Abstract
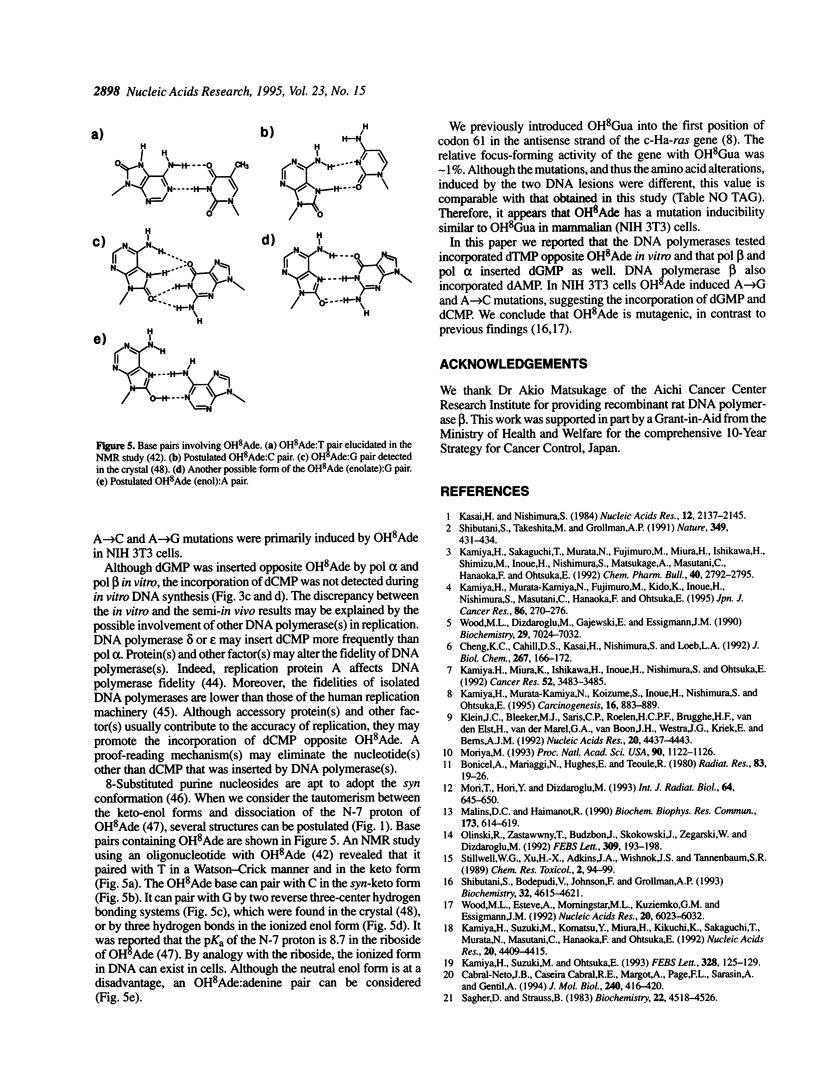
An oligodeoxyribonucleotide containing 8-hydroxyadenine (OH8Ade) was chemically synthesized and single- and double-stranded c-Ha-ras gene fragments with OH8Ade at the second position of codon 61 were prepared. The single-stranded ras gene fragment was used as a template for in vitro DNA synthesis with the Klenow fragment of Escherichia coli DNA polymerase I, Taq DNA polymerase, rat DNA polymerase beta and mouse DNA polymerase alpha. The former two enzymes exclusively incorporated dTMP opposite OH8Ade. The DNA polymerases alpha and beta misinserted dGMP, and dAMP and dGMP, respectively. The c-Ha-ras gene was constructed using the double-stranded ras gene fragment containing OH8Ade and was transfected into NIH 3T3 cells. The gene with OH8Ade induced focus formation, indicating that OH8Ade elicited point mutations in cells. When c-Ha-ras genes present in transformed cells were analyzed, an A-->G transition and an A-->C transversion were detected. These results indicate that OH8Ade induced misincorporation in in vitro DNA synthesis and mutations in mammalian cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessho T., Tano K., Kasai H., Ohtsuka E., Nishimura S. Evidence for two DNA repair enzymes for 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in human cells. J Biol Chem. 1993 Sep 15;268(26):19416–19421. [PubMed] [Google Scholar]
- Bonicel A., Mariaggi N., Hughes E., Teoule R. In vitro gamma irradiation of DNA: identification of radioinduced chemical modifications of the adenine moiety. Radiat Res. 1980 Jul;83(1):19–26. [PubMed] [Google Scholar]
- Cabral Neto J. B., Cabral R. E., Margot A., Le Page F., Sarasin A., Gentil A. Coding properties of a unique apurinic/apyrimidinic site replicated in mammalian cells. J Mol Biol. 1994 Jul 29;240(5):416–420. doi: 10.1006/jmbi.1994.1457. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Cho B. P., Evans F. E. Structure of oxidatively damaged nucleic acid adducts. 3. Tautomerism, ionization and protonation of 8-hydroxyadenosine studied by 15N NMR spectroscopy. Nucleic Acids Res. 1991 Mar 11;19(5):1041–1047. doi: 10.1093/nar/19.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W., Duplaa A. M., Guy A., Téoule R., Fazakerley G. V. Structure and in vitro replication of DNA templates containing 7,8-dihydro-8-oxoadenine. Nucleic Acids Res. 1991 Apr 25;19(8):1753–1758. doi: 10.1093/nar/19.8.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R. G., Ochman H. Production of single-stranded DNA templates by exonuclease digestion following the polymerase chain reaction. Nucleic Acids Res. 1989 Jul 25;17(14):5865–5865. doi: 10.1093/nar/17.14.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H., Miura H., Kato H., Nishimura S., Ohtsuka E. Induction of mutation of a synthetic c-Ha-ras gene containing hypoxanthine. Cancer Res. 1992 Apr 1;52(7):1836–1839. [PubMed] [Google Scholar]
- Kamiya H., Miura K., Ishikawa H., Inoue H., Nishimura S., Ohtsuka E. c-Ha-ras containing 8-hydroxyguanine at codon 12 induces point mutations at the modified and adjacent positions. Cancer Res. 1992 Jun 15;52(12):3483–3485. [PubMed] [Google Scholar]
- Kamiya H., Murata-Kamiya N., Fujimuro M., Kido K., Inoue H., Nishimura S., Masutani C., Hanaoka F., Ohtsuka E. Comparison of incorporation and extension of nucleotides in vitro opposite 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in hot spots of the c-Ha-ras gene. Jpn J Cancer Res. 1995 Mar;86(3):270–276. doi: 10.1111/j.1349-7006.1995.tb03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H., Murata-Kamiya N., Koizume S., Inoue H., Nishimura S., Ohtsuka E. 8-Hydroxyguanine (7,8-dihydro-8-oxoguanine) in hot spots of the c-Ha-ras gene: effects of sequence contexts on mutation spectra. Carcinogenesis. 1995 Apr;16(4):883–889. doi: 10.1093/carcin/16.4.883. [DOI] [PubMed] [Google Scholar]
- Kamiya H., Murata N., Murata T., Iwai S., Matsukage A., Masutani C., Hanaoka F., Ohtsuka E. Cyclobutane thymine dimers in a ras proto-oncogene hot spot activate the gene by point mutation. Nucleic Acids Res. 1993 May 25;21(10):2355–2361. doi: 10.1093/nar/21.10.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H., Sakaguchi T., Murata N., Fujimuro M., Miura H., Ishikawa H., Shimizu M., Inoue H., Nishimura S., Matsukage A. In vitro replication study of modified bases in ras sequences. Chem Pharm Bull (Tokyo) 1992 Oct;40(10):2792–2795. doi: 10.1248/cpb.40.2792. [DOI] [PubMed] [Google Scholar]
- Kamiya H., Suzuki M., Komatsu Y., Miura H., Kikuchi K., Sakaguchi T., Murata N., Masutani C., Hanaoka F., Ohtsuka E. An abasic site analogue activates a c-Ha-ras gene by a point mutation at modified and adjacent positions. Nucleic Acids Res. 1992 Sep 11;20(17):4409–4415. doi: 10.1093/nar/20.17.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H., Suzuki M., Ohtsuka E. Mutation-spectrum of a true abasic site in codon 12 of a c-Ha-ras gene in mammalian cells. FEBS Lett. 1993 Aug 9;328(1-2):125–129. doi: 10.1016/0014-5793(93)80979-5. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. C., Bleeker M. J., Saris C. P., Roelen H. C., Brugghe H. F., van den Elst H., van der Marel G. A., van Boom J. H., Westra J. G., Kriek E. Repair and replication of plasmids with site-specific 8-oxodG and 8-AAFdG residues in normal and repair-deficient human cells. Nucleic Acids Res. 1992 Sep 11;20(17):4437–4443. doi: 10.1093/nar/20.17.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Borden A., Banerjee S. K., LeClerc J. E. Mutation frequency and spectrum resulting from a single abasic site in a single-stranded vector. Nucleic Acids Res. 1990 Apr 25;18(8):2153–2157. doi: 10.1093/nar/18.8.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard G. A., Guy A., Brown T., Téoule R., Hunter W. N. Conformation of guanine-8-oxoadenine base pairs in the crystal structure of d(CGCGAATT(O8A)GCG). Biochemistry. 1992 Sep 15;31(36):8415–8420. doi: 10.1021/bi00151a004. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. 4,6-Diamino-5-formamidopyrimidine, 8-hydroxyguanine and 8-hydroxyadenine in DNA from neoplastic liver of English sole exposed to carcinogens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):614–619. doi: 10.1016/s0006-291x(05)80079-8. [DOI] [PubMed] [Google Scholar]
- Miura K., Kamiya H., Tominaga M., Inoue Y., Ikehara M., Noguchi S., Nishimura S., Ohtsuka E. Overproduction of cellular and activated Ha-ras proteins by mutating a synthetic gene. Chem Pharm Bull (Tokyo) 1987 Dec;35(12):4878–4882. doi: 10.1248/cpb.35.4878. [DOI] [PubMed] [Google Scholar]
- Mori T., Hori Y., Dizdaroglu M. DNA base damage generated in vivo in hepatic chromatin of mice upon whole body gamma-irradiation. Int J Radiat Biol. 1993 Dec;64(6):645–650. doi: 10.1080/09553009314551881. [DOI] [PubMed] [Google Scholar]
- Moriya M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G.C-->T.A transversions in simian kidney cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1122–1126. doi: 10.1073/pnas.90.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya M., Zhang W., Johnson F., Grollman A. P. Mutagenic potency of exocyclic DNA adducts: marked differences between Escherichia coli and simian kidney cells. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11899–11903. doi: 10.1073/pnas.91.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinski R., Zastawny T., Budzbon J., Skokowski J., Zegarski W., Dizdaroglu M. DNA base modifications in chromatin of human cancerous tissues. FEBS Lett. 1992 Sep 7;309(2):193–198. doi: 10.1016/0014-5793(92)81093-2. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Purmal A. A., Kow Y. W., Wallace S. S. Major oxidative products of cytosine, 5-hydroxycytosine and 5-hydroxyuracil, exhibit sequence context-dependent mispairing in vitro. Nucleic Acids Res. 1994 Jan 11;22(1):72–78. doi: 10.1093/nar/22.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. K., Eritja R., Kaplan B. E., Petruska J., Goodman M. F. Nucleotide insertion kinetics opposite abasic lesions in DNA. J Biol Chem. 1987 May 15;262(14):6864–6870. [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Bodepudi V., Johnson F., Grollman A. P. Translesional synthesis on DNA templates containing 8-oxo-7,8-dihydrodeoxyadenosine. Biochemistry. 1993 May 4;32(17):4615–4621. doi: 10.1021/bi00068a019. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Stillwell W. G., Xu H. X., Adkins J. A., Wishnok J. S., Tannenbaum S. R. Analysis of methylated and oxidized purines in urine by capillary gas chromatography-mass spectrometry. Chem Res Toxicol. 1989 Mar-Apr;2(2):94–99. doi: 10.1021/tx00008a004. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Izuta S., Yoshida S. DNA polymerase alpha overcomes an error-prone pause site in the presence of replication protein-A. J Biol Chem. 1994 Apr 8;269(14):10225–10228. [PubMed] [Google Scholar]
- Takada-Takayama R., Tada S., Hanaoka F., Ui M. Peptide mapping of the four subunits of the mouse DNA polymerase alpha-primase complex. Biochem Biophys Res Commun. 1990 Jul 31;170(2):589–595. doi: 10.1016/0006-291x(90)92132-j. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Tchou J., Bodepudi V., Shibutani S., Antoshechkin I., Miller J., Grollman A. P., Johnson F. Substrate specificity of Fpg protein. Recognition and cleavage of oxidatively damaged DNA. J Biol Chem. 1994 May 27;269(21):15318–15324. [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Roberts J. D., Sabatino R. D., Myers T. W., Tan C. K., Downey K. M., So A. G., Bambara R. A., Kunkel T. A. Fidelity of mammalian DNA replication and replicative DNA polymerases. Biochemistry. 1991 Dec 24;30(51):11751–11759. doi: 10.1021/bi00115a003. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Ikehara M. Carbon-13 magnetic resonance spectra of 8-substituted purine nucleosides. Characteristic shifts for the syn conformation. J Am Chem Soc. 1977 May 11;99(10):3250–3253. doi: 10.1021/ja00452a008. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Esteve A., Morningstar M. L., Kuziemko G. M., Essigmann J. M. Genetic effects of oxidative DNA damage: comparative mutagenesis of 7,8-dihydro-8-oxoguanine and 7,8-dihydro-8-oxoadenine in Escherichia coli. Nucleic Acids Res. 1992 Nov 25;20(22):6023–6032. doi: 10.1093/nar/20.22.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]