Abstract
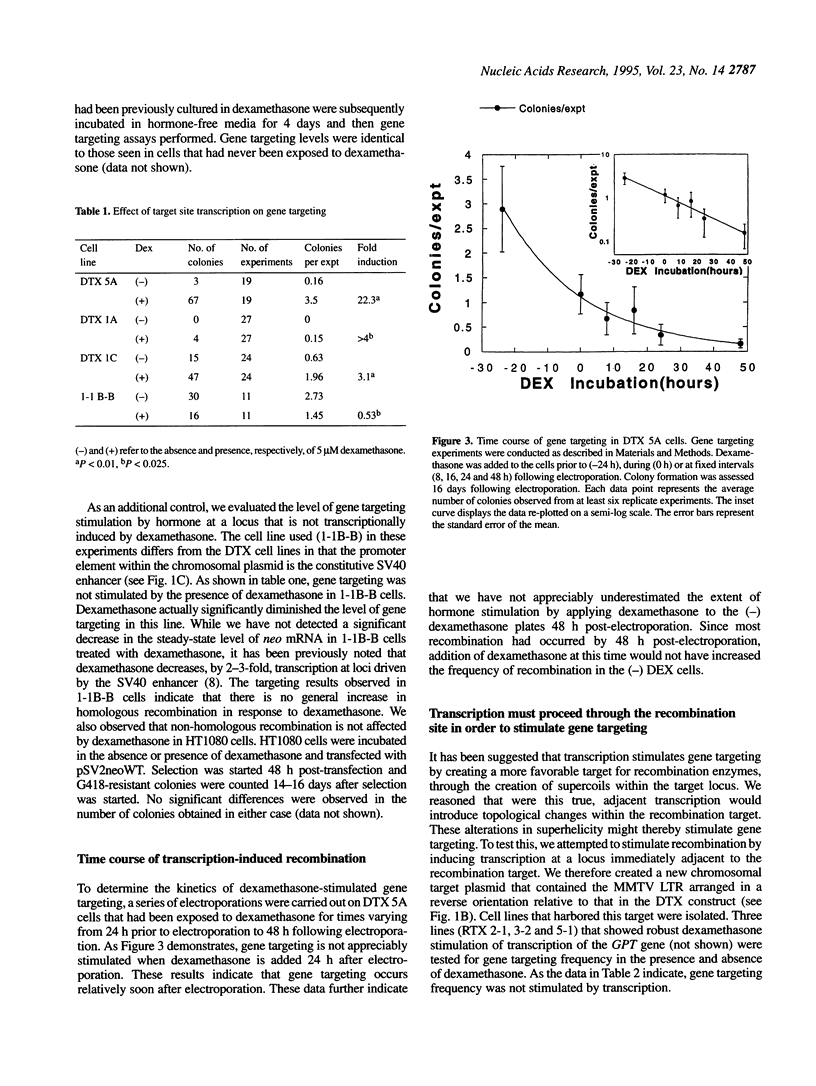
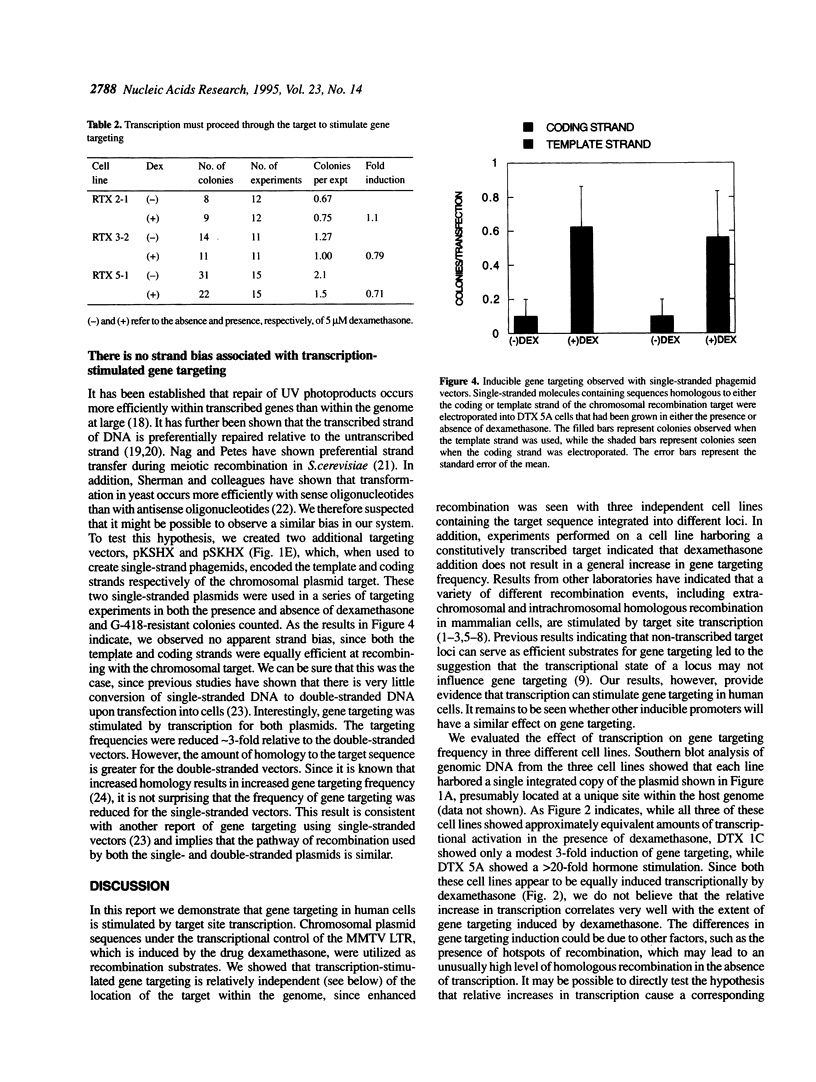
We evaluate the effect of target site transcription on gene targeting in cultured human fibrosarcoma cells. A number of cell lines that harbored a plasmid recombination substrate within their chromosomal DNA were created. Gene targeting frequency was then measured at these different loci in the presence and absence of an agent that stimulated target site transcription. We observed that gene targeting was significantly enhanced by RNA transcription. The magnitude of transcription-stimulated gene targeting varied from 3-fold to > 20-fold. No increase in gene targeting was observed, however, when transcription proceeded away from, rather than through, the recombination site. Transcription-stimulated gene targeting was also observed when single-stranded plasmid vectors complementary to either the coding or template strand were used as recombination substrates. Our results indicate that gene targeting, like other forms of DNA recombination, can be stimulated by target site transcription. The implications of our observations on current models of transcription-stimulated recombination are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell A. J., Bardwell L., Iyer N., Svejstrup J. Q., Feaver W. J., Kornberg R. D., Friedberg E. C. Yeast nucleotide excision repair proteins Rad2 and Rad4 interact with RNA polymerase II basal transcription factor b (TFIIH). Mol Cell Biol. 1994 Jun;14(6):3569–3576. doi: 10.1128/mcb.14.6.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Buratowski S. DNA repair and transcription: the helicase connection. Science. 1993 Apr 2;260(5104):37–38. doi: 10.1126/science.8465198. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Feaver W. J., Svejstrup J. Q., Bardwell L., Bardwell A. J., Buratowski S., Gulyas K. D., Donahue T. F., Friedberg E. C., Kornberg R. D. Dual roles of a multiprotein complex from S. cerevisiae in transcription and DNA repair. Cell. 1993 Dec 31;75(7):1379–1387. doi: 10.1016/0092-8674(93)90624-y. [DOI] [PubMed] [Google Scholar]
- Fujioka K., Aratani Y., Kusano K., Koyama H. Targeted recombination with single-stranded DNA vectors in mammalian cells. Nucleic Acids Res. 1993 Feb 11;21(3):407–412. doi: 10.1093/nar/21.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas K. D., Donahue T. F. SSL2, a suppressor of a stem-loop mutation in the HIS4 leader encodes the yeast homolog of human ERCC-3. Cell. 1992 Jun 12;69(6):1031–1042. doi: 10.1016/0092-8674(92)90621-i. [DOI] [PubMed] [Google Scholar]
- Hanawalt P., Mellon I. Stranded in an active gene. Curr Biol. 1993 Jan;3(1):67–69. doi: 10.1016/0960-9822(93)90156-i. [DOI] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Bradley A. The length of homology required for gene targeting in embryonic stem cells. Mol Cell Biol. 1991 Nov;11(11):5586–5591. doi: 10.1128/mcb.11.11.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. S., Sheng M., Greenberg M. E., Kolodner R. D., Papaioannou V. E., Spiegelman B. M. Targeting of nonexpressed genes in embryonic stem cells via homologous recombination. Science. 1989 Sep 15;245(4923):1234–1236. doi: 10.1126/science.2506639. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. RNA polymerase marching backward. Science. 1993 Feb 12;259(5097):944–945. doi: 10.1126/science.7679800. [DOI] [PubMed] [Google Scholar]
- Kawasaki I., Bae Y. S., Eki T., Kim Y., Ikeda H. Homologous recombination of monkey alpha-satellite repeats in an in vitro simian virus 40 replication system: possible association of recombination with DNA replication. Mol Cell Biol. 1994 Jun;14(6):4173–4182. doi: 10.1128/mcb.14.6.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Abraham J. A. Involvement of double-strand chromosomal breaks for mating-type switching in Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1984;49:77–88. doi: 10.1101/sqb.1984.049.01.011. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Altering genes in animals by gene targeting. Annu Rev Immunol. 1992;10:705–730. doi: 10.1146/annurev.iy.10.040192.003421. [DOI] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D. K., Petes T. D. Genetic evidence for preferential strand transfer during meiotic recombination in yeast. Genetics. 1990 Aug;125(4):753–761. doi: 10.1093/genetics/125.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff J. A., Reynolds R. J. Transcription stimulates homologous recombination in mammalian cells. Mol Cell Biol. 1990 Sep;10(9):4837–4845. doi: 10.1128/mcb.10.9.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff J. A. Transcription enhances intrachromosomal homologous recombination in mammalian cells. Mol Cell Biol. 1992 Dec;12(12):5311–5318. doi: 10.1128/mcb.12.12.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Song K. Y., Schwartz F., Maeda N., Smithies O., Kucherlapati R. Accurate modification of a chromosomal plasmid by homologous recombination in human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6820–6824. doi: 10.1073/pnas.84.19.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Reynolds P., Prakash L., Prakash S. Purification and characterization of the Saccharomyces cerevisiae RAD1/RAD10 endonuclease. J Biol Chem. 1993 Dec 15;268(35):26391–26399. [PubMed] [Google Scholar]
- Sweder K. S., Hanawalt P. C. Transcription-coupled DNA repair. Science. 1993 Oct 15;262(5132):439–440. doi: 10.1126/science.8211165. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Bardwell A. J., Bardwell L., Tappe N. J., Friedberg E. C. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature. 1993 Apr 29;362(6423):860–862. doi: 10.1038/362860a0. [DOI] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- White M. A., Dominska M., Petes T. D. Transcription factors are required for the meiotic recombination hotspot at the HIS4 locus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6621–6625. doi: 10.1073/pnas.90.14.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Moerschell R. P., Wakem L. P., Komar-Panicucci S., Sherman F. Strand-specificity in the transformation of yeast with synthetic oligonucleotides. Genetics. 1992 Aug;131(4):811–819. doi: 10.1093/genetics/131.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Y., Ou B. R., Forsberg N. E. Effects of dexamethasone on muscle protein homeostasis and on calpain and calpastatin activities and gene expression in rabbits. J Endocrinol. 1994 May;141(2):209–217. doi: 10.1677/joe.0.1410209. [DOI] [PubMed] [Google Scholar]



