayexpress/browse.html?keywords=E-TABM-26] [DOI] [PubMed]
- 25.Dalgaard P. Statistics and Computing: Introductory Statistics with R. Springer-Verlag Inc.; NY: 2002. p. 260. [Google Scholar]
- 26. SPECS website Available from : http://www.pathology.uci.edu/faculty/mercola/UCISPECSHome.html.
- 27.Guo Y, Hastie T, Tibshirani R. Regularized linear discriminant analysis and its application in microarrays. Biostatistics. 2007;8(1):86–100. doi: 10.1093/biostatistics/kxj035. [DOI] [PubMed] [Google Scholar]
- 28.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99(10):6567–72. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Xia Xiao-Qin, Jia Zhenyu, Sawyers Anne, Yao Huazhen, Wang-Rodriquez Jessica, McClelland Michael, Mercola Dan. In silico estimates of tissue components in surgical samples based on expression profiling data using. Cancer Research. 2010 doi: 10.1158/0008-5472.CAN-10-0021. (in press) [algorythm available at http://webarraydborg/webarray/indexhtml] [DOI] [PMC free article] [PubMed]
- 30.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166(6):2472–83. [PubMed] [Google Scholar]
- 31.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17(4):411–9. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 32.Yanagisawa N, Li R, Rowley D, et al. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol. 2007;38(11):1611–20. doi: 10.1016/j.humpath.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Shariat SF, Scardino PT, Lilja H. Screening for prostate cancer: an update. Can J Urol. 2008;15(6):4363–74. [PMC free article] [PubMed] [Google Scholar]
- 34.Dennis G, Jr., Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 35.Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR. Inhibition of transforming growth factor-beta activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res. 2002;62(21):6021–5. [PubMed] [Google Scholar]
- 36.Zhang Q, Helfand BT, Jang TL, et al. Nuclear factor-kappaB-mediated transforming growth factor-beta-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2009;15(10):3557–67. doi: 10.1158/1078-0432.CCR-08-1656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.