Abstract
Background
Preclinical evidence suggests that commonly used anesthetic agents induce long-lasting neurobehavioral changes when administered early in life but there has been virtually no attention to the neurodevelopmental consequences for the fetus of maternal anesthesia. This study tested the hypothesis that fetal rats exposed to isoflurane during maternal anesthesia on gestational day 14, which corresponds to the second trimester in humans, would be behaviorally abnormal as adults.
Methods
Timed, pregnant rats were randomly assigned on gestational day 14 to receive 1.4% isoflurane in 100% oxygen (N = 3) or 100 % oxygen (N = 2) for 4 h. Beginning at 8 weeks of age, male offspring were evaluated for spontaneous locomotor activity, hippocampal dependent learning and memory (spontaneous alternations, novel object recognition, and radial arm maze), and anxiety (elevated plus maze).
Results
Isoflurane anesthesia was physiologically well tolerated by the dams. Adult rats exposed prenatally to isoflurane were not different than controls on spontaneous locomotor activity, spontaneous alternations, or object recognition memory but made more open arm entries on the elevated plus maze and took longer and made more errors of omission on the radial arm maze.
Conclusions
Rats exposed to isoflurane in utero at a time that corresponds to the second trimester in humans have impaired spatial memory acquisition and, reduced anxiety compared to controls. This suggests the fetal brain may be adversely affected by maternal anesthesia and raises the possibility that vulnerability to deleterious neurodevelopmental effects of isoflurane begins much earlier in life than previously recognized.
INTRODUCTION
Preclinical studies demonstrate that commonly used sedatives and anesthetic agents administered at the extremes of life induce long-lasting neurobehavioral changes,1,2 and recent clinical studies support the possibility.3–5 General anesthetics administered during the critical ‘growth spurt’ phase of brain synaptogenesis cause apoptosis-mediated neurodegeneration and synapse loss, whereas they increase synaptogenesis later in neurodevelopment.1,6,7 However, brain development is well underway as early as the second trimester of pregnancy; neurogenesis, neuronal migration, and corticogenesis are major neurodevelopmental events at this stage.8 This is significant because most nonobstetric surgeries and fetal intervention procedures are performed during the second trimester.9,10 Nevertheless, there has been virtually no attention to the neurodevelopmental consequences for the fetus of maternal anesthesia.
There are several reasons for concern. First, most general anesthetic agents are lipophilic and cross the placenta easily.11 Second, fetal intervention procedures are relatively long, and general anesthesia is, of course, necessary. Third, high concentrations of anesthetic (~1.5 minimum alveolar concentration) are usually required to facilitate uterine quiescence and minimize the risk of preterm labor.12 Thus, clinical necessity and practice may inadvertently put the fetal brain at risk for neurodevelopmental abnormalities. Perhaps most importantly, the processes occurring early in fetal neurodevelopment are exquisitely sensitive to environmental and pharmacological influences.13–15 γ aminobutyric acid (GABA) receptor modulators are of particular interest in this regard because GABA is a trophic factor in the developing brain,16 and excessive or prolonged GABAergic stimulation during early neurodevelopment is capable of causing life-long behavioral consequences by altering neural connectivity.13,17 This is potentially important because while all of the volatile general anesthetics are pleiotropic agents that act at multiple receptors, the GABAA receptor is one of their principal sites of action.18 Accordingly, we hypothesized that early gestational exposure to isoflurane during maternal anesthesia may have adverse effects on the fetal brain that lead to behavioral abnormalities in adulthood.
MATERIALS AND METHODS
Subjects
With the approval of the Institutional Animal Care and Use Committee of Longwood Medical Area (Boston, Massachusetts), experiments were conducted on five timed-pregnant Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) and their respective offspring. The dams, weighing between 230–280 g, were acclimated to the approved housing facility for 3 days prior to anesthetic treatment on embryonic day 14 (E 14). This age was chosen as E 14 - 16 in a pregnant rat corresponds to the second trimester of human pregnancy,19,20 the period when most nonobstetric surgeries and fetal interventions are performed,10,12 and when both species show similar neurodevelopmental profiles.8,20 Dams were housed in standard polypropylene cages, exposed to a 12 h light/dark cycle in a temperature and humidity regulated room, with access to standard rat chow and water ad libitum. Appropriate care was taken to minimize the number of animals used and their suffering.
Anesthesia
On E 14, the dams were randomized to control (N = 2) or anesthesia (N = 3) groups. Animals randomized to anesthesia received 1.4% isoflurane in 100% oxygen for 4 h whereas control animals received 100% oxygen for 4 h in identical anesthetizing chambers. This concentration of isoflurane was selected because it represents 1 minimum alveolar concentration in the pregnant rodent.21 The animals breathed spontaneously and the isoflurane concentration was measured continuously with an agent analyzer (Datex, Tewksbury, MA; Ohmeda, Madison, WI). During isoflurane anesthesia, maternal blood pressure was measured non-invasively every hour, rectal temperature was maintained at 37 ± 0.5°C with heating pads, and venous blood gas and plasma glucose were measured at the end of the anesthetic. These time points were chosen after we confirmed in a preliminary study (N = 2 dams) with continuous invasive blood pressure monitoring, hourly blood gases, and serial plasma glucose determinations that maternal physiology remained stable throughout a 4 h isoflurane anesthetic. Following anesthesia, the anesthetized and control dams recovered in 100% oxygen for 20 min following return of righting reflex. Maternal weight was monitored daily until delivery of the pups on gestational day 22. Each litter was group-housed with the corresponding dam until postnatal day 28 at which time pups were weaned and housed in pairs. To eliminate the influence of the estrus stage on cognitive behavior, only adult male offspring were used for these experiments. Animals were singly housed during behavioral testing.
All animals were acclimated to the experimental environment for 1 week prior to behavioral characterization. Blinded investigators performed the behavioral experiments, and all equipments used for behavioral testing were wiped with 70% ethanol between animal uses to eliminate olfactory trails. The behavioral tasks were designed to detect changes in locomotor activity (spontaneous locomotor activity task), exploratory behavior (spontaneous alternation task, novel object recognition task), anxiety (elevated plus maze task), and spatial working memory (radial arm maze task).
Spontaneous Locomotor Activity
Spontaneous locomotor activity was tested in a black Plexiglas chamber (50 cm × 50 cm × 40 cm) illuminated from below with a 25W incandescent bulb (approximately 50 lux). The animal was placed in the box and allowed to move spontaneously for 5 min. Animal movement was tracked using Any-maze video-tracking software (Stoelting Co., Wood Dale, IL) to evaluate distance traveled, mean speed, total number of immobile episodes, and the total duration of immobility.
Spontaneous Alternation
This task depends on the rodents’ innate tendency to explore new areas and tests spatial working memory.22 The task was performed on a Y-maze with arm dimensions of 50 × 10 × 30 cm. The rat was placed at the end of one arm and spontaneous movement observed for a period of 5 min. Arm entry was scored when all four paws were completely inside the arm, and an alternation was scored when there were successive entries into three consecutive arms (a triplet). The total number of possible alternations was the total number of arm entries minus 2. The percent alternation score was calculated by dividing the actual number of alternations by the number of possible alternations, expressed as a percentage.
Elevated Plus Maze Task
The elevated plus maze task evaluates anxiety-like behaviors.23 The maze, elevated 60 cm off the floor by wooden legs, consists of four arms, each 50 × 10 cm, intersecting each other; a pair of opposing arms has walls 50 cm in height (the ‘closed’ arms) and the other pair has a 2 cm ridge (the ‘open’ arms) to prevent the rat from falling off the maze. The entire testing chamber was uniformly illuminated with ambient fluorescent lighting at an intensity of approximately 200 lux. After randomization, rats were placed in the center of the maze and allowed to explore freely for 5 min. This task pits the inherent tendency of the rat to explore novel areas against innate avoidance of open spaces. Increased time in the ‘closed’ relative to the ‘open’ arms is interpreted as greater levels of anxiety, usually measured by the extent to which the animals explore the open arms (frequency and duration of visits) relative to their exploration of all arms.
Novel Object Recognition Task
This task relies on rodents’ inherent preference for novelty and is not reliant on motivational state but is dependent on both the perirhinal cortex and the hippocampus.24 During the first stage of this task, the rat was allowed to explore two identical objects, placed at a distance of 25 cm from each other in a plexiform glass chamber (50 cm × 50 cm × 40 cm), for 5 min, and its performance was tracked using an automated tracking device and accompanying Any-Maze software before returning it to the home cage for 2 h. All objects and the maze were wiped with 70% ethanol and the novel object (different in shape, size and texture) was placed in the position of the object that was least explored; the rat was now returned to the testing chamber where he was allowed to explore the objects for 2 min. Data were manually scored by an observer blinded to treatment condition and confirmed using an automated tracking device and accompanying Any-Maze software (Stoelting Co.). The number of approaches to the novel and familiar object, and the time spent exploring them were recorded. Exploration was scored when the rat sniffed the object or placed its nose within a zone 2 cm (marked by the computer) around the object. Sitting or standing on the object were not considered exploratory behaviors. ‘Discrimination ratio’, defined as the difference in exploration time for objects divided by the total exploration time, was subsequently calculated.
Radial Arm Maze Task
The radial arm maze task is used to assess spatial memory in rodents.25 It consists of a central arena elevated 1 m off the floor, with 12 radiating arms each of which has a recessed cup at the end for hiding a food reward (quarter pieces of Froot Loop cereal, Kellog’s, Battle Creek, MI). To facilitate spatial navigation, fixed, extra-maze cues in the form of simple geometrical designs were placed on the walls of the maze room. To ensure motivated performance all rats were food restricted to 85% of their baseline weight, with free access to water in the home cage. Rats were habituated to the maze daily for 7 days. Habituation consisted of placing the animal in the maze without extra-maze clues and allowing it to freely explore and eat randomly scattered food rewards on the maze. Each habituation trial was terminated when the rat had explored all 12 arms, or 10 min had elapsed. Formal testing consisted of a daily 10-min session in which the rat was placed on the central arena of the maze with all arms baited with food reward. The rat was allowed to choose arms in any order until all 12 arms were visited or 10 min had elapsed. A correct choice was defined as one in which the rat entered a baited arm not previously explored, whereas an error was scored when the rat entered an arm it had previously explored (commission) or failed to enter the arm in 10 min (omission). The number of correct choices to first error, the total number of errors, and the time to complete the maze was recorded daily for 7 days.
Statistical Analysis
Behavioral data from pups born to anesthetized and control dams were analyzed with linear mixed models in which anesthetic condition (control or anesthetized) was modeled as a fixed effect and dam was modeled as a random, repeated-measures effect to account for non-independence of observations among pups born to the same dam. This analysis allowed for simultaneous modeling of effects of anesthetic condition and dam even though these variables are not independent (i.e., it was not possible for a particular dam to have both control and anesthetized offspring). In models in which behavioral testing occurred over several days, day was also entered as a random, repeated measures effect. All models were computed in SPSS 18.0.2 (SPSS Inc., Chicago, IL) using the MIXED command using a scaled identity matrix covariance structure for random subject effects, and a first-order autoregressive covariance structure for repeated effects (training day for radial maze testing), because this resulted in models that converged with superior fit indices compared to models with alternate covariance structures. Results from these analyses are presented as F-statistics from the estimates of fixed effects of anesthetic condition for locomotor activity, spontaneous alternation, elevated plus maze, and object recognition tasks; and F-statistics from the estimates of fixed effects of anesthetic condition, training day, and day×anesthesia interaction for the measures of radial maze performance. All other data were analyzed with Student’s t-test.
For comparisons of behavioral measures between the pups born to anesthetized and control dams, we employed a procedure described by Benjamini and Hochberg26 to control the overall false discovery rate to the 0.05 level. This procedure controls the expected proportion of falsely rejected hypotheses, and provides greater statistical power relative to procedures that control the family-wise error rate (such as Bonferroni corrections). In this study, we reject the null hypothesis, and treat as statistically significant, P-values less than 0.0075, the three smallest P-values from the 20 comparisons of behavioral measures between groups.
RESULTS
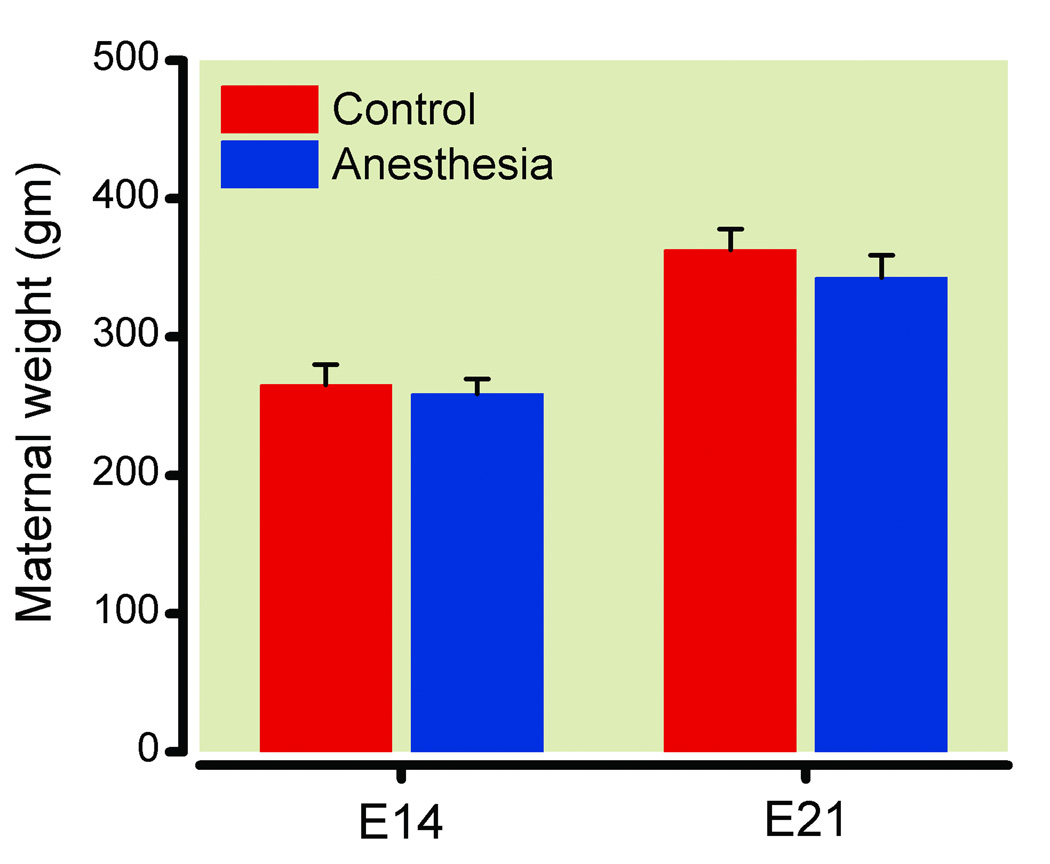
Blood pressure was stable in the dams during anesthesia, and oxygenation, ventilation, and plasma glucose were within normal limits (table 1). Weight of the control and anesthetized dams did not differ at the time of anesthesia on E 14 or at E 21, the day prior to delivery (fig. 1, P > 0.05); all pups were viable and there were no differences in litter size between the control and anesthetized dams (13 ± 0.8 vs. 10.3 ± 0.7 in control and isoflurane-exposed dams, respectively; P > 0.05, mean ± SEM). The weight of the adult male offspring was not different between the groups prior to behavioral testing (377 ± 10 vs. 376 ± 6 g in control and isoflurane-exposed animals, respectively; P > 0.05, mean ± SEM). Two rats exposed to isoflurane in utero were excluded from all behavioral analyses, as their spontaneous locomotor activity was 2 SD lower than the mean making them statistical outliers. A total of 26 adult male rats (N = 12 and 14 in control and anesthesia groups, respectively) were included in the final analysis.
TABLE 1.
Maternal Physiology during Isoflurane Anesthesia
| MAP 1 h (mm Hg) |
MAP 2 h (mm Hg) |
MAP 3 h (mm Hg) |
MAP 4 h (mm Hg) |
pH | PvCO2 (mm Hg) |
PvO2 (mm Hg) |
Glucose (mg/dl) |
|---|---|---|---|---|---|---|---|
| 109 ± 3 | 105 ± 6 | 100 ± 2 | 106 ± 3 | 7.41 ± 0.02 | 41.7 ± 1.6 | 356 ± 45 | 105 ± 10 |
Maternal physiology during isoflurane anesthesia. Blood gases and glucose were measured at 4 h of anesthesia on a maternal tail vein sample.
MAP = mean arterial blood pressure measured hourly.
Data are expressed as mean ± SEM for three pregnant rats.
Figure 1.
Maternal weight at the time of anesthesia (E 14) and one day before delivery (E 21). There were no differences between the groups (P > 0.05). Data are expressed as mean ± SEM for 2 control and 3 anesthetized dams.
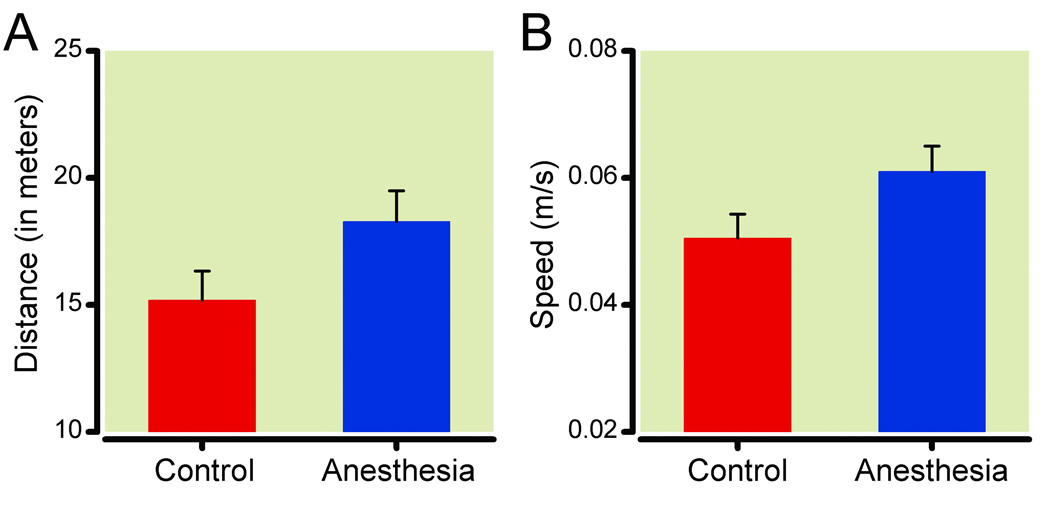
Rats anesthetized in utero were no more or less active than controls on the spontaneous locomotor activity test, as measured by increases in the distance traveled [F(1,26) = 3.664, P = 0.067; fig. 2A], mean speed [F(1,26) = 3.81, P = 0.062; fig. 2B], or the frequency or duration of immobile episodes [Fs(1,26) < 1.452, Ps > 0.15]. There were no differences in the number of arm entries during spontaneous alternation testing between isoflurane-exposed rats and control rats [16.7 ± 1.1 vs. 14.3 ± 1.6, mean ± SEM; F(1,26) = 5.023; P = 0.034, fig. 3], nor was there a difference in spontaneous alternations between the groups [F(1,26) = 0.005; P = 0.945].
Figure 2.
Spontaneous locomotor activity. There were no differences in either the distance traveled (Panel A) or the mean speed (Panel B) between adult rats exposed to isoflurane in utero (N = 14) and age-matched controls (N = 12) (P = 0.07 and 0.06, respectively). Data are expressed as mean ± SEM.
Figure 3.
Spontaneous alternation behavior. Adult rats exposed to isoflurane in utero (N = 14) were no different than controls (N = 12) either in the number of arm entries (P = 0.03, not significant after Benjamini-Hochberg correction) or in the percent alternation score, calculated by dividing the actual number of alternations by the number of possible alternations (not shown). Data are expressed as mean ± SEM.
The performance of one control rat was excluded from the elevated plus maze task because the time spent in the arms was > 2 SD from the mean of the control group (making this case a statistical outlier), and one previously anesthetized rat was excluded because it fell off the maze during testing. Isoflurane-treated rats made significantly more entries into the open arms, [F(1,24) = 9.448, P = 0.005, (fig. 4A)], which is consistent with decreased anxiety. However, time in the open arms [F(1,24) = 6.448, P = 0.018, (fig. 4B)], total arm entries [F(1,24) = 5.404, P = 0.029], and entries into the closed arms [F(1,24) = 1.143, P = 0.296] were not different between the control and isoflurane-exposed animals after applying the Benjamini-Hochberg correction.
Figure 4.
Performance in the elevated plus maze. Adult rats exposed to isoflurane in utero (N = 13) made more open arm entries (P = 0.005, significant after Benjamini-Hochberg correction; Panel A) though there were no differences in the time spent in the open arms (P = 0.02, not significant; Panel B) compared to controls (N = 11). Data are expressed as mean ± SEM.
In the object recognition task, control and isoflurane-treated rats did not differ in time spent exploring the objects during the first (sample) phase, [F(1,26) = 1.712, P = 0.202]. Similarly, there were no differences in the number of approaches to the novel object [F(1,26) = 0.899, P = 0.352], the familiar object [F(1,26) = 4.516, P = 0.043], or in the total number of approaches during the second (novel object) phase of testing [F(1,26) = 4.717, P = 0.039]. Isoflurane-exposed animals also were no different from controls for recognition memory as measured by the ‘discrimination ratio’ based on exploration time [F(1,26) = 0.735, P = 0.399]. Thus, isoflurane-exposed rats do not have a deficit in object recognition memory as tested in this paradigm.
In contrast, based on performance on the radial arm maze task, isoflurane-exposed pups had a spatial working memory deficit. This deficit was manifest as a main effect of anesthetic condition, with rats exposed to isoflurane in utero making more omission errors [F(1, 50.726) = 8.494; P = 0.005, fig. 5A], and taking longer to complete the task, [F(1, 61.794) = 12.705; P = 0.001, fig. 5B], both significant after Benjamini-Hochberg correction, than controls. In contrast, there was no day × anesthesia interaction [F(1,90.93) = 4.046, P = 0.047] and no difference in correct choices before the first error [F(1,63.969) = 4.781; P = 0.032, fig. 5C], total arm entries [F(1, 52.264) = 0.038, P = 0.845], or the total number of errors [F(1, 60.364) = 2.303; P = 0.134] (data not shown) after the Benjamini-Hochberg correction was applied. Thus, the effect of anesthetic treatment on time to complete the maze cannot be ascribed to reduced locomotor activity or drive to explore the maze. Notably, with the exception of total number of arm entries [F(1,100.091) = 0.074, P = 0.787], all measures of performance demonstrated the expected main effects of testing day [Fs(1,90.93–103.371) > 4.244, Ps ≤ 0.042], indicating progressively greater mastery of the task with continued training. The increase in time taken to complete the maze along with an increase in omission errors despite a similar number of arm entries is consistent with a spatial working memory deficit in isoflurane-exposed animals in this paradigm.
Figure 5.
Performance on the radial arm maze. There were no differences in the total number of arm entries on the maze between the groups but animals exposed to isoflurane in utero (N = 14) made more omission errors than the controls (N = 11; P = 0.005, significant after Benjamini-Hochberg correction)(A). On time to complete the maze (B), there was an effect of anesthesia condition (P = 0.001, significant after Benjamini-Hochberg correction), with rats exposed to isoflurane in utero taking longer to complete the maze than age-matched controls. There were no differences in the number of choices made before the first error in both groups (P = 0.03; C). Data are expressed as mean ± SEM and were analyzed using linear mixed models in which anesthetic condition (control or anesthetized) was modeled as a fixed effect and dam was modeled as a random, repeated-measures effect to account for non-independence of observations among pups born to the same dam.
DISCUSSION
The current study demonstrates that male rats exposed in utero to a clinically relevant concentration of isoflurane during maternal anesthesia are behaviorally abnormal as adults. Our behavioral phenotype was characterized by impaired acquisition of spatial working memory; there were no differences in spontaneous locomotor activity, spontaneous alternations, or novel object recognition memory and the increase in open arm entries on the elevated plus maze, which suggests reduced anxiety, was not apparent on other indices of anxiety (e.g., time in open arms). The behavioral changes are unlikely to be explained by an indirect adverse effect of isoflurane on maternal well-being because maternal systemic physiology was normal, and there were no differences in noncognitive variables such as litter size, viability, and weight between the isoflurane exposed and control animals. Nor are the behavioral deficits explained by defects in locomotor ability because animals exposed to isoflurane in utero made a similar number of arm entries on the radial arm maze task and, if anything, tended to be more active than controls in other behavioral tasks. Therefore, we conclude that during a time believed to correspond to the second trimester of human fetal development the fetal rat brain is adversely affected by maternally administered isoflurane.
Early neural development is genetically determined but precisely choreographed and highly regulated. Consequently, one would expect the brain to be differentially vulnerable depending upon the timing of a potential insult, with behavioral outcome varying as a function of the neurodevelopmental events occurring at the time of exposure. We selected E14 as our investigative time point for several reasons. First, it corresponds to the second trimester of human neurodevelopment,19,20 the period when most nonobstetric surgeries and fetal interventions are performed.9,10 Second, GABAA receptor subunits appear around E14 whereas N-methyl-D-aspartic receptors are believed to be non-functional at this age.27–29 Thus, the effects of isoflurane, which is a N-methyl-D-aspartic receptor-antagonist as well as GABA receptor modulator, are presumably largely GABA-mediated at E 14. Furthermore, GABA plays a major role in normal neurodevelopment at this age, implying it may be a time of particular vulnerability to GABAA receptor agonists or modulators.16 GABA is a trophic factor in the developing brain, where it regulates key developmental processes including proliferation of neural stem / progenitor cells, neuronal differentiation, and migration.16,30 As such, GABA controls the organization of three-dimensional architecture of the fetal brain.
Given this background, it is not surprising that excessive or prolonged GABAergic stimulation during fetal neurodevelopment is capable of causing life-long behavioral consequences by altering neural connectivity. 13,17 In fact, repeated exposure to high concentrations of halothane and enflurane during early and mid-gestation has been shown to cause learning dysfunction in adolescent mice.31 Moreover, behavioral abnormalities similar to those we describe have been demonstrated after maternal consumption of ethanol or valproic acid during early to mid gestation (E11-14); both agents have GABAergic properties and have been demonstrated to disrupt the cortical architecture of the fetal brain and produce behavioral abnormalities in adulthood.14,15,32,33 Changes in GABAA receptor subunit composition, distribution, and concentration of the neurotrophin brain derived neurotrophic factor have also been reported following fetal exposure to GABA receptor modulators such as the benzodiazepines and ethanol,34–36 and such changes have been implicated in anxiety-related behaviors in adulthood.37–40 Existing behavioral data, however, are inconsistent on this point; some studies report increased anxiety-like behavior following prenatal treatment with agents that have GABAergic properties37,38 whereas others find reduced anxiety later in life.39,40 Unfortunately, our study does not resolve this issue; although one feature of performance on the elevated plus maze (i.e., arm entries) supports the idea that isoflurane-exposed pups were less anxious as adults than controls, the phenotype was not evident on all measures of anxiety testing. Because of the complexity of early neurodevelopment and the pleiotropic nature of most sedatives and anesthetics, however, it is possible that their adverse neurodevelopmental effects are not entirely GABA mediated and plausible that subtle differences in the agent, timing, and length of exposure produce vastly different behavioral phenotypes.
The behavioral deficits in isoflurane-exposed rats consisted of taking longer to complete the radial arm maze and making more omission errors than control animals. These differences abated by the end of the testing period, however, indicating that isoflurane-exposed rats were capable of learning, albeit more slowly than controls. Inasmuch as there was no evidence of memory impairment in the spontaneous alternation task, one might speculate that the impairments in the radial maze indicate inadequate or impaired mastery of task rules rather than a true spatial working memory deficit per se. However, compared to the radial maze, the spontaneous alternation task is much simpler and can be solved by strategies that do not require activation of hippocampal circuits (e.g., directional sense, olfaction).41 As such, the spontaneous alternation task may be insufficiently sensitive to detect subtle learning impairment.
Our results contradict those of a previous report in which no behavioral impairment was observed following exposure of pregnant E 20 rats to isoflurane.42 However, that study differed from ours in one important respect: it focused on a later stage of gestation (E20).42 This is relevant because neurodevelopmental events at E20 are different than they are at E14.19,20,42,43 Consequently, because the neurodevelopmental events occurring at the time of isoflurane exposure in the two studies differed, it is neither surprising nor unexpected that vulnerability to adverse effects of the agent would also differ. Our findings may also seem to be at odds with a retrospective epidemiological study that explored potential association between the type of anesthesia for cesarean delivery and the incidence of learning disability;44 the study did not find any evidence for learning disability in childhood after general anesthetic exposure in utero. However, the exposure times were short and the exposure to general anesthesia occurred near or at term.
This study is limited in several important respects. First, it was a behavioral study and therefore provides no insight into the causative mechanisms. Second, our study was underpowered to detect differences in locomotor activity and we did not comprehensively phenotype animals on noncognitive behaviors so the effect of fetal isoflurane exposure on other aspects of neurodevelopment is unresolved. Third, because in vitro evidence suggests that hyperoxia may be harmful to developing neurons,45 our use of 100% oxygen as a carrier gas may be questioned. This is unlikely to explain our results, however, because the control dams also received 100% oxygen for the same period of time and the fetal brain was probably not exposed to a high oxygen concentration because of the arterial-venous admixture in the placenta. Furthermore, it is common practice to use 100% oxygen during maternal anesthesia in humans. Fourth, because we investigated behavioral changes only in males, we can draw no conclusions regarding the vulnerability of females to in utero isoflurane exposure.
With pregnant women often needing surgery for nonpregnancy related illness12 and growing evidence that general anesthetics may be harmful at various stages of neurodevelopment,46–48 there is clearly a need for better understanding of how maternal general anesthesia affects the developing fetal brain. Our preclinical study examines this issue and provides evidence that rats exposed to isoflurane in utero at a time that corresponds to the second trimester of humans have a learning disability as adults. This is consistent with the contemporary view that altered developmental programming in utero may contribute to a host of cognitive deficits, psychiatric disturbances, and other diseases that manifest later in life49–51 and suggests that the fetal brain may be adversely affected by maternal anesthesia. More to the point, these results add to the growing body of evidence indicating that exposure to anesthetic agents during critical periods of neural development cause persistent behavioral impairment and suggest that vulnerability to these deleterious neurodevelopmental effects may begin earlier in life than previously recognized.
What we already know about this topic
General anesthesia during infancy affects neural development in animals and may disrupt development in humans
Whether fetal exposure to general anesthesia produces similar effects in humans is unknown
What this article tells us that is new
In pregnant rats during mid-gestation, anesthesia with isoflurane for 4 h resulted in deficits in spatial memory and reduced anxiety behavior in the offspring at adulthood
Acknowledgments
Financial Support:
This study was supported by National Institutes of Health (Bethesda, Maryland) Grants RO1 AG20253 and RO1 GM088817 (Gregory Crosby); K08NS048140, R21AG029856 and R01 GM088801 (Zhongcong Xie); KO8 GM077057 (Deborah J. Culley) and by an intramural fellowship awarded by the Department of Anesthesiology, Perioperative, and Pain Medicine of the Brigham & Women’s Hospital (Arvind Palanisamy).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting(s) where the work has been presented:
Poster presentation, Association of University Anesthesiologists 57th Annual Meeting, April 7–9, 2010 at Denver, Colorado
Oral Presentation, Society for Obstetric Anesthesia and Perinatology 42nd Annual Meeting, May 12–16 at San Antonio, Texas
REFERENCES
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 5.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Roo M, Klauser P, Briner A, Nikonenko I, Mendez P, Dayer A, Kiss JZ, Muller D, Vutskits L. Anesthetics rapidly promote synaptogenesis during a critical period of brain development. PLoS One. 2009;4:e7043. doi: 10.1371/journal.pone.0007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 8.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: What is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Goodman S. Anesthesia for nonobstetric surgery in the pregnant patient. Semin Perinatol. 2002;26:136–145. doi: 10.1053/sper.2002.32203. [DOI] [PubMed] [Google Scholar]
- 10.Tran KM. Anesthesia for fetal surgery. Semin Fetal Neonatal Med. 2010;15:40–45. doi: 10.1016/j.siny.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Dwyer R, Fee JP, Moore J. Uptake of halothane and isoflurane by mother and baby during caesarean section. Br J Anaesth. 1995;74:379–383. doi: 10.1093/bja/74.4.379. [DOI] [PubMed] [Google Scholar]
- 12.Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anestesiol. 2007;73:235–240. [PubMed] [Google Scholar]
- 13.Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, Ben-Ari Y, Represa A. Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia. 2007;48:684–693. doi: 10.1111/j.1528-1167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 14.Hausknecht KA, Acheson A, Farrar AM, Kieres AK, Shen RY, Richards JB, Sabol KE. Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci. 2005;119:302–310. doi: 10.1037/0735-7044.119.1.302. [DOI] [PubMed] [Google Scholar]
- 15.Kuwagata M, Ogawa T, Shioda S, Nagata T. Observation of fetal brain in a rat valproate-induced autism model: A developmental neurotoxicity study. Int J Dev Neurosci. 2009;27:399–405. doi: 10.1016/j.ijdevneu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metin C, Vallee RB, Rakic P, Bhide PG. Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci. 2008;28:11746–11752. doi: 10.1523/JNEUROSCI.3860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 19.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 20.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 21.Mazze RI, Rice SA, Baden JM. Halothane, isoflurane, and enflurane MAC in pregnant and nonpregnant female and male mice and rats. Anesthesiology. 1985;62:339–341. doi: 10.1097/00000542-198503000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Wenk GL. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8):5A. doi: 10.1002/0471142301.ns0805as26. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 27.Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- 28.Cobas A, Fairen A, Alvarez-Bolado G, Sanchez MP. Prenatal development of the intrinsic neurons of the rat neocortex: a comparative study of the distribution of GABA-immunoreactive cells and the GABAA receptor. Neuroscience. 1991;40:375–397. doi: 10.1016/0306-4522(91)90127-a. [DOI] [PubMed] [Google Scholar]
- 29.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 31.Chalon J, Tang CK, Ramanathan S, Eisner M, Katz R, Turndorf H. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg. 1981;60:794–797. [PubMed] [Google Scholar]
- 32.Gibson MA, Butters NS, Reynolds JN, Brien JF. Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal guinea pig. Neurotoxicol Teratol. 2000;22:183–192. doi: 10.1016/s0892-0362(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 33.Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–1864. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martire M, Altobelli D, Cannizzaro C, Maurizi S, Preziosi P. Prenatal diazepam exposure functionally alters the GABA(A) receptor that modulates [3H]noradrenaline release from rat hippocampal synaptosomes. Dev Neurosci. 2002;24:71–78. doi: 10.1159/000064947. [DOI] [PubMed] [Google Scholar]
- 35.Iqbal U, Dringenberg HC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters hippocampal GABA(A) receptors and impairs spatial learning in the guinea pig. Behav Brain Res. 2004;150:117–125. doi: 10.1016/S0166-4328(03)00246-8. [DOI] [PubMed] [Google Scholar]
- 36.Kellogg CK, Yao J, Pleger GL. Sex-specific effects of in utero manipulation of GABA(A) receptors on pre- and postnatal expression of BDNF in rats. Brain Res Dev Brain Res. 2000;121:157–167. doi: 10.1016/s0165-3806(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 37.Livezey GT, Marczynski TJ, Isaac L. Prenatal diazepam: Chronic anxiety and deficits in brain receptors in mature rat progeny. Neurobehav Toxicol Teratol. 1986;8:425–432. [PubMed] [Google Scholar]
- 38.Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kellogg CK, Primus RJ, Bitran D. Sexually dimorphic influence of prenatal exposure to diazepam on behavioral responses to environmental challenge and on gamma-aminobutyric acid (GABA)-stimulated chloride uptake in the brain. J Pharmacol Exp Ther. 1991;256:259–265. [PubMed] [Google Scholar]
- 40.Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, Viana GS. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology. 2007;53:942–950. doi: 10.1016/j.neuropharm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 44.Sprung J, Flick RP, Wilder RT, Katusic SK, Pike TL, Dingli M, Gleich SJ, Schroeder DR, Barbaresi WJ, Hanson AC, Warner DO. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–310. doi: 10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, Piening T, Moysich A, Grune T, Thor F, Heumann R, Buhrer C, Ikonomidou C. Oxygen causes cell death in the developing brain. Neurobiol Dis. 2004;17:273–282. doi: 10.1016/j.nbd.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 48.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 49.Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, Anokhin KV, Bougneres P, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet. 2009;373:1654–1657. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- 50.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, Krebs MO. Peri-pubertal maturation after developmental disturbance: A model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]