Abstract
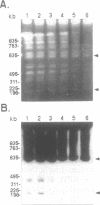
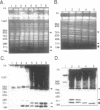
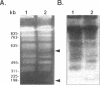
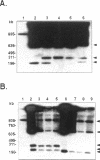
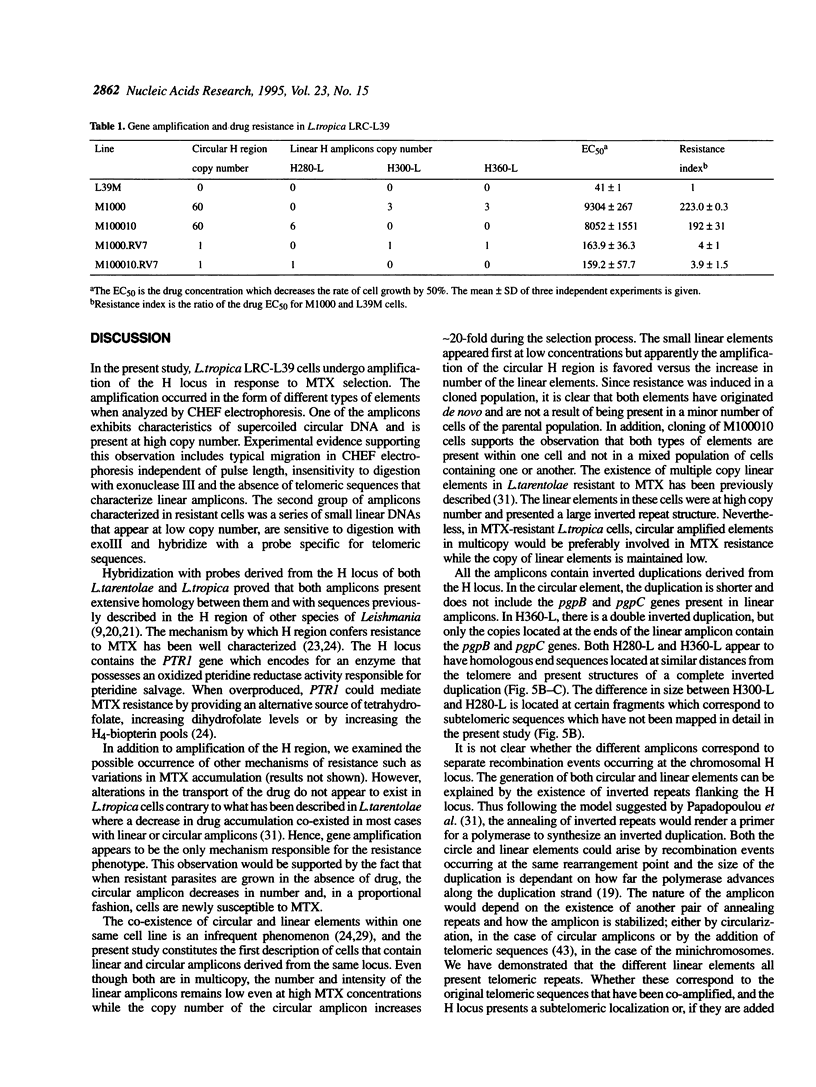
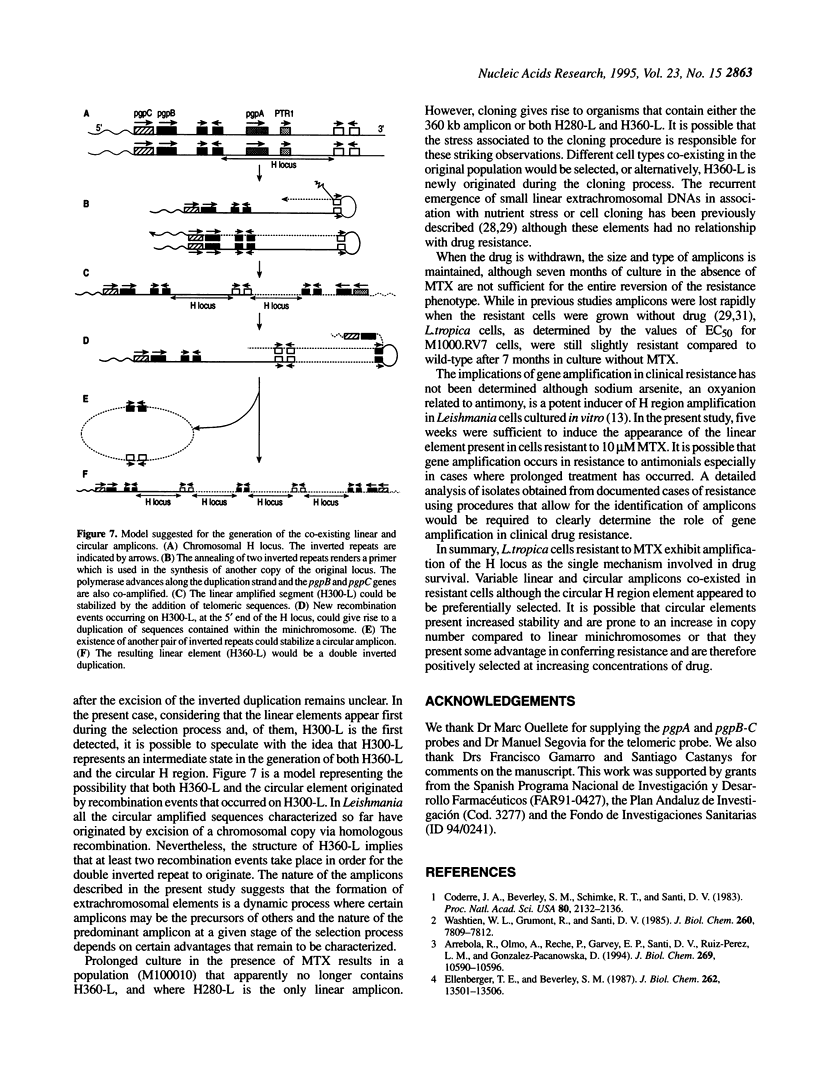
Circular and linear amplicons were analyzed in detail in Leishmania tropica cells resistant to methotrexate (MTX). Both types of elements presented sequences related to the H locus and coexisted in resistant cells. The linear amplicons appeared first during the selection process (at 10 microM MTX) and varied with regard to size and structure in cells exposed to increasing concentrations of drug. The circular element was evident at higher concentrations (50 microMs) but was the major amplified DNA in cells resistant to 1000 microM MTX while the level of amplification of the linear elements remained low. The extrachromosomal DNAs were unstable in the absence of drug and their disappearance coincided with an increase in sensitivity to MTX. Mapping of the minichromosomes and the circular element showed that they were all constituted by inverted duplications. The circular amplicon contained an inverted repeat derived from the H locus that encompassed the pteridine reductase gene (PTR1) responsible for MTX resistance. The amplified segment in the linear amplicons was longer and included the pgpB and pgpC genes that encode P-glycoproteins of unknown function previously characterized in different Leishmania species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrebola R., Olmo A., Reche P., Garvey E. P., Santi D. V., Ruiz-Perez L. M., Gonzalez-Pacanowska D. Isolation and characterization of a mutant dihydrofolate reductase-thymidylate synthase from methotrexate-resistant Leishmania cells. J Biol Chem. 1994 Apr 8;269(14):10590–10596. [PubMed] [Google Scholar]
- Bello A. R., Nare B., Freedman D., Hardy L., Beverley S. M. PTR1: a reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11442–11446. doi: 10.1073/pnas.91.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coburn C. M. Recurrent de novo appearance of small linear DNAs in Leishmania major and relationship to extra-chromosomal DNAs in other species. Mol Biochem Parasitol. 1990 Aug;42(1):133–141. doi: 10.1016/0166-6851(90)90121-2. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blaineau C., Bastien P., Rioux J. A., Roizès G., Pagès M. Long-range restriction maps of size-variable homologous chromosomes in Leishmania infantum. Mol Biochem Parasitol. 1991 Jun;46(2):292–302. doi: 10.1016/0166-6851(91)90053-9. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. A member of the aldoketo reductase family confers methotrexate resistance in Leishmania. J Biol Chem. 1992 Dec 5;267(34):24165–24168. [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Callahan H. L., Roberts W. L., Rainey P. M., Beverley S. M. The PGPA gene of Leishmania major mediates antimony (SbIII) resistance by decreasing influx and not by increasing efflux. Mol Biochem Parasitol. 1994 Nov;68(1):145–149. doi: 10.1016/0166-6851(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Chiquero M. J., Olmo A., Navarro P., Ruiz-Perez L. M., Castanys S., Gonzalez-Pacanowska D., Gamarro F. Amplification of the H locus in Leishmania infantum. Biochim Biophys Acta. 1994 Nov 29;1227(3):188–194. doi: 10.1016/0925-4439(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Reductions in methotrexate and folate influx in methotrexate-resistant lines of Leishmania major are independent of R or H region amplification. J Biol Chem. 1987 Oct 5;262(28):13501–13506. [PubMed] [Google Scholar]
- Ellis J., Crampton J. Characterisation of a simple, highly repetitive DNA sequence from the parasite Leishmania donovani. Mol Biochem Parasitol. 1988 May;29(1):9–17. doi: 10.1016/0166-6851(88)90114-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Coderre J. A., Santi D. V. Selection and properties of Leishmania tropica resistant to 10-propargyl-5,8-dideazafolate, an inhibitor of thymidylate synthetase. Mol Biochem Parasitol. 1985 Oct;17(1):79–91. doi: 10.1016/0166-6851(85)90129-x. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Grondin K., Papadopoulou B., Ouellette M. Homologous recombination between direct repeat sequences yields P-glycoprotein containing amplicons in arsenite resistant Leishmania. Nucleic Acids Res. 1993 Apr 25;21(8):1895–1901. doi: 10.1093/nar/21.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S., Beverley S. M., Wagner W., Ullman B. Unstable amplification of two extrachromosomal elements in alpha-difluoromethylornithine-resistant Leishmania donovani. Mol Cell Biol. 1992 Dec;12(12):5499–5507. doi: 10.1128/mcb.12.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Ruiz-Perez L. M., Wong M. L., Santi D. V. Extrachromosomal elements in the lower eukaryote Leishmania. J Biol Chem. 1988 Nov 15;263(32):16970–16976. [PubMed] [Google Scholar]
- Iovannisci D. M., Beverley S. M. Structural alterations of chromosome 2 in Leishmania major as evidence for diploidy, including spontaneous amplification of the mini-exon array. Mol Biochem Parasitol. 1989 May 1;34(2):177–188. doi: 10.1016/0166-6851(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Ullman B. High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J Parasitol. 1983 Aug;69(4):633–636. [PubMed] [Google Scholar]
- Jackson P. R., Wohlhieter J. A., Jackson J. E., Sayles P., Diggs C. L., Hockmeyer W. T. Restriction endonuclease analysis of Leishmania kinetoplast DNA characterizes parasites responsible for visceral and cutaneous disease. Am J Trop Med Hyg. 1984 Sep;33(5):808–819. doi: 10.4269/ajtmh.1984.33.808. [DOI] [PubMed] [Google Scholar]
- Katakura K., Chang K. P. H DNA amplification in Leishmania resistant to both arsenite and methotrexate. Mol Biochem Parasitol. 1989 May 1;34(2):189–191. doi: 10.1016/0166-6851(89)90010-8. [DOI] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Légaré D., Hettema E., Ouellette M. The P-glycoprotein-related gene family in Leishmania. Mol Biochem Parasitol. 1994 Nov;68(1):81–91. doi: 10.1016/0166-6851(94)00156-1. [DOI] [PubMed] [Google Scholar]
- Navarro M., Maingon R., Hamers R., Segovia M. Dynamics and size polymorphisms of minichromosomes in Leishmania major LV-561 cloned lines. Mol Biochem Parasitol. 1992 Oct;55(1-2):65–74. doi: 10.1016/0166-6851(92)90127-6. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Borst P. Drug resistance and P-glycoprotein gene amplification in the protozoan parasite Leishmania. Res Microbiol. 1991 Jul-Aug;142(6):737–746. doi: 10.1016/0923-2508(91)90089-s. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Hettema E., Wüst D., Fase-Fowler F., Borst P. Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. EMBO J. 1991 Apr;10(4):1009–1016. doi: 10.1002/j.1460-2075.1991.tb08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993 May;9(5):150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Mourad W., Leblanc E., Ouellette M. Changes in folate and pterin metabolism after disruption of the Leishmania H locus short chain dehydrogenase gene. J Biol Chem. 1994 Mar 11;269(10):7310–7315. [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Ouellette M. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 1992 Oct;11(10):3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Ouellette M. Frequent amplification of a short chain dehydrogenase gene as part of circular and linear amplicons in methotrexate resistant Leishmania. Nucleic Acids Res. 1993 Sep 11;21(18):4305–4312. doi: 10.1093/nar/21.18.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo-Peixoto M. L., Beverley S. M. Amplified DNAs in laboratory stocks of Leishmania tarentolae: extrachromosomal circles structurally and functionally similar to the inverted-H-region amplification of methotrexate-resistant Leishmania major. Mol Cell Biol. 1988 Dec;8(12):5188–5199. doi: 10.1128/mcb.8.12.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi M., Janse C. J., Dore E., Scotti R., Pace T., Reterink T. J., van der Berg F. M., Mons B. Generation of chromosome size polymorphism during in vivo mitotic multiplication of Plasmodium berghei involves both loss and addition of subtelomeric repeat sequences. Mol Biochem Parasitol. 1990 Jun;41(1):73–82. doi: 10.1016/0166-6851(90)90098-7. [DOI] [PubMed] [Google Scholar]
- Rovai L., Tripp C., Stuart K., Simpson L. Recurrent polymorphisms in small chromosomes of Leishmania tarentolae after nutrient stress or subcloning. Mol Biochem Parasitol. 1992 Jan;50(1):115–125. doi: 10.1016/0166-6851(92)90249-j. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stuart K. D. Circular and linear multicopy DNAs in Leishmania. Parasitol Today. 1991 Jul;7(7):158–159. doi: 10.1016/0169-4758(91)90119-9. [DOI] [PubMed] [Google Scholar]
- Tripp C. A., Wisdom W. A., Myler P. J., Stuart K. D. A multicopy, extrachromosomal DNA in Leishmania infantum contains two inverted repeats of the 27.5-kilobase LD1 sequence and encodes numerous transcripts. Mol Biochem Parasitol. 1992 Oct;55(1-2):39–50. doi: 10.1016/0166-6851(92)90125-4. [DOI] [PubMed] [Google Scholar]
- Washtien W. L., Grumont R., Santi D. V. DNA amplification in antifolate-resistant Leishmania. The thymidylate synthase-dihydrofolate reductase gene and abundant mRNAs. J Biol Chem. 1985 Jul 5;260(13):7809–7812. [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Wilson K., Beverley S. M., Ullman B. Stable amplification of a linear extrachromosomal DNA in mycophenolic acid-resistant Leishmania donovani. Mol Biochem Parasitol. 1992 Oct;55(1-2):197–206. doi: 10.1016/0166-6851(92)90140-f. [DOI] [PubMed] [Google Scholar]