Abstract
Objective
To determine whether parental periodontal disease history is a risk factor for periodontal disease in adult offspring.
Methods
Proband periodontal examination (combined attachment loss (CAL) at age 32, and incidence of CAL from ages 26–32) and interview data were collected during the age-32 assessments in the Dunedin Study. Parental data were also collected. The sample was divided into two familial-risk groups for periodontal disease (high- and low-risk) based on parents’ self-reported periodontal disease.
Results
Periodontal risk analysis involved 625 proband-parent(s) groups. After controlling for confounding factors, the high-familial-risk periodontal group was more likely to have 1+ sites with 4+mm CAL (RR 1.45; 95% CI 1.11–1.88), 2+ sites with 4+mm CAL (RR 1.45; 95% CI 1.03–2.05), 1+ sites with 5+mm CAL (RR 1.60; 95% CI 1.02–2.50) and 1+ sites with 3+mm incident CAL (RR 1.64; 95% CI 1.01–2.66) than the low-familial-risk group. Predictive validity was enhanced when information was available from both parents.
Conclusions
Parents with poor periodontal health tend to have offspring with poor periodontal health. Family/parental history of oral health is a valid representation of the shared genetic and environmental factors that contribute to an individual’s periodontal status, and may help predict patient prognosis and preventive treatment need.
Keywords: periodontal, intergenerational, risk, family history
Introduction
The concept of intergenerational continuity in periodontal health is not new. It was observed almost a century ago, and during the 1940s and 1950s, researchers conducted investigations into intergenerational effects, including family studies and twin studies (Gorlin et al. 1967, Hassell & Harris 1995). Clear evidence for a substantial genetic component in periodontal disease susceptibility was demonstrated in animal models (Baer et al. 1961). However, the main focus of research from the 1960s to 1990s shifted from hereditary factors to the role of bacteria and other environmental factors in disease risk (Löe 1993). More recently, the idea that virtually all characteristics are the result of gene-environment interaction has become the paradigm for considering many common, preventable disorders of adulthood (Collins 2004, Hunter 2005, Moffitt et al. 2005). An increasing interest in gene-environment interactions is reflected in greater awareness of the role of family history and intergenerational continuity in health as a practical, inexpensive approach to categorising gene-environment risk for these disorders, including periodontal disease (Khoury et al. 2005, Scheuner et al. 1997, Valdez et al. 2010).
Research suggests that the health status of one generation can have a profound effect on that of the next. Studies have found intergenerational and familial associations for cardiovascular disease (Greenlund et al. 1997, Parikh et al. 2007, Rose 1967, Sesso et al. 2001), non-insulin-dependent diabetes mellitus (Dallo & Weller 2003, Meigs et al. 2000, Newman et al. 1987, Srinivasan et al. 2003), metabolic syndrome (Lascaux-Lefebvre et al. 2001), cancer (Hsieh & Albertsen 2003, Jonsson et al. 2004, Pharoah et al. 1997), asthma (Arshad et al. 2005), obesity (Reilly et al. 2005, Whitaker et al. 1997), health-related behaviours, including smoking, drug and alcohol use (Chassin et al. 1998, Hill et al. 2005, Merikangas et al. 1998, Shenassa et al. 2003), diet and exercise (Hood et al. 2000, Mattocks et al. 2008), and other health-related influences such as socio-economic status (Corcoran 1995, Zimmerman 1992).
Is family history a risk factor for oral disease? Over the past few decades, the small amount of research that has been carried out on intergenerational transmission of oral health suggests that it may be a risk factor for caries in children (Shearer & Thomson 2010). Regarding periodontal disease, a number of studies have examined familial aggregation of aggressive periodontitis (Nibali et al. 2008). However, there is a shortage of studies investigating the intergenerational transmission of chronic periodontal disease (which generally does not present until the fourth decade) (Kinane & Hart 2003). This is a particular deficiency, because genetic and epigenetic1 factors are thought to play a major role in the aetiology of periodontal disease (Barros & Offenbacher 2009, Gomez et al. 2009, Meisel et al. 2004, Michalowitz et al. 2000, Page 1999). While it is probable that the periodontal health status of one generation has an effect on that of the next, the nature and extent of this effect is unclear.
The importance of investigating periodontal intergenerational associations is highlighted when consideration is made of the impact in most developed countries of effective population-based oral health strategies over the past 40 years, advances in restorative dentistry, expectations of retaining a functional dentition for life, and aging populations. Increasingly elderly populations are now retaining teeth which would previously have been lost to dental caries; in effect, more teeth are at risk of periodontal disease for longer. Does this matter? Recent research has found associations between periodontal disease and systemic disease (Cullinan et al. 2009, Kandelman et al. 2008). In particular, the bidirectional link between periodontal disease and diabetes mellitus suggests that a higher prevalence of periodontal disease in a population may adversely affect its overall health (with consequential suffering, costs and use of scarce resources). In addition, periodontal disease may have a direct impact on quality of life (Cunha-Cruz et al. 2007, Needleman et al. 2004).
Not all individuals are equally susceptible to periodontal disease, and the identification of those who will progress to advanced disease is desirable, but not straightforward. Family history reflects the results of shared genetic variations, and shared non-genetic factors (environmental factors, exposures and common behaviours) (Khoury 2003). Possibly, a family history of periodontal disease may be an early marker of shared genetic, epigenetic and environmental influences associated with periodontal disease risk, and allow for early intervention to minimise adverse environmental factors. The aim of this study was to determine whether an individual’s periodontal health and disease risk is predicted by that of his or her parents.
Methods
Study design and participants
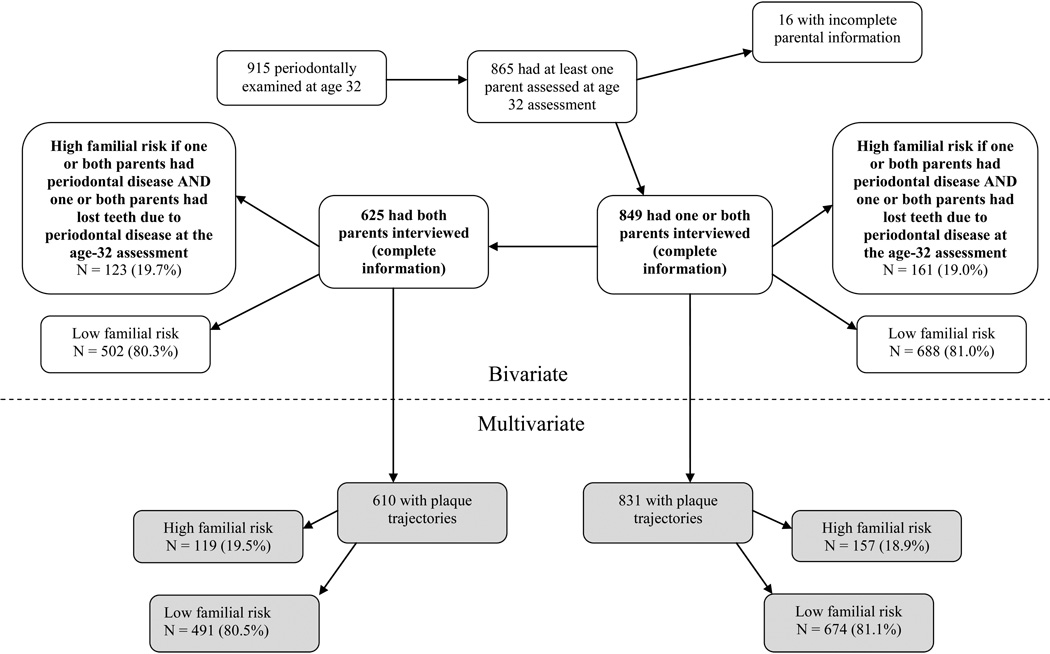
This study is an analysis of data from the Dunedin Multidisciplinary Health and Development Study (DMHDS) using periodontal data collected from Study members (hereafter referred to as “probands”) and their parents during the age-32 assessments. The DMHDS is a longitudinal epidemiological study of a birth cohort of 1,037 children born at the Queen Mary Hospital, Dunedin, New Zealand between 1 April 1972 and 31 March 1973. These 1037 children represent 91% of the 1139 eligible children born between these dates, and 972 (96% of the surviving 1014) were assessed at age 32. Of these, 932 were dentally examined, and 915 were periodontally examined. Māori (7.5%) were under-represented (in comparison to 15% in the total New Zealand population) in the cohort at age 32. Ethics approval for the study was granted by the Otago Research Ethics Committee, and participants gave informed consent.
Measurements
The study used data collected from probands’ oral examinations and interviews, and from interviews with their parents, at the age-32 assessments.
Proband examinations
Periodontal disease
A full-mouth periodontal examination was conducted on 915 probands (17 individuals with a history of cardiac valvular anomalies or rheumatic fever were not included). Three sites (mesio-buccal, buccal and disto-lingual) per tooth (barring third molars) were examined in all four quadrants, using a National Institute of Dental Research (NIDR) probe. Two measures were recorded: gingival recession (the distance in millimetres from the gingival margin to the cemento-enamel junction) and probing depth (the distance from the gingival margin to the tip of the probe). Gingival recession was recorded as a negative where the gingival margin was situated more than 1mm coronally to the cemento-enamel junction (as would be the case in gingival hyperplasia). The combined attachment loss (CAL) for each site was calculated by summing gingival recession and probing depth.
The prevalence of periodontal disease was determined using three different case definitions: one or more sites with 4+mm CAL; two or more sites with 4+mm CAL; and one or more sites with 5+mm CAL (Thomson et al. 2007). In addition, individuals who had experienced one or more sites with 3+mm incident CAL between ages 26 and 32 were classified as incident cases (Thomson et al. 2008).
Other periodontal measures
Gingival bleeding on probing was assessed for each tooth by observing the presence or absence of bleeding at each of the three probing sites, 10 seconds after probing. The percentage of teeth showing bleeding on probing (BOP) was computed. The simplified oral hygiene index was used to quantify plaque accumulation on six index teeth (Greene & Vermillion 1964), and the overall plaque score was the sum of the scores divided by the number of teeth scored. Long-term plaque exposure was described through trajectory analysis. The longitudinal data on plaque scores measured at ages 5, 9, 15, 18, 26, and 32 years were used to split the cohort into three distinct ‘plaque groups’ using a group-based trajectory analysis model, based on the censored normal distribution, in SAS 9.2. The scores were as follows: group 1, low levels of plaque (group mean = 0.59, N = 328, 39.5% of the cohort); group 2, moderate levels of plaque (group mean = 0.93, N = 408, 49.1%); and group 3, high levels of plaque (group mean = 1.45, N = 95, 11.4%). Overall, plaque trajectory data were available for 953 Study members, but analyses were restricted to those 831 Study members who were periodontally examined at age 32, who’d had at least one parent attend for interview, and for whom plaque data were available at age 32 years (Broadbent et al. 2010).
Proband interviews
Probands were questioned on their smoking history; in addition, tobacco usage data had been collected during previous assessments. Current and ex-smokers were asked about the number of cigarettes smoked per day, and the number of years at this level of consumption. These data were used to compute an individual’s exposure as the number of pack-years to age 32.
A measure of socioeconomic status (SES) at age 32 was obtained from each study member using standard New Zealand indices which apply a six-interval classification according to occupation; for example, a doctor scores “1” and a labourer scores “6” (Elley & Irving 1985, Irving & Elley 1977). Study members with a score of “1” or “2” were allocated to the “high SES group; those with a score of “3” or “4” were assigned to the “medium SES group; and those with a score of “5” or “6” were assigned to the “low SES group. Participants were asked to indicate whether they were routine or episodic users of dental care services. Routine users were those who usually visited for a check-up, and had made a dental visit in the previous 12 months (Thomson et al. 2010).
Parental interviews
Around the same time as the age-32 assessment (2003 to 2006), the parents of probands took part in an interview on their oral health status and history (Milne et al. 2008a). They were asked whether they had ever been told they had periodontal disease, whether they had lost any teeth (for any reason) and if so, how many. Finally, they were asked about the main reason for their tooth loss (tooth decay, periodontal disease, trauma or another reason). Two of these variables (prevalence of periodontal disease, and prevalence of tooth loss due to periodontal disease) formed the basis of the familial-risk grouping for periodontal disease (Figure 1). Probands were allocated to the high-familial-risk category if one or both of their parents reported having periodontal disease, and one or both parents had lost teeth due to periodontal disease, at the age-32 assessment. All other probands were grouped in the low-familial-risk category.
Figure 1.
Periodontal Disease Familial-risk Groups
Statistical analysis
The parental interview information was used to allocate each proband (their child) to either a “high-familial-risk” group or a “low-familial-risk” group for periodontal disease (Figure 1). The utility of those familial-risk groups was evaluated by examining gradients across them in probands’ periodontal disease experience (for example, by comparing CAL prevalence in the two family risk categories). In addition, analyses were carried out for two samples. The first sample comprised probands who had one or both parents interviewed at the age-32 assessment (generalizable to one- or two-parent families); the second consisted of probands who had both parents interviewed at the age-32 assessment (a more complete parental history is obtained, but is only generalizable to two-parent families). The first sample encompassed the second, with the addition of those who had only one parent attend.
Descriptive and bivariate analyses were conducted using SPSS version 16.0 (SPSS Inc. Chicago, Illinois). Multivariate analyses used Stata version 10.0 (StataCorp, College Station, Texas 77845, USA). Chi-square tests were used to examine the statistical significance of associations observed between categorical variables. Independent sample t-tests were used for continuous dependent variables. Statistical tests were two-tailed and the threshold for statistical significance was set at p<0.05. In the multivariate analysis, the generalized linear model (GLM) command with modified Poisson regression analysis was used to estimate relative risk and confidence intervals, using a robust error variance procedure. Model selection was done on the basis of biological plausibility and by stepwise regression. Effect modification between variables was explored, and any interaction between variables that improved the model was included.
Results
Of the original 1037 participants, 915 (90.1% of the surviving cohort) were periodontally examined at age 32. Of those who were periodontally examined, the majority (865, 94.5%) had one or both parents participate in the family health history study; two-thirds (633, 69.2%) had both parents participate. Data from 16 probands were excluded from the analysis due to incomplete parental information. For the periodontal risk analysis, the sample size was 849 for the “one or both parents interviewed” sample, and 625 for the “both parents interviewed” sample. These groups were further refined for the multivariate analyses according to whether the probands had been assigned to a plaque trajectory (Figure 1).
Periodontal disease by familial-risk category
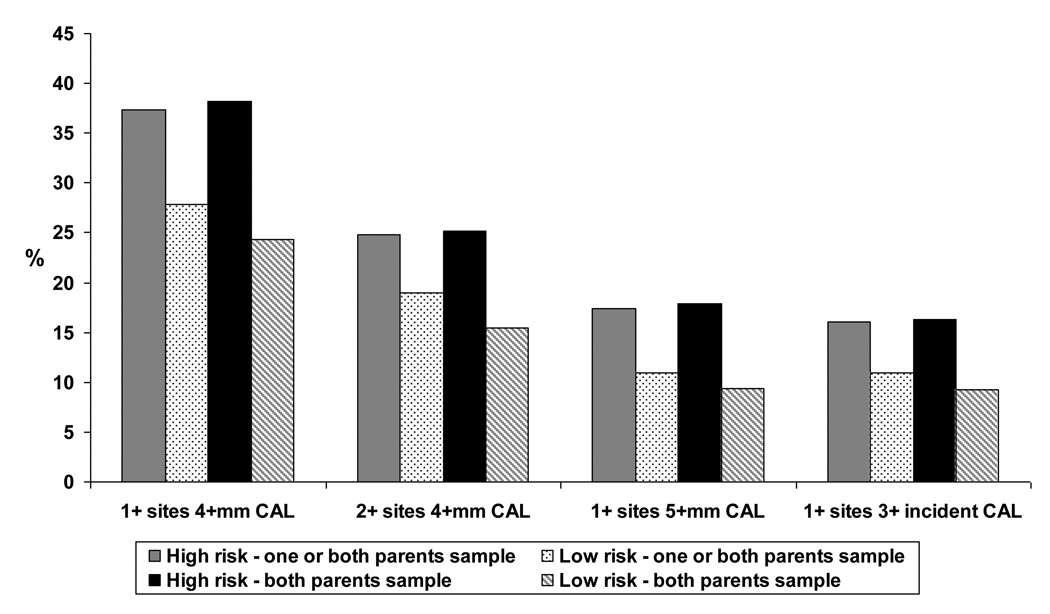
In bivariate analyses for the “one or both parents interviewed” sample, the risk category for periodontal disease was significantly associated with the prevalence of 1+ sites with 4+mm CAL, the prevalence of 1+ sites with 5+mm CAL, the mean percentage of sites with bleeding on probing (BOP), and the mean plaque score at age 32 (Table 1 and Figure 2). In bivariate analyses for the “both parents interviewed” sample, the risk category for periodontal disease was significantly associated with the age-32 prevalence of 1+ and 2+ sites with 4+mm CAL, the age-32 prevalence of 1+ sites with 5+mm CAL, the prevalence of 1+ sites with 3+mm incident CAL between ages 26 and 32, the mean percentage of sites with 4+ CAL, and the mean percentage of sites with BOP at age 32 (Table 1 and Figure 2). Associations were generally stronger for the “both parents interviewed” sample.
Table 1.
Proband periodontal disease prevalence, extent of periodontal disease, bleeding on probing (BOP) and plaque score, and proband caries and tooth loss prevalence and severity, at age 32, and prevalence of one or more sites with 3+mm incident CAL between age 26 and age 32, by familial-risk category for periodontal disease.
| Risk category for periodontal disease according to parental periodontitis history | ||||||
|---|---|---|---|---|---|---|
| One or both parents sample | Both parents sample | |||||
| High risk | Low risk | Total | High risk | Low risk | Total | |
| Proband periodontal disease prevalence (%) | ||||||
| 1+ sites with 4+mm CAL | 60 (37.3) | 192 (27.9)a | 252 (29.7) | 47 (38.2) | 122 (24.3)b | 169 (27.0) |
| 2+ sites with 4+mm CAL | 40 (24.8) | 131 (19.0) | 171 (20.1) | 31 (25.2) | 78 (15.5)a | 109 (17.4) |
| 1+ sites with 5+mm CAL | 28 (17.4) | 76 (11.0)a | 104 (12.2) | 22 (17.9) | 47 (9.4)c | 69 (11.0) |
| 1+ sites with 3+mm incident CAL | 26 (16.1) | 76 (11.0) | 102 (12.0) | 20 (16.3) | 46 (9.2)a | 66 (10.6) |
| Proband periodontal disease extent (SD) | ||||||
| Mean % of sites with 4+mm CAL | 2.8 (7.1) | 1.8 (5.7) | 2.0 (6.0) | 2.6 (5.5) | 1.3 (4.4)d | 1.6 (4.7) |
| Mean % of sites with 5+mm CAL | 0.7 (3.1) | 0.5 (2.6) | 0.5 (2.7) | 0.6 (1.5) | 0.4 (2.5) | 0.4 (2.4) |
| Other periodontal measures (SD) | ||||||
| Mean % sites with BOP | 10.6 (8.0) | 8.1 (7.0)e | 8.6 (7.2) | 10.3 (7.8) | 7.5 (6.4)e | 8.1 (6.8) |
| Mean plaque score | 0.8 (0.6) | 0.7 (0.5)d | 0.8 (0.5) | 0.8 (0.6) | 0.7 (0.5) | 0.7 (0.5) |
| Smoker at 32 | 64 (39.8) | 216 (31.4) | 280 (33.0) | 45 (36.6) | 139 (27.7) | 184 (29.4) |
p<0.05; chi-square test.
p<0.005; chi-square test.
p<0.01; chi-square test.
p<0.05; independent samples t-test.
p<0.001; independent samples t-test.
Figure 2.
Proband periodontal disease prevalence by periodontal familial-risk category at age 32
Multivariate analyses
Multivariate modelling was used to determine the relative risk (RR) for having one or more sites with 4+mm CAL, two or more sites with 4+mm CAL, one or more sites with 5+mm CAL at age 32, and one or more sites with 3+mm incident CAL between ages 26 and 32 in the high-familial-risk group for periodontal disease (using the low-familial-risk group as a referent) while controlling for the confounding factors of sex, episodic user of dental services, SES, plaque trajectory, and tobacco use. For the “one or both parents interviewed” sample, the RR for those in the high-familial-risk group did not reach statistical significance for any of these variables (Table 2).
Table 2.
Outcomes of multivariate modelling, and smoking-plaque effect modification, for proband prevalence of one or more sites with 4+mm combined attachment loss (CAL), prevalence of two or more sites with 4+mm CAL, and prevalence of one or more sites with 5+mm CAL at age 32, and prevalence of one or more sites with 3+mm incident CAL between age 26 and age 32.
| High familial-risk group for periodontal disease | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted Model 1a |
Adjusted Model 2b |
Adjusted Model 3c |
|
| Periodontal disease prevalence at 32 | ||||
| One or both parents sample | ||||
| RR 1+ sites with 4+mm CAL (95% CI) | 1.33 (1.05,1.69) | 1.32 (1.05,1.67) | 1.24 (0.98,1.55) | 1.23 (0.98,1.54) |
| RR 2+ sites with 4+mm CAL (95% CI) | 1.32 (0.96,1.80) | 1.31 (0.96,1.78) | 1.20 (0.89,1.63) | 1.18 (0.88,1.57) |
| RR 1+ sites with 5+mm CAL (95% CI) | 1.59 (1.06,2.38) | 1.57 (1.05,2.33) | 1.40 (0.94,2.09) | 1.36 (0.92,1.99) |
| RR 1+ sites with 3+mm incident CAL (95% CI) | 1.47 (0.97,2.21) | 1.45 (0.97,2.18) | 1.35 (0.90,2.04) | 1.34 (0.90,2.01) |
| Both parents sample | ||||
| RR 1+ sites with 4+mm CAL (95% CI) | 1.57 (1.19,2.08) | 1.55 (1.19,2.03) | 1.46 (1.12,1.89) | 1.45 (1.11,1.88) |
| RR 2+ sites with 4+mm CAL (95% CI) | 1.65 (1.14,2.40) | 1.62 (1.12,2.33) | 1.51 (1.07,2.12) | 1.45 (1.03,2.05) |
| RR 1+ sites with 5+mm CAL (95% CI) | 1.93 (1.19,3.11) | 1.88 (1.18,2.99) | 1.64 (1.04,2.59) | 1.60 (1.02,2.50) |
| RR 1+ sites with 3+mm incident CAL (95% CI) | 1.79 (1.10,2.92) | 1.75 (1.08,2.83) | 1.65 (1.01,2.69) | 1.64 (1.01,2.66) |
Model 1 adjusted for sex and SES.
Model 2 adjusted for sex, SES, use of dental services, plaque trajectory, and pack years to age 32 (smoking history).
Model 3 adjusted for sex, SES, use of dental services, and interaction between smoking and plaque trajectory.
Reference categories: male (female, coded 0), medium or low SES at age 32 (high SES coded 0), episodic user of dental services at age 32 (routine user of dental services at age 32, coded 0), moderate or high plaque trajectory (low plaque trajectory coded 0), non-smoker at age 32 + moderate plaque trajectory, non-smoker at age 32 + high plaque trajectory, smoker at age 32 + low plaque trajectory, smoker at age 32 + moderate plaque trajectory or smoker at age 32 + high plaque trajectory (non-smoker at age 32 + low plaque trajectory coded 0), high familial-risk for periodontal disease (low familial-risk for periodontal disease coded 0)
RR, relative risk; CAL, combined attachment loss; CI, confidence interval; SES, socioeconomic status. Significant findings in bold type.
For the “both parents interviewed” sample, the RR for having one or more sites with 4+mm CAL by age 32 for those in the high-familial-risk group was 1.45 times greater than that for the low-familial-risk group (Table 2). The RR for having two or more sites with 4+mm CAL by age 32 for those in the high-familial-risk group was 1.45 times greater than that for the low-familial-risk group. For those in the high-familial-risk group, the RR for having one or more sites with 5+mm CAL by age 32 was 1.60 times that of the low-familial-risk group, and the RR for having one or more sites with 3+mm incident CAL between ages 26 and 32 was 1.64.
Multivariate modelling revealed effect modification between plaque and smoking to substantially increase smokers’ relative risk (in either sample) of having having one or more sites with 4+mm CAL, two or more sites with 4+mm CAL, and one or more sites with 5+mm CAL, by age 32; and of having one or more sites with 3+mm incident CAL between ages 26 and 32 (Supplementary Tables 1–8 and Supplementary Figures 1–6). Likewise, effect modification was found between familial-risk group and smoking whereby there was no difference between the reference group and the high-familial-risk group in non-smokers (with the exception of the prevalence of 1+ sites with 3+mm incident CAL between ages 26 and 32, in the "both parents in" sample), but both high- and low-familial-risk groups experienced greater risk for all outcomes in smokers. In general, effect modification between familial-risk group and plaque trajectory was not found.
Discussion
These data from a prospective cohort study suggest a degree of continuity of periodontal health across generations within families. Study members (probands) were grouped according to their parents’ self-reported periodontal health status, recorded by interview, when probands were aged 32. It was found, if both parents were interviewed, and after controlling for confounding factors, that those in the high-familial-risk group for periodontal disease had significantly greater risk (than the low-familial-risk group) of having 1+ or 2+ sites with 4+mm CAL by age 32, of having 1+ sites with 5+mm CAL by age 32, and of having one or more sites with 3+mm incident CAL between ages 26–32. Analysis of the unadjusted data found associations between familial-risk grouping and the prevalence of 1+ sites with 4+mm CAL, and 1+ sites with 5+mm CAL, when the “one or both parents interviewed” sample was used. However, when confounding factors were controlled for, the high-familial-risk group in this sample showed no statistically significant greater risk of having periodontal disease (over that of the low-familial-risk group).
This study had some limitations. We relied on parental self-report data to categorise the proband into familial-risk groups and on proband self-report data on tobacco use, and use of dental services. The issue of the reliability and validity of self-report data has been addressed by others (Blicher et al. 2005, Gilbert & Litaker 2007). Interview/examiner-based assessments, as used in the family health history study, are more likely to yield valid data than “self-completed” data. In addition, Dunedin Study participants and their parents are familiar with interviews, are aware of the importance of accurate responses, and there is a long history of mutual trust and respect between participants and researchers. However, the possibility of error due to parents being unaware of their oral health status at the age-32 assessment of probands must be considered. This error would most likely have been in the direction of undiagnosed periodontal disease leading to misclassification, and is most likely to have favoured the null hypothesis (although there is no way of knowing this for sure). In addition, there was the potential for recall bias as parents may not have remembered whether or not they had been diagnosed with periodontal disease). In the case of the “one or both parents interviewed” sample, the possibility of error due to lack of data on the periodontal health status of a non-attending co-parent cannot be overlooked.
Turning to the study findings, we believe that these are unprecedented. Until now, it has not been possible to examine the nature and extent of intergenerational continuity in periodontal health because such data have not been available. The Dunedin Study is unique in its longevity, sample size, retention rate, oral health data (including intergenerational data), and information on a range of potential risk, ameliorating, exacerbating and confounding factors. It offers a particularly valuable opportunity to investigate intergenerational associations in periodontal health, and to broaden our understanding of the causal associations between parental periodontal health and the periodontal health of their offspring.
The use of a birth cohort, and the high retention rate, means that the sample is representative of its source population (the South Island of New Zealand). The issue of whether the findings can be generalized to the New Zealand population, and to other populations (particularly the United States), has been addressed by another paper using data from this sample (Thomson et al. 2006). It was cautiously concluded that findings from the DMHDS can be generalized to these populations. While this sample under-represents Maori with respect to the total New Zealand Maori population, it is representative of the South Island. According to the 2006 Census, 7.6% of adults aged 25–34 self-identified as Maori in Otago (Dunedin is the capital of the Otago region); this is in accord with the DMHDS sample. Furthermore, as Maori, on average, suffer poorer oral health than the general population (Broughton 1993), the under-representativeness of Maori in the DMHDS may have led to an under-estimation of the strength of the observed associations.
While the longitudinal and intergenerational findings are unique, the DMHDS cross-sectional and descriptive findings are reasonably consistent with the limited data available from other studies (Brennan et al. 2001, Oral Health U.S. 2002, Slade et al. 2007). This is true also of the findings for the parents at the age-32 assessment (Slade et al. 2007). This increases confidence in the validity of the intergenerational findings.
Familial risk assessment tools for chronic diseases (such as coronary artery disease, diabetes, cancer, and psychiatric disorders) are derived from empirical data which have accumulated in the literature over the past 20–30 years (Yoon et al. 2009). More recently, the formal assessment of the validity and utility of family history as a tool to improve health has being considered (Berg et al. 2009, Milne et al. 2008a). To date, however, such information has not been available for periodontal disease; there was little experimental data to guide the construction of the periodontal disease familial-risk groups. Therefore, in this study, the grouping of individuals into risk categories was based on familial/parental risk assessment tools from other disciplines (Milne et al. 2008b, Scheuner et al. 1997, Yoon et al. 2002). The age-32 assessment bivariate data provided support for the groupings. In addition, they were informed by cross-sectional and longitudinal studies which indicate associations between parental and child oral health more generally (Shearer & Thomson 2010). The grouping used seemed intuitive, and ensured sufficient statistical power for the analyses (in terms of having sufficient numbers in each familial-risk group). It was kept in mind that the main objective of the risk grouping was to identify high-risk individuals who may benefit from earlier, more frequent, and more costly preventive care. Such early intervention, while initially more costly, should prove to be more cost-effective in the long run.
Essentially, a largely consistent pattern of higher prevalence and greater extent of disease was seen across the familial-risk groups. The gradients were clear, and in the expected direction (although not all associations reached statistical significance). The findings indicate that the familial-risk categorisation is generally valid for the two groups, particularly so for the “both parents interviewed” sample, and less so for the “one or both parents interviewed” sample. It is possible that future assessments as the Dunedin Study cohort ages may find greater distinction between the periodontal disease familial-risk groups, due both to continued disease progression with age, and to the parental histories becoming more defined as the parents themselves age. Future assessments may also highlight stronger associations for periodontal disease familial-risk grouping when data are available from one parent only, and stronger associations between familial-risk group and the extent of periodontal disease.
It is not surprising that more (and stronger) associations were found in the “both parents interviewed” sample than in the “one or both parents interviewed” sample. It seems reasonable to assume that the potential for misclassification in the direction of undiagnosed disease would be an issue for periodontal disease. While most people are aware of having lost a tooth (for example), a substantial proportion may be unaware of having periodontal disease. It is possible that this error is compounded in the “one or both parents interviewed” sample, whereby no data were obtained from a non-attending parent (although it is not possible to speculate on which direction this misclassification may lie; that is, whether or not it favoured the null hypothesis). This potential error may be one reason why the associations differ for the two samples; however, it is likely that other unknown factors may also be involved. In any case, it appears that predictive validity is enhanced if data from both parents can be obtained.
The interaction between smoking and plaque trajectory is not an unexpected finding. Smokers were more likely to be at greater risk of periodontal disease than non-smokers, and smokers with high plaque trajectories were at greatest risk. These findings suggest that smoking and plaque trajectory combine to raise the risk of periodontal disease to a higher level than either of these factors acting independently, and highlight the necessity of assessing effect modification between smoking and plaque levels in periodontal disease research (Hyman 2006). Likewise, effect modification between smoking and familial-risk group is a reasonable finding; smokers in both low- and high-familial risk groups are at greater risk of periodontal disease than non-smokers, and smokers in the high-familial risk group are at greatest risk. Smoking exacerbates the impact of being in the high-familial risk group for periodontal disease.
While the mechanisms underlying intergenerational continuity in periodontal health are unclear and are undoubtedly complex, there are a number of potential pathways whereby disease risk can be transmitted across generations. Intergenerational transmission of genetically- or epigenetically-determined traits may be one mechanism (Nadeau 2009, Skinner et al. 2010). Risk factors such as socio-economic status, smoking and episodic use of dental care services may continue across generations, manifesting as poor health capital (Chassin et al. 1998, Corcoran 1995, Hill et al. 2005, Shenassa et al. 2003, Zimmerman 1992). Poor maternal health before and during pregnancy (and/or during the early post-natal period) can have an unfavourable impact on intrauterine fetal growth and neonatal development, in turn leading to adverse outcomes for the offspring later in the life course (Barker 1998, De Stavola et al. 2000, Frankel et al. 1996, Lithell et al. 1996, Power & Jefferis 2002). In fact, poor maternal periodontal health has been associated with an increased risk of pre-term birth and low birth weight in some populations (Wimmer & Pihlstrom 2008). Another mechanism involving a genetic predisposition coupled with exposure to environmental risk factors forms the basis for the gene-environment interaction model; that is, the situation where both genetic and environmental factors interact to produce health outcomes in individuals and populations (Collins 2004, Hunter 2005, Moffitt et al. 2005).
Our findings provide evidence to suggest a causal association between parental periodontal health and proband periodontal health. Generally, periodontal disease has a later onset than other oral disease such as caries, and the association in this cohort between parental periodontal health and proband periodontal health may accordingly strengthen with age. The predictive validity of parental periodontal health information is enhanced if data from both parents can be obtained.
Conclusions
This study suggests that the children of parents with poor periodontal oral health are more likely to have poor periodontal health in adulthood than the children of parents with good periodontal health. Family/parental history of periodontal health appears to be a valid representation of the complex interplay between shared genetic factors and shared environmental factors, exposures and behaviours that contribute to an individual’s periodontal health status. Generally, it could be quickly and inexpensively assessed by clinicians, and along with assessment of SES and smoking history, may improve prediction of patient prognosis and preventive treatment need.
Clinical relevance
Scientific rationale for the study
Family history of periodontal disease may be an early marker of shared genetic, epigenetic and environmental influences associated with periodontal disease risk.
Principal findings
The children of parents with poor periodontal oral health are more likely to have poor periodontal health in adulthood than the children of parents with good periodontal health.
Practical implications
Generally, family/parental history of periodontal health could be quickly and inexpensively assessed by clinicians to improve prediction of patient prognosis and preventive treatment need.
Supplementary Material
Acknowledgments
We thank the Dunedin Study members, their families and friends, Unit research staff, and study founder Phil Silva. The study protocol was approved by the institutional review boards of the participating universities. Study members gave informed consent before participating.
Source of funding
This work was supported by: Grants R01 DE-015260 and R03 DE018716 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, USA; UK Medical Research Council grant G0100527, and G0601483; US National Institute of Aging grant AG032282; US-NIMH grants MH077874, MH45070 and MH49414; programme grants 03/271 and 09/086 from the Health Research Council of New Zealand; The Lady Davis Fellowship of the Hebrew University; and The Caselberg Trust. Avshalom Caspi is a Royal Society-Wolfson Merit Award holder.
Footnotes
Conflict of interest
No conflict of interest declared.
Inherited changes in phenotype caused by mechanisms other than changes in the underlying DNA sequence
References
- 1.Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127:502–508. doi: 10.1378/chest.127.2.502. [DOI] [PubMed] [Google Scholar]
- 2.Baer P, Crittenden L, Jay GE, jr, Lieberman J. Studies on periodontal disease in the mouse. II. Genetic and maternal effects. Journal of Dental Research. 1961;40:23–33. doi: 10.1177/00220345610400011901. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJP. Mothers, Babies and Health in Later Life. 2nd edition. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- 4.Barros SP, Offenbacher S. Epigenetics: connecting environment and genotype to phenotype and disease. Journal of Dental Research. 2009;88:400–408. doi: 10.1177/0022034509335868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg A, Baird MA, Botkin JR, Driscoll DA, Fishman PA, Guarino PD, Hiatt RA, Jarvik GP, Millon-Underwood S, Morgan TM, Mulvihill JJ, Pollin TI, Schimmel SR, Stefanek ME, Vollmer WM, Williams JK. National Institutes of Health State-of-the-Science Conference Statement: Family History and Improving Health. Annals of Internal Medicine. 2009;151:872–877. doi: 10.7326/0003-4819-151-12-200912150-00165. [DOI] [PubMed] [Google Scholar]
- 6.Blicher B, Joshipura K, Eke P. Validation of self-reported periodontal disease: a systematic review. Journal of Dental Research. 2005;84:881–890. doi: 10.1177/154405910508401003. [DOI] [PubMed] [Google Scholar]
- 7.Brennan DS, Spencer AJ, Slade GD. Prevalence of periodontal conditions among public-funded dental patients in Australia. Australian Dental Journal. 2001;46:114–121. doi: 10.1111/j.1834-7819.2001.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 8.Broadbent JM, Thomson WM, Boyens JV, Poulton R. Dental plaque and oral health over the first 30 years of life. Journal of the American Dental Association. 2010 doi: 10.14219/jada.archive.2011.0197. In press. [DOI] [PubMed] [Google Scholar]
- 9.Broughton JR. Te niho waiora me te iwi Maori: dental health and the Maori people. New Zealand Dental Journal. 1993;89:15–18. [PubMed] [Google Scholar]
- 10.Chassin L, Presson CC, Todd M, Rose JS, Sherman SJ. Maternal socialization of adolscent smoking: the intergenerational transmission of parenting and smoking. Developmental Psychology. 1998;34:1189–1201. doi: 10.1037//0012-1649.34.6.1189. [DOI] [PubMed] [Google Scholar]
- 11.Collins FS. The case for a US prospective cohort study of genes and environment. Nature. 2004;429:478–477. doi: 10.1038/nature02628. [DOI] [PubMed] [Google Scholar]
- 12.Corcoran M. Rags to rags: poverty and mobility in the United States. Annual Review of Sociology. 1995;21:237–267. [Google Scholar]
- 13.Cullinan MP, Ford PJ, Seymour GJ. Periodontal disease and systemic health: current status. Australian Dental Journal. 2009;54:S62–S69. doi: 10.1111/j.1834-7819.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 14.Cunha-Cruz J, Hujoel PP, Kressin NR. Oral health-related quality of life of periodontal patients. Journal of Periodontal Research. 2007;42:169–176. doi: 10.1111/j.1600-0765.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 15.Dallo FJ, Weller SC. Effectiveness of diabetes mellitus screening recommendations. Proceedings of the National Academy of Science of the USA. 2003;100:10574–10579. doi: 10.1073/pnas.1733839100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Stavola BL, Hardy R, Kuh D, Silva IS, Wadsworth MEJ, Swerdlow AJ. Birthweight, childhood growth and risk of breast cancer in a British cohort. British Journal of Cancer. 2000;83:964–968. doi: 10.1054/bjoc.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elley W, Irving J. The Elley-Irving socio-economic index 1981 census revision. New Zealand Journal of Educational Studies. 1985;20:115–128. [Google Scholar]
- 18.Frankel S, Elwood P, Sweetnam P, Yarnell J, Davey Smith G. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–1480. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert GH, Litaker M. Validity of self-reported periodontal status in the Florida Dental Care Study. Journal of Periodontology. 2007;78:1429–1438. doi: 10.1902/jop.2007.060199. [DOI] [PubMed] [Google Scholar]
- 20.Gomez RS, Dutra WO, Moreira PR. Epigenetics and periodontal disease: future perspectives. Inflammation Research. 2009;58:625–629. doi: 10.1007/s00011-009-0041-7. [DOI] [PubMed] [Google Scholar]
- 21.Gorlin RJ, Stallard RE, Shapiro BL. Genetics and periodontal disease. Journal of Periodontology. 1967;38:5–10. doi: 10.1902/jop.1967.38.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Greene JC, Vermillion JR. The Simplified Oral Hygiene Index. Journal of the American Dental Association. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 23.Greenlund KJ, Valdez R, Bao W, Wattigney W, Srinivaan SR, Berenson GS. Verification of parental history of coronary artery disease and associations with adult offspring risk factors in a community sample: The Bogalusa Heart Study. American Journal of Medical Science. 1997;313:220–227. doi: 10.1097/00000441-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Hassell TM, Harris EL. Genetic influences in caries and periodontal diseases. Critical reviews in oral biology and medicine. 1995;6:319–342. doi: 10.1177/10454411950060040401. [DOI] [PubMed] [Google Scholar]
- 25.Hill KG, Hawkins JD, Catalano RF, Abbott RD, Guo J. Family influences on the risk of daily smoking initiation. Journal of Adolescent Health. 2005;37:202–210. doi: 10.1016/j.jadohealth.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Hood MY, Moore LL, Sundarajan-Ramamurti A, Singer M, Cupples LA, Ellison RC. Parental eating attitudes and the development of obesity in children. The Framingham Children's Study. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000;24:1319–1325. doi: 10.1038/sj.ijo.0801396. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh K, Albertsen PC. Populations at high risk for prostate cancer. Urologic Clinics of North America. 2003;30:669–676. doi: 10.1016/s0094-0143(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DJ. Gene-environment interactions in human diseases. Nature. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 29.Hyman J. The importance of assessing confounding and effect modification in research involving periodontal disease and systemic diseases. Journal of Clinical Periodontology. 2006;33:102–103. doi: 10.1111/j.1600-051X.2005.00881.x. [DOI] [PubMed] [Google Scholar]
- 30.Irving J, Elley W. A socio-economic index for the female labour force in New Zealand. New Zealand Journal of Educational Studies. 1977;12:154–163. [Google Scholar]
- 31.Jonsson S, Thorsteinsdottir U, Gudbjartsson DF, Jonsson HH, Kristjansson K, Arnason S, Gudnason V, Isaksson HJ, Hallgrimsson J, Gulcher JR, Amundadottir LT, Kong A, Stefansson K. Familial risk of lung carcinoma in the Icelandic population. Journal of the American Medical Association. 2004;292:2977–2983. doi: 10.1001/jama.292.24.2977. [Erratum appears in Journal of the American Medical Association (2005) 293, 163] [DOI] [PubMed] [Google Scholar]
- 32.Kandelman D, Petersen PE, Ueda H. Oral health, general health, and quality of life in older people. Special Care in Dentistry. 2008;28:224–236. doi: 10.1111/j.1754-4505.2008.00045.x. [DOI] [PubMed] [Google Scholar]
- 33.Khoury MJ. Genetics and genomics in practice: The continuum from genetic disease to genetic information in health and disease. Genetics in Medicine. 2003;5:261–268. doi: 10.1097/01.GIM.0000076977.90682.A5. [DOI] [PubMed] [Google Scholar]
- 34.Khoury MJ, Davis R, Gwinn M, Lindegren M, Yoon P. Do we need genomic research for the prevention of common diseases with environmental causes? American Journal of Epidemiology. 2005;161:799–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- 35.Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Critical Reviews in Oral Biology and Medicine. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- 36.Lascaux-Lefebvre V, Ruidavets JB, Arveiler D, Amouyel P, Haas B, Cottel D, Bingham A, Ducimetiere P, Ferrieres J. Influence of parental histories of cardiovascular risk factors on risk factor clusters in the offspring. Diabetes & Metabolism. 2001;27:503–509. [PubMed] [Google Scholar]
- 37.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependant diabetes and insulin concentrations in men aged 50–60 years. British Medical Journal. 1996;312:406–410. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Löe H. Periodontal diseases: a brief historical perspective. Periodontology 2000. 1993;2:7–12. doi: 10.1111/j.1600-0757.1993.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 39.Mattocks C, Ness A, Deere K, Tilling K, Leary S, Blair SN, Riddoch C. Early life determinants of physical activity in 11 to 12 year olds: cohort study. British Medical Journal. 2008;336:26–29. doi: 10.1136/bmj.39385.443565.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 41.Meisel P, Schwahn C, Gesch D, Bernhardt O, John U, Kocher T. Dose-effect relation of smoking and the Interleukin-1 gene polymorphism in periodontal disease. Journal of Periodontology. 2004;75:236–242. doi: 10.1902/jop.2004.75.2.236. [DOI] [PubMed] [Google Scholar]
- 42.Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 43.Michalowitz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE, Califano JV, Burmeister JA, Schenkein HA. Evidence of a substantial genetic basis for risk of adult periodontitis. Journal of Periodontology. 2000;71:1699–1707. doi: 10.1902/jop.2000.71.11.1699. [DOI] [PubMed] [Google Scholar]
- 44.Milne BJ, Caspi A, Crump R, Poulton R, Rutter M, Sears MR, Moffitt TE. The validity of the Family History Screen for assessing family history of mental disorders. American Journal of Medical Genetics. 2008a;Part B:41–49. doi: 10.1002/ajmg.b.30764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milne BJ, Moffitt TE, Crump R, Poulton R, Rutter M, Sears MR, Taylor A, Caspi A. How should we construct family history scores? A comparison of alternative approaches from the Dunedin Family Health History Study. Psychological Medicine. 2008b;38:1793–1802. doi: 10.1017/S0033291708003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 47.Nadeau JH. Transgenerational genetic effects on phenotypic variation and disease risk. Human Molecular Genetics. 2009;18:R202–R210. doi: 10.1093/hmg/ddp366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Needleman I, McGrath C, Floyd P, Biddle A. Impact of oral health on the life quality of periodontal patients. Journal of Clinical Periodontology. 2004;31:454–457. doi: 10.1111/j.1600-051X.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 49.Newman B, Selby JV, King MC, Slemenda C, Fabsitz R, Friedman GD. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia. 1987;30:763–768. doi: 10.1007/BF00275741. [DOI] [PubMed] [Google Scholar]
- 50.Nibali L, Donos N, Brett PM, Parkar M, Ellinas T, Llorente M, Griffiths GS. A familial analysis of aggressive periodontitis –clinical and genetic findings. Journal of Periodontal Research. 2008;43:627–634. doi: 10.1111/j.1600-0765.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 51.Oral Health U.S. Dental, Oral and Craniofacial Data Resource Center. Bethesda, Maryland: 2002 http://drc.hhs.gov/report/index.htm.
- 52.Page RC. Milestones in periodontal research and the remaining critical issues. Journal of Periodontal Research. 1999;34:331–339. doi: 10.1111/j.1600-0765.1999.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 53.Parikh NI, Hwang SJ, Larson MG, Cupples LA, Fox CS, Manders ES, Murabito JM, Massaro JM, Hoffmann U, O'Donnell CJ. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 54.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. International Journal of Cancer. 1997;71:800–809. doi: 10.1002/(sici)1097-0215(19970529)71:5<800::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 55.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. International journal of Epidemiology. 2002;31:413–419. [PubMed] [Google Scholar]
- 56.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A for the Avon Longitudinal Study of Parents and Children Study Team. Early life risk factors for obesity in childhood: cohort study. British Medical Journal. 2005;330 doi: 10.1136/bmj.38470.670903.E0. (published 670920 May 672005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose G. Familial concentration of ischaemic heart disease. British Medical Journal. 1967;2:4–5. doi: 10.1136/bmj.2.5543.4-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheuner MT, Wang SJ, Raffel LJ, Larabell SK, Rotter JI. Family history: a comprehensive genetic risk assessment method for the chronic conditions of adulthood. American Journal of Medical Genetics. 1997;71:315–324. doi: 10.1002/(sici)1096-8628(19970822)71:3<315::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 59.Sesso HD, Lee I, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 60.Shearer DM, Thomson WM. Intergenerational continuity in oral health: a review. Community Dentistry and Oral Epidemiology. 2010;38:479–486. doi: 10.1111/j.1600-0528.2010.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shenassa ED, McCaffery JM, Swan GE, Khroyan TV, Shakib S, Lerman C, Lyons M, Mouttapa M, Niaura RS, Buka SL, Leslie F, Santangelo SL. Intergenerational transmission of tobacco use and dependence: a transdisciplinary perspective. Nicotine & Tobacco Research. 2003;5:S55–S69. doi: 10.1080/14622200310001625500. [DOI] [PubMed] [Google Scholar]
- 62.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends in Endocrinology & Metabolism. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slade G, Spencer AJ, Roberts-Thomson KF, editors. Australia's Dental Generations: The Australian National Survey of Adult Oral Health 2004–2006. Canberra: Australian Institute of Health and Welfare; 2007 (Dental Statistics and Research series No.34)
- 64.Srinivasan SR, Frontini MG, Berenson GS. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: the Bogalusa Heart Study. Metabolism: Clinical & Experimental. 2003;52:443–450. doi: 10.1053/meta.2003.50065. [DOI] [PubMed] [Google Scholar]
- 65.Thomson WM, Broadbent JM, Poulton R, Beck J. Changes in periodontal disease experience from 26 to 32 years of age in a birth cohort. Journal of Periodontology. 2006;77:947–954. doi: 10.1902/jop.2006.050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomson WM, Broadbent JM, Welch D, Beck J, Poulton R. Cigarette smoking and periodontal disease among 32-year-olds: a prospective study of a representative birth cohort. Journal of Clinical Periodontology. 2007;34:828–834. doi: 10.1111/j.1600-051X.2007.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomson WM, Poulton R, Broadbent JM, Moffitt TE, Caspi A, Beck J, Welch D, Hancox R. Cannabis smoking and periodontal disease among young adults. Journal of the American Medical Association. 2008;299:525–531. doi: 10.1001/jama.299.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomson WM, Williams SM, Broadbent JM, Poulton R, Locker D. Long-term dental visiting patterns and adult oral health. Journal of Dental Research. 2010;89:307–311. doi: 10.1177/0022034509356779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: a genomic tool for disease prevention and health promotion. Annual Review of Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 70.Whitaker RC, Wright JA, Pepe MS, Siedel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. The New England Journal of Medicine. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 71.Wimmer G, Pihlstrom BL. A critical assessment of adverse pregnancy outcome and periodontal disease. Journal of Clinical Periodontology. 2008;35:380–397. doi: 10.1111/j.1600-051X.2008.01284.x. [DOI] [PubMed] [Google Scholar]
- 72.Yoon P, Scheuner MT, Jorgensen C, Khoury MJ. Developing Family Healthware, a family history screening tool to prevent common chronic diseases. Preventing Chronic Disease: Public Health Research, Practice and Policy. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 73.Yoon P, Scheuner MT, Peterson-Oehlke MS, Gwinn M, Faucett MS, Khoury MJ. Can family history be used as a tool for public health and preventive medicine? Genetics in Medicine. 2002;4:304–310. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Zimmerman DJ. Regression toward mediocrity in economic stature. The American Economic Review. 1992;82:409–429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.