Abstract
The most potent outcomes for cannabis use disorders have been observed with a combination of three evidence-based interventions, motivational enhancement therapy (MET), cognitive-behavioral therapy (CBT), and abstinence-based contingency-management (CM). Access to this intervention remains limited because of cost and service availability issues. This report describes the initial stages of a project designed to develop and test a computer-assisted version of MET/CBT/CM that could address many of the current barriers to its dissemination. A nonrandomized, 12-week comparison study assigned 38 adults seeking treatment for a cannabis use disorder to either therapist-delivered (n=22) or computer-delivered (n=16) MET/CBT/CM. Attendance, retention, and cannabis use outcomes did not differ significantly between groups, and there were no indications of superior outcomes favoring therapist delivery. Participants provided positive ratings of the computer-delivered sessions. These preliminary findings suggest that computer-assisted delivery of MET/CBT/CM is acceptable to outpatients and does not adversely impact compliance or outcomes achieved during treatment with MET/CBT/CM for cannabis use disorders. Assessment of post-treatment outcomes and replication in randomized trials are needed to determine reliability and longer-term effects. As observed in a growing number of studies, computerized therapies have the potential to increase access to, reduce costs, and enhance fidelity of providing evidence-based treatments without sacrificing and possibly enhancing effectiveness.
Keywords: cannabis, marijuana, treatment, computer delivered, contingency management, internet
1. Introduction
Cannabis remains the most widely used illicit substance in most developed countries (EMCDDA, 2008; UNODC, 2009). The need for interventions to address cannabis-related problems among youth and adults has become clear as treatment admissions for primary cannabis problems increase (SAMHSA, 2009). Cumulative results across randomized trials for cannabis use disorders in adults indicate that behaviorally-based treatment approaches, in particular, motivational enhancement therapy (MET), cognitive behavioral therapy (CBT), and contingency management (CM), are efficacious (Budney et al., 2007).
The most potent of the tested behavioral interventions appears to be a combination of MET, CBT, and abstinence-based CM. Five trials have demonstrated enhanced rates of cannabis abstinence when integrating these approaches for adults and adolescents (Budney et al., 2000; Budney et al., 2006; Carroll et al., 2006; Kadden et al., 2007; Stanger et al., 2009). Each of these trials included manualized MET/CBT (9–14 weekly individual sessions) and an abstinence-based CM incentive program that delivered tangible, monetary-based reinforcement contingent on cannabis abstinence documented by a structured once or twice weekly urine testing program.
Although these interventions have proven efficacious, at least two important limitations to translation must be addressed: (1) availability of this treatment is low because many counselors are not trained to provide high quality MET, CBT, or CM, and many geographic locations have little or no easy access to outpatient services; (2) the cost of adding CM to treatment is considered a barrier (Carroll and Rounsaville, 2007; Kirby et al., 2006; McLellan et al., 2003).
To begin to address some of these barriers and limitations, we are developing a computer-assisted version of MET/CBT/CM for cannabis use disorders that could potentially enhance access, reduce costs, and reduce relapse. Computerized interventions have demonstrated promising outcomes for numerous health behaviors and psychological disorders (Pull, 2006; Rosser et al., 2009; Titov, 2007). Multiple studies have evaluated computerized interventions targeting alcohol and tobacco. A recent meta-analysis of this literature concluded that computer-delivered treatments have a significant effect, and may be a cost-effective and highly accessible means of treating uncomplicated substance use and related problems (Rooke et al., 2010). A recent example of an innovative, computer-delivered program that enhanced treatment engagement and abstinence when added to standard drug counseling is called CBT4CBT (Carroll et al., 2008; Carroll et al., 2009). This program involves a series of 6 videos that use a narrator to provide didactic training of CBT skills followed by movie vignettes in which actors demonstrate how to practice and use of these skills as well as explain how they have worked for them. Others have shown that a computerized brief motivational intervention can reduce illicit drug use in postpartum women (Ondersma et al., 2005; 2007). For opioid-dependent, buprenorphine maintained outpatients, a computerized CBT/CM program readily substituted for therapist-delivered CBT with no adverse effects on outcomes (Bickel et al., 2008). Last, a recent report indicated that a computerized MET/CBT treatment for cannabis/alcohol problems and depression demonstrated equivalent positive outcomes to a therapist providing a similar intervention (Kay-Lambkin et al., 2009).
The present project involved substantial modifications of and additions to the CBT software program used in the aforementioned opioid dependence study for use with persons seeking treatment for cannabis use disorders. This report describes the development of the computerized intervention and findings from an initial, non-randomized trial comparing it with therapist-delivered MET/CBT/CM. Randomization was not involved in because the study was crafted using a “convenience” sample of participants that was dictated by the timelines for development of the interventions (see Treatment Assignment section). If found efficacious, such an intervention would have the potential to increase the overall effectiveness of treatment services for cannabis use disorders by increasing availability of and access to empirically-based interventions.
2. Method
2.1. Software Development and Content
A computerized program was developed to deliver a 12-week, 9-session MET/CBT/CM intervention for cannabis use disorders. The content was derived from three treatment manuals: (1) CRA plus Vouchers for Cocaine Dependence (Budney and Higgins, 1998), (2) Brief Counseling for Marijuana Dependence (BCMD) (Steinberg et al., 2005), and (3) MET/CBT/CM for Marijuana Dependence (see unpublished manual; Budney et al., 2000; 2006). First, two MET sessions were developed. This involved computer presentation of a personalized feedback report and goal setting exercises that increased the interactivity of the program to emulate a motivational interviewing style. Second, the program engaged the participant in a lifestyle goal-setting/management exercise at each session that prompted responses on progress, plans to continue or revise current goals, and desire to create new goals. Third, the content of CBT sessions paralleled those described in aforementioned BCMD and the unpublished marijuana treatment manuals, which included modules on understanding marijuana-use patterns, coping with craving, managing thoughts about using drugs, problem solving, drug-refusal skills, coping with lapses, managing negative moods, assertiveness skills, and coping with other life problems.
The programming employed three technological styles. Computer-assisted instruction selectively presented information, required active responses to queries designed to assess knowledge acquisition, and evaluated and provided immediate feedback to user responses. Computer simulation involved video-based simulation in which actors model coping behavior to enhance learning (e.g., drug-refusal skills). Third, interactive exercises and worksheets were developed to better enhance learning and personalize content. For example, in MET sessions, participants rated the importance of costs and benefits of quitting marijuana use and developing a personalized change plan. In each CBT session, responses to a question on whether or not cannabis was used prompted completion of a “functional analysis” routine to assess events related to the drug use and possible ways to avoid or cope with drug triggers in the future.
Participants used a unique password to access their program via the Internet using one of three computers set up in cubicles in a designated clinic office. Prior computer experience was not necessary as the first module provided training. Participants could access their program remotely to practice or review elements of their program between sessions. The system enabled secure access from any computer with high-speed Internet access.
2.2. Feedback
During the development phase of the project, the investigators, clinicians, and research staff provided feedback on each module to the programmer. After multiple modifications, modules were then sent to three cannabis behavioral treatment experts for review and feedback. Modifications were made as suggested, if feasible. Most of the feedback suggested further increases in the interactive and reflective components of the modules, particularly for MET; however, the majority of these were not implemented because of budget/programming limitations.
2.3. Therapist Training and Treatment Fidelity
Two therapists, one masters level counselor (5 years experience) and one licensed substance abuse counselor (15 years experience) conducted the MET/CBT sessions, which paralleled the computerized modules. Therapists participated in extensive training focusing on explication, demonstration, and role-playing of the therapy components. Therapists attended an initial 2-day workshop by an expert (DW) in the training and delivery of MET and CBT and then received ongoing training and weekly supervision (AJB, SF, DW). Weekly supervision of training cases included detailed feedback based on videotape review of sessions. Therapists were assigned study participants only after they were judged as acceptable or better in conducting the interventions by the supervisors. The masters level counselor had previous experience with MET and CBT, and thus required fewer pilot cases (n=3) than did the licensed substance abuse counselor (n=5). During the study, all sessions were videotaped and one session per week was reviewed by a supervisor and discussed with the therapist to monitor treatment integrity and fidelity.
2.4. Participants
Participants (n=38) were recruited from advertisements in local newspapers, notices mailed to local professionals, and posters located throughout the local community. Individuals had to be 18 years or older, meet criteria for a current DSM-IV diagnosis of cannabis abuse or dependence, and report use of cannabis on at least 50 of the previous 90 days. Exclusion criteria included current dependence on alcohol or any other drug except nicotine, current participation in treatment for substance abuse, or severe psychological distress. Other exclusion criteria included legal status that might result in imminent incarceration, plans to move out of the area in the next 12 months, living with someone enrolled in the project, not living within 30 miles of the research site, and not being fluent in English.
Participants were screened for eligibility during an initial phone contact, and final eligibility was determined at the baseline interview. Ineligible participants were referred to other community agencies. Informed consent for the assessment was obtained prior to the intake interview and consent for trial participation was obtained following the baseline assessment.
2.5. Treatment Assignment
The first 16 participants were assigned to the therapist-delivered condition (tMET/CBT/CM) while the computer program was under development. When the program became available, the next 10 were assigned to the computer-assisted condition (cMET/CBT/CM), and the final 12 were quasi-randomly assigned to tMET/CBT/CM based on therapist caseloads and availability.
2.6. Measures
2.6.1. Substance Use
A modified version of the structured interview, Form 90I, was used to obtain information on history, frequency, quantity, and patterns of substance use (Miller et al., 1994). This included a timeline follow-back interview to elicit daily information on substance use during the prior 90 days (Sobell et al., 1992). The Psychoactive Substance Use Disorders section of the Structured Clinical Interview for DSM-IV Disorders was used to determine lifetime and current diagnoses of substance use disorders (First et al., 1995).
The Marijuana Problems Scale assessed negative consequences associated with cannabis use (Stephens et al., 2000). Summed scores provide indices with good internal consistency and sensitivity to treatment effects (Marijuana Treatment Project Research Group, 2004). The Marijuana Self-efficacy Scale measured efficacy for avoiding use in situations involving negative affect, social discomfort, and presence of others using marijuana (Litt et al., 2005). The Readiness to Change Questionnaire (RTC; Rollnick et al., 1992) results in 3 subscales related to three stages of change: precontemplation, contemplation, and action. The Coping Strategies Scale assesses use of coping skills taught in CBT. Ratings of frequency of use for various coping strategies provides a Total Coping score and four subscale scores (active–behavioral, active–cognitive, avoidant–behavioral, and avoidant–cognitive) (Litt et al., 2005).
2.6.2. Urine Toxicology
Urine specimens were collected under staff observation and were tested for 11-nor-delta-9-THC-9-carboxylic acid (THCCOOH), the primary marijuana metabolite on a MGC 240 analyzer (Microgenics Corporation). A cutoff level of 50ng/ml for THCCOOH was used to determine cannabis abstinence. Creatinine level (< 30ng/ml) was assessed as a proxy for specimens too dilute for valid testing, and an invalid specimen prompted requests to provide another specimen within 4–24 hours. At intake and every 4 weeks during treatment specimens were tested for cocaine, opioids, benzodiazepines, and amphetamines.
2.7. Therapist-Delivered Intervention (tMET/CBT/CM)
Participants assigned to tMET/CBT/CM received a 9-session individual therapy intervention (Budney et al., 2006; Steinberg et al., 2005). During Session 1 (60–90 minutes), participants received MET which involved discussion of a personalized feedback report, cannabis use, and change plans using a motivational interviewing style. Session 2 (45–60 minutes) continued this process and reviewed efforts to reduce cannabis use and planned for adjustments in strategy if desired. During sessions 3–9 (45–60 minutes), therapists provided structured CBT Sessions that included lifestyle goal setting/case management, treatment contracting, functional analysis training, and a set of core coping skills focused on the participant’s cannabis use.
2.8. Computer-Delivered therapy (cMET/CBT/CM)
Participants assigned to cMET/CBT/CM received the same 9-session intervention via the computerized program. Clinic visits for computerized therapy sessions followed the same weekly schedule, although sessions were generally shorter in duration than those in therapist condition see Table 1. In addition, cMET/CBT/CM participants were assigned a therapist and received a total of three 15–30 minute sessions. The initial session occurred immediately prior to the first computerized therapy session. The therapist prompted discussion of the circumstances for entering treatment, oriented participants to the intervention, explained how the computer program worked, provided details on the CM procedure, and discussed the plan for therapist contact throughout treatment. The two other sessions occurred during weeks 4 and 12. These involved discussion of progress, issues with the computer, need for referrals or clinical assistance, and encouragement to use their computer program remotely. Seven of sixteen participants accessed their program remotely (M = 2 occurrences). A research staff member was available at each computer session to assist with any problems and ensure that participants were not intoxicated or experiencing any crises in need of attention.
Table 1.
Participant Characteristics
| Participant Characteristics | Therapist-Delivered MET/CBT/CM (n=22) | Computer-Delivered MET/CBT/CM (n=16) |
|---|---|---|
| Age (years) a | 32.7 (10.5) | 32.9 (8.7) |
| Female | 45% | 50% |
| Ethnicity | ||
| White | 73% | 56% |
| African American | 27% | 44% |
| Days of cannabis use (in past 90) a | ||
| Mean (SD) | 72.7 (18.1) | 72.8 (16.8) |
| Met DSM criteria for cannabis abuse | 23% | 25% |
| Met DSM criteria for cannabis dependence | 77% | 75% |
| Marijuana Problem Scale (Item Count) a | 8.8 (4.0) | 10.2 (4.9) |
| Self-Efficacy (Overall Mean) a | 4.4 (1.5) | 3.8 (1.3) |
| Readiness to Change (Action score) a | 4.3 (0.8) | 3.9 (0.6) |
Note: No significant differences between groups for any baseline variables
Mean (standard deviation)
2.9. Abstinence-based Incentives (CM)
Participants in both groups provided urine specimens on a twice-weekly schedule, and typically received results within 15 minutes from the research staff. An abstinence-based voucher CM program, during which participants earned monetary-based vouchers, was used to motivate and reinforce cannabis abstinence. Because many regular cannabis users will test positive for cannabis for 14 days or more at the test cutoff level used in this study (50ng/ml) Weeks 1–2 of treatment were considered a washout phase (Budney et al., 2006). During this time, participants received vouchers worth $5 for each urine specimen provided, independent of test results facilitating compliance with provision of urine specimens during the initial 2 weeks. During weeks 3–12, participants earned vouchers contingent on providing urine specimens that tested negative for cannabinoids. The first negative specimen was worth $1.50, and values for each subsequent consecutive negative specimen increased by $1.50. A $10 bonus voucher was earned for each week of abstinence (2 consecutive negative specimens). Specimens positive for cannabis or failure to submit a specimen reset the value of vouchers to the initial $1.50 value, where they could escalate again according to the same schedule. Participants remaining continuously abstinent during Weeks 3–12 earned vouchers worth $435. Earnings were redeemed in the clinic for gift cards or certificates available from local merchants. Spending was encouraged throughout treatment by therapists in tMET/CBT/CM or research staff in cMET/CBT/CM.
For cMET/CBT/CM participants, staff entered urine test results into a computer linked with the computer program. Each time participants logged into the computer, the program displayed earnings for each day, cumulative earnings, and amount available to spend.
2.10. Data Analysis
Comparisons between treatment groups on baseline characteristics were performed using t-tests for continuous measures and chi-square tests for nominal measures (Table 1). All primary outcome analyses used an intent-to-treat approach. The primary outcome was longest duration of continuous cannabis abstinence (LDA) achieved during treatment for each participant documented via urinalysis [e.g., 7 consecutive negative specimens = 3.5 weeks of abstinence (week = 2 consecutive samples)]. A t test examined mean LDA between groups, and chi square and logrank tests were performed to assess differences in the number of participants who achieved specific durations of continuous abstinence. For these analyses, missing urine specimens were regarded as positive for cannabis to provide a conservative estimate of use. Use of extensive outreach procedures and a flexible schedule for specimen collection suggests that the great majority of missing specimens were indicators of recent use.
Secondary outcomes included days of cannabis use reported on the TLFB, Marijuana Problem Scale scores, and Marijuana Self-Efficacy Scale scores, and the Coping Strategies Scale scores. For these analyses, only participants with end of treatment assessment data were included (cMET/CBT/CM = 12; tMET/CBT/CM=11). Alternative analyses that would include all cases were deemed inappropriate given the high rate of missing data at this assessment point.
3.0. Results
3.1. Participants
Sociodemographic, substance use and psychosocial functioning data for the two groups are presented in Table 1. Participants were approximately half women, two-thirds White and one third African American, with a mean age of 32.9 years. Most used marijuana almost daily (M=72 of the last 90 days) and reported long histories of cannabis use (mean age of initiation = 14.9 yrs). Seventy-six percent met DSM-IV criteria for cannabis dependence, while 24% met criteria for abuse. Approximately half of the participants were current cigarette smokers. The two treatment groups did not differ on any demographic or substance use measures (Table 1).
3.2. Retention and Attendance
Treatment acceptability defined as attending 3 or more urine testing appointments (greater than one week of participation) did not differ significantly between conditions (tMET/CBT/CM = 73%; cMET/CBT/CM=94% p = .91). The mean number of specimens submitted did not differ between groups: tMET/CBT/CM (12.3, SD=9.5) and cMET/CBT/CM (16.3 SD=8.5); t(36) = −1.34, p = .19. The mean number of MET/CBT sessions attended (total possible = 9) did not differ significantly [tMET/CBT/CM=5.1 (3.4); cMET/CBT/CM=6.9 (2.8), p = .09]. Last, completion of treatment defined as providing a urine specimen during Week 12 also did not differ between groups (tMET/CBT/CM: 41%; cMET/CBT/CM=50%, p = .19).
3.3. Time Spent in Therapy Sessions
Mean participant time spent in MET/CBT/CM sessions was significantly greater in tMET/CBT/CM (see Table 2). Mean duration across the 9 computerized sessions was 23.9 (8.9) minutes ranging from 39.5 (MET1) to 12.3 (CBT9) minutes. As expected, estimated mean total time spent with a therapist for tMET/CBT/CM participants (Sessions 1–9: 482 minutes) was much greater than therapist total time for cMET/CBT/CM (3 supportive sessions: 47 minutes).
Table 2.
Secondary Outcomes
| Variables | Therapist-MET/CBT/CM Intake | Therapist-MET/CBT/CM End of Treatment | Computer-MET/CBT/CM Intake | Computer-MET/CBT/CM End of Treatment |
|---|---|---|---|---|
| % days cannabis use a, b | 82.0% (14.9) | 12.6% (29.1) | 78.5% (18.9) | 10.3% (14.2) |
| Marijuana Problem Scale c | 11.0 (6.6, 15,4) | 6.4 (1.9, 10.8) | 15.0 (10.1, 19.9) | 10.6 (4.7, 16.5) |
| Marijuana Self-Efficacy Scale (Overall Mean) c | 4.9 (3.8, 6.0) | 5.6 (1.7) | 4.0 (3.3, 4.6) | 5.7 (1.6) |
| Coping Strategies Scales c | ||||
| Active-Behavior | 2.8 (2.3, 3.2) | 3.0 (2.5, 3.5) | 2.7(2.3, 3.1) | 2.9 (2.5, 3.2) |
| Active-Cognitive | 2.9 (2.4, 3.4) | 3.1 (2.5, 3.7) | 2.9 (2.4, 3.3) | 3.0 (2.6, 3.5) |
| Avoidant-Behav | 2.6 (2.0, 3.2) | 3.0 (2.4, 3.6) | 2.6 (2.2, 3.1) | 3.0 (2.6, 3.4) |
| Active-Cognitive | 2.9 (2.3, 3.4) | 3.2 (2.6, 3.8) | 2.8 (2.3, 3.2) | 3.2 (2.7, 3.6) |
| Voucher Earnings a | --- | $161 (170) | --- | $197 (196) |
| Mean minutes per MET/CBT/CM session d | --- | 48.2 (6.8) | --- | 29.3 (14.9) |
| Mean Total minutes with therapist | 482 | 47 | ||
Note: Analyses of repeated measures include only participants who provided intake and end of treatment data (tMET/CBT/CM=11; cMET/CBT/CM=12)
Mean (standard deviation)
Significant main effect for time indicating positive change in both treatment groups
Mean (95% confidence interval)
p < .01
3.4. Therapist Effects
Comparison of outcomes by therapist for tMET/CBT/CM revealed no main effects of therapist on session attendance (p=.46) or longest period of abstinence (p=.89).
3.5. Cannabis Use
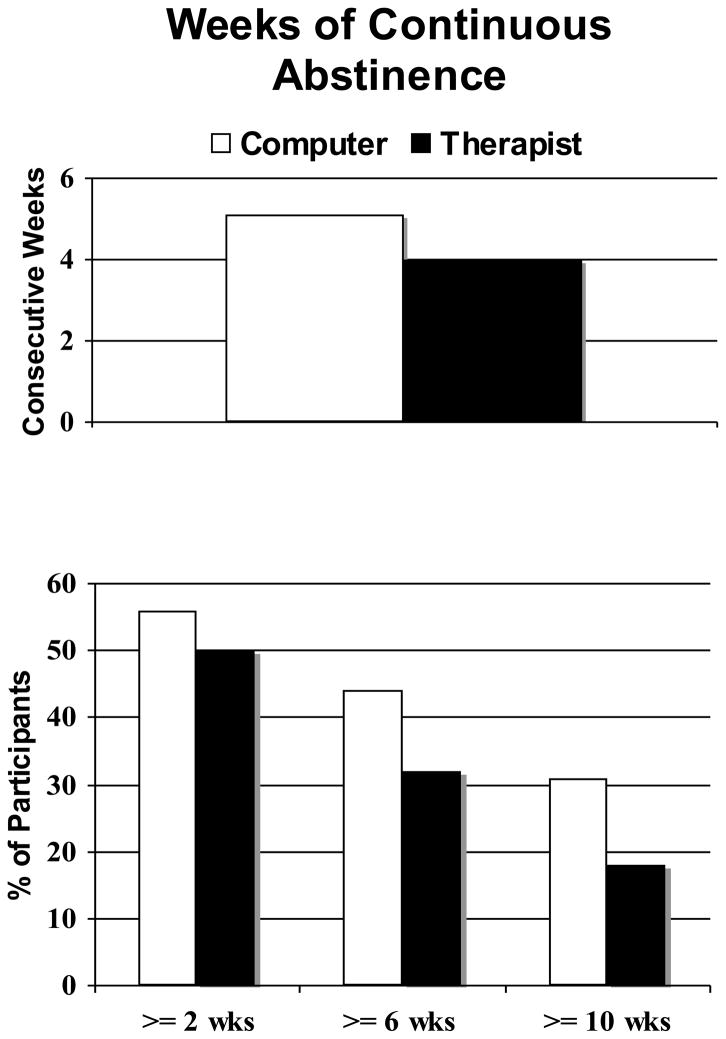
Figure 1 presents the primary cannabis abstinence outcomes observed during the 12-week treatment period. No difference between treatments was observed for mean weeks of continuous abstinence (t (36)=.07, p=.48), nor were any significant group differences observed on the percentage of participants achieving at least 4, 6 or 8 weeks of abstinence (p > .05 for chi square tests). Survival analysis of continuous abstinence showed no significant difference between the treatment groups (log-rank chi-square = 1.5, p=0.22). Mean percentage of cannabis-negative urine specimens provided during treatment also did not differ between treatments [tMET/CBT/CM = 34% (SD=38); cMET/CBT/CM = 43% (SD=44), p = .48].
Figure 1.
The top panel illustrates the group means for longest documented period of abstinence during treatment; no significant group difference was observed. The bottom panel illustrates the percentage of participants in each group that achieved specified periods of abstinence; no significant group differences were observed.
3.6. Secondary outcomes
Note that comparisons between those who provided end of treatment assessment data (n=23) were significantly younger, had greater readiness to change scores, and were more likely to be Caucasian than those who did not (n=15). Thus, findings from secondary analyses comparing changes on the Marijuana Problem Scale, Self-Efficacy, Coping Skill Strategy scale scores should be interpreted conservatively as this subsample was not highly representative of the entire sample. No main effects for group or group by time interactions were observed for any of these outcome variables. Mean percent days of cannabis use and mean voucher earnings from the CM program also did not differ by group. Group comparisons of all secondary outcome measures are shown in Table 2.
3.7. Consumer Feedback
cMET/CBT/CM participants responded to a 13-item consumer feedback questionnaire after each computer session. Each item was rated on a 1–10 scale with 10 being the most positive response. Across all computer sessions and items, scores ranged from 7.1 to 9.1 reflecting highly positive responses. The lowest average ratings received (7.1) were for liking the video and answering questions you had about the topic; the highest rating (9.1) was for easy to understand. Other ratings included: provided new information (7.5), interesting (8.0), how it compares to previous education/training sessions (8.2), useful (8.3), likely to help with quitting marijuana (8.3), teaching/quizzes (8.3), likely to help others (8.8), useful as part of a treatment program (8.7), like using the computer (8.8), important in your own life (8.9). Note that only a few participants provided comments suggesting substantial changes. Comments were similar to those of the experts, that is, they desired more interactive experiences.
4. Discussion
The findings from this nonrandomized comparison of computer- and therapist-delivered MET/CBT/CM suggest that the computer can effectively substitute for the therapist without adversely affecting during treatment outcomes when used in combination with an abstinence-based reinforcement program. Notwithstanding the limitations discussed below, compared with tMET/CBT/CM, cMET/CBT/CM did not adversely impact participant engagement, attendance, cannabis use outcomes, reports of cannabis-related problems, or reported use of coping skills associated with reducing or stopping cannabis use. In addition, the participants rated the computer program highly and voiced few complaints. Such findings are highly consistent with a similar comparative study of CBT delivery during outpatient treatment for opioid dependence that included buprenorphine and a CM program (Bickel et al., 2008).
The possibility of using a computer to deliver MET/CBT/CM gives rise to potential implications for clinical practice of great consequence. First, we believe our data suggest substantial cost savings related to greatly reduced therapist time (approximately 10 fold or 7 hours less therapist time than the tMET/CBT/CM), which has great importance given that many believe that the cost of incentive-based CM programs provides the greatest barrier to their implementation in community settings. Although we did not conduct a cost analysis, a study of computer-assisted treatment for panic disorder, that reported a comparable reduction in therapist time, estimated savings of 32% or $540 per patient (Newman et al., 1997). Computerized treatments have costs associated with development and computer purchase, but have minimal maintenance, space, and personnel costs relative to therapist-delivered interventions. Most relevant to the delivery of MET/CBT/CM, such cost savings could be used to fund CM incentive programs that enhance outcomes when added to psychosocial counseling for cannabis and other types of substance use disorders (Higgins et al., 2008).
Second, computerized delivery of specific treatment components would allow therapist time and effort to be redirected toward more severe cases, dual diagnosis interventions, family interventions, or provision of supportive group therapies, all of which might result in greater overall effectiveness of substance use treatment programs. Third, computer delivery could increase access of evidence-based interventions to persons in remote areas who otherwise would not have treatment available, and would increase availability where there is a lack of trained providers. Moreover, typical problems associated with scheduling issues, missed sessions, and availability of highly desirable session times with therapists would be greatly reduced. Fourth, the integrity and fidelity of MET/CBT/CM would be standardized, consistent, and of known quality. Computerized MET/CBT/CM would also address the difficulties, expense, and viability associated with training a clinical workforce to deliver these interventions.
This said, a number of limitations with the present and prior studies merit consideration. The present study included only a small sample size, was non-randomized, did not have a control group for comparison with the two active interventions, and did not include post-treatment follow-up assessments. The groups did not appear to differ on any of the measured baseline variables that could impact outcome, which provides some reassurance that biased group assignment did not affect the results. The lack of a control group does not allow one to directly determine if the interventions produced equally positive outcomes or had no effect at all. However, 4 previous experimental demonstrations of the efficacy of tMET/CBT/CM for CUD would suggest the former (Budney et al., 2000, 2006; Carroll et al., 2006; Kadden et al., 2007). The need for post-treatment evaluation raises perhaps the most critical issue. Prior studies of the treatment components evaluated in this study (CM and MET/CBT) have shown that CM with no counseling produced during treatment outcomes that were equivalent to tMET/CBT/CM (Budney et al., 2006; Carroll et al., 2006; Kadden et al., 2007; Rawson et al., 2002). Given that finding, one should not be surprised by the present study demonstrating that computerized MET/CBT sessions combined with CM produced during treatment outcomes equivalent to tMET/CBT/CM. These prior studies, however, showed that MET/CBT/CM produced better outcomes than CM alone in the year following treatment, suggesting that the MET/CBT was important to enhancing maintenance of treatment gains. Whether or not computerized MET/CBT/CM maintains abstinence outcomes as well as therapist delivered MET/CBT/CM has yet to be tested. Last, the present study did not include objective measures of fidelity or integrity of the therapist-delivered treatment, which does not allow assessment of the quality of tMET/CBT/CM that was compared with the computerized intervention. That said, the therapists were experienced, well-trained, closely supervised, and the assessment of our MET/CBT expert supervisors was that their performance reflected at least an average level of competence and quality as compared with community providers. The conclusion that cMET/CBT/CM can substitute for tMET/CBT/CM in clinical practice awaits additional study and replication. An ongoing randomized trial in our clinic will address many of these limitations of this initial study.
Although the present study provides only preliminary information on the efficacy of cMET/CBT/CM for cannabis use disorders, the findings support prior data indicating that computer-assisted interventions for substance use offer great promise for enhancing the overall effectiveness of our treatment services delivery systems. Computers and other digital technology platforms offer innovative methods to at least partially address many of the issues that present barriers to effectively meeting the needs of those with substance use problems (e.g., cost, access to and availability of evidence-based interventions, consistent delivery of high quality interventions, availability of real time assistance, access to continued care to enhance long-term recovery). Creative methods for integrating such technology with the current delivery system will offer additional challenges.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Exp Clin Psychopharmacol. 2008;16:132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. National Institute on Drug Abuse; Bethesda, MD: 1998. [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping-skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: a 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100:178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. A vision of the next generation of behavioral therapies research in the addictions. Addiction. 2007;102:850–862. doi: 10.1111/j.1360-0443.2007.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA. Annual report on the state of the drugs problem. European Monitoring Centre for Drugs and Drug Addiction; Lisbon: 2008. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis 1 disorders--patient edition SCID I/P. Biometricts Research Department: New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. The Guilford Press; New York, NY: 2008. [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Lewin TJ, Carr VJ. Computer-based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction. 2009;104:378–388. doi: 10.1111/j.1360-0443.2008.02444.x. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend. 2006;85:19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Stephens RS. Coping and self-efficacy in marijuana treatment: results from the marijuana treatment project. J Consult Clin Psychol. 2005;73:1015–1025. doi: 10.1037/0022-006X.73.6.1015. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group. Brief Treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public’s demand for quality care? J Subst Abuse Treat. 2003;25:117–121. [PubMed] [Google Scholar]
- Miller WR, DelBoca FK. Measurement of drinking behavior using the form-90 family of instruments. J Stud Alcohol. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Newman MG, Kenardy J, Herman S, Barr-Taylor C. Comparison of Palmtop-Computer-assisted Brief Cognitive-Behavioral Treatment for Panic Disorder. J Consult Clin Psychol. 1997;65:178–183. doi: 10.1037//0022-006x.65.1.178. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat. 2005;28:305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med. 2007;32:231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull CB. Self-help Internet interventions for mental disorders. Curr Opin Psychiatry. 2006;19:50–53. doi: 10.1097/01.yco.0000198103.31155.73. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Heather N, Gold R, Hall W. Development of a short “readiness to change” questionnaire for use in brief opportunistic interventions. Br J Addict. 1992;87:743–754. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- Rooke S, Thorsteinsson E, Karpin A, Copeland J, Allsop D. Computer-delivered interventions for alcohol and tobacco use: a meta-analysis. Addiction. 2010;105:1381–1390. doi: 10.1111/j.1360-0443.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- Rosser BA, Vowles KE, Keogh E, Eccleston C, Mountain GA. Technologically-assisted behaviour change: a systematic review of studies of novel technologies for the management of chronic illness. J Telemed Telecare. 2009;15:327–338. doi: 10.1258/jtt.2009.090116. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Treatment Episode Data Set (TEDS) Highlights - - 2007 National Admissions to Substance Abuse Treatment Services. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2009. [Google Scholar]
- Sobell LC, Sobell MB, Litten R, Allen J. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg KL, Roffman R, Carroll KM, McRee B, Babor TF, Miller M, Kadden R, Duresky MA, Stephens R. Substance Abuse and Mental Health Services Administration . Brief counseling for marijuana dependence: A manual for treating adults. Department of Health and Human Services; 2005. [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Titov N. Status of computerized cognitive behavioural therapy for adults. Aust N Z J Psychiatry. 2007;41:95–114. doi: 10.1080/00048670601109873. [DOI] [PubMed] [Google Scholar]
- UNODC. World Drug Report - 2009. United Nations Office on Drugs and Crime; New York: 2009. [Google Scholar]