Abstract
Alveolar rhabdomyosarcoma (ARMS) are characterized by the expression of chimeric transcription factors Pax3-FKHR and Pax7-FKHR, due to chromosomal translocations fusing PAX3 or PAX7 with the FKHR gene. Although ARMS exhibits a muscle lineage phenotype, the cells evade terminal differentiation despite expressing the potent myogenic transcriptional regulator MyoD. Here we show that while Pax7-FKHR inhibits MyoD-dependent transcription, MyoD enhances Pax7-FKHR activity in myogenic cell cultures. Importantly, this effect is not recapitulated by close related transcription factor myogenin and involves specific MyoD functional domains, distinct from those required for Pax7 to regulate MyoD during muscle formation. Together, these results suggest that although repressed as a myogenic regulatory factor, MyoD can play an active role in ARMS by augmenting Pax7-FKHR function.
Keywords: Pax7-FKHR, MyoD, ARMS, rhabdomyosarcoma, Pax7, C3H10T1/2
INTRODUCTION
The transcription factors Pax3 and Pax7 have essential roles during muscle development and the formation of the satellite cell pool, which represents the adult stem cell population responsible for muscle growth and regeneration [Buckingham and Relaix 2007;Le Grand and Rudnicki 2007;Holterman and Rudnicki 2005]. Despite genetic evidence supporting their critical function in regulating muscle progenitors, the underlying molecular mechanisms remain unsolved. This is particularly true in the case of Pax7, which appears to regulate myogenic progression, despite being a poor transcriptional activator [Bennicelli et al. 1999]. Moreover, Pax7 can negatively regulate the expression and activity of the muscle regulatory factor (MRF) MyoD, independently of its transcriptional activity [Olguin and Olwin 2004;Olguin et al. 2007]. Recent findings indicate that Pax7 is able to recruit a histone methyltransferase complex to the myf5 promoter, activating transcription of this MRF involved in early muscle commitment [McKinnell et al. 2008]. Thus, it appears that Pax7 may activate the myogenic program inducing expression of early MRFs, while preventing muscle differentiation through direct inhibition of MyoD function.
It is believed that Pax3 and Pax7 function(s) are altered in alveolar rhabdomyosarcomas (ARMS), an aggressive pediatric soft tissue sarcoma. The most common cytogenetic feature of this cancer are the chromosomal translocations leading to fusion between PAX3 or PAX7 and FKHR (FOXO1) gene. The Pax-FKHR fusion proteins contain intact Pax3 or Pax7 DNA binding domains fused to the FKHR transcriptional activation domain [Barr 2001]. The resultant fusion proteins are more abundant and transcriptionally active than their wild type counterparts [Davis and Barr 1997;Fredericks et al. 1995;Bennicelli et al. 1996;Bennicelli et al. 1995], suggesting that deregulation of Pax3/Pax7 downstream target genes contributes to tumorigenesis. Intriguingly, Pax-FKHR proteins induce expression of MyoD, yet inhibits terminal differentiation [Davicioni et al. 2006;Cao et al. 2010;Graf Finckenstein et al. 2008;Khan et al. 1999]. Although MyoD is expressed in rhabdomyosarcomas, it is transcriptionally inactive and cannot drive myogenesis [Tapscott et al. 1993;Yang et al. 2009]. In this scenario, it remains unclear if MyoD expression reflects the cellular origin of the tumor (i.e. a committed muscle cell/progenitor) or if arises from Pax-FKHR dependent transcription in vivo.
The retention of MyoD expression in ARMS may affect Pax7-FKHR function since a functional relationship exists between Pax7 and MyoD in muscle progenitors. Here we show that Pax7-FKHR can effectively repress MyoD-dependent myogenesis, yet expression of MyoD is required for Pax7-FKHR to activate transcription in a myogenic cell culture system. This MyoD requirement is specific, since myogenin has no significant effect on Pax7-FKHR activity. Remarkably, MyoD-E12 swap-domain proteins demonstrate that the MyoD domains sensitive to repression by Pax7 and those that cooperate with Pax7-FKHR are distinct.
MATERIALS AND METHODS
Cell lines
C3H10T1/2 cells were cultured in DMEM, 10% fetal bovine serum; at 37°C and 5% CO2. For myogenic conversion assays, cultures were induced to differentiate in DMEM, 2% fetal bovine serum for 48h or as specified.
Myogenic conversion and reporter assays
Myogenic conversion of C3H10T1/2 cells was induced by transfecting (Superfect, Qiagen) 1 μg/well (12 well plate) of the pRSV-MyoD vector. Differentiation was triggered 24h after transfection for 24h or 48h, as indicated. When required, pcDNA3-hPax7, pcDNA3-mPAx7 or pBluescript-Pax7FKHR vectors were co-transfected along with pRSV-MyoD or pEMS-ratmyogenin vectors at the indicated molar ratios. pcDNA3 was used as control DNA. To evaluate MyoD transcriptional activity, the myogenin-luc reporter gene was transfected in the absence or the presence of MyoD expression vector and in the absence or presence of Pax7 or Pax7-FKHR expression vectors, in triplicate for each condition. Unless specified, the MyoD-to-Pax7 ratio was 1:2 according to the protocol described earlier [Olguin and Olwin 2004;Olguin et al. 2007]. The CMV-LacZ expression vector was used as marker for transfection efficiency and pcDNA3 was used as control DNA. After differentiation induction, whole cell lysates were obtained and luciferase and β-galactosidase activities determined using the Dual-Light System (Applied Biosystems) as reported previously [Olguin and Olwin 2004;Olguin et al. 2007]. Total protein content was estimated (micro BCA, Pierce) for subsequent analyses. Where indicated, difference between background activity (reporter gene vector alone) and the activation in different experimental conditions was represented as fold activation.
Pax7 and Pax7-FKHR were tested for transcriptional activation in C3H10T1/2 cells as described previously [Olguin et al. 2007], by co-transfection with the 6xPRS9-luc reporter gene and pcDNA3, pRSV-MyoD or pEMS-myogenin.
When indicated, Pax7 or Pax7-FKHR was co-transfected with MyoD-E12 swap constructs (kindly donated by Dr. Stephen Tapscott, Fred Hutchinson Cancer Research Center) and transcriptional activity was tested as described above.
Immunofluorescence
Cells were seeded on glass coverslips placed in 6 well plates. After the specified treatments, cells were fixed in 4% paraformaldehyde for 20 min., permeabilized by incubation in 0.5% Triton X-100/PBS for 5 min. and processed for indirect immunofluorescence according to standard procedures. Primary antibodies and dilutions used were: Mouse monoclonal anti-Pax7 (Hybridoma Bank, Iowa University) at 1:5 (cell culture supernatant); mouse monoclonal anti-MyHC (MF20, Hybridoma Bank, Iowa University) at 1:5 (cell culture supernatant); rabbit polyclonal anti-β-galactosidase (Abcam). Secondary antibodies conjugated to Alexa 594, Alexa 488 were from Molecular Probes. Vectashield with DAPI (Vector Laboratories), was used as mounting media and nuclei counterstaining. Micrographs were taken from a Nikon (Eclipse E800) epifluorescence microscope (using Nikon 20x/0.50 and 40x/0.75 objectives) at RT, using Slidebook v3.0 acquisition software (Intelligent Imaging Innovations Inc.) coupled to a Cooke Sensicam digital camera. Digital deconvolution for single plane images (no neighbors) was applied (when required) to acquired images (Slidebook v3.0).
Image processing and figure Preparation
For figure preparation, images were exported into Photoshop, if necessary the brightness and contrast was adjusted to the entire image, the image cropped and individual color channels extracted (when required) without color correction adjustments or gamma adjustments. Final figures were prepared in Keynote ’09 (Apple Inc.) and Adobe Illustrator CS3 (Adobe Systems Inc).
RESULTS
Pax7-FKHR expression inhibits MyoD function
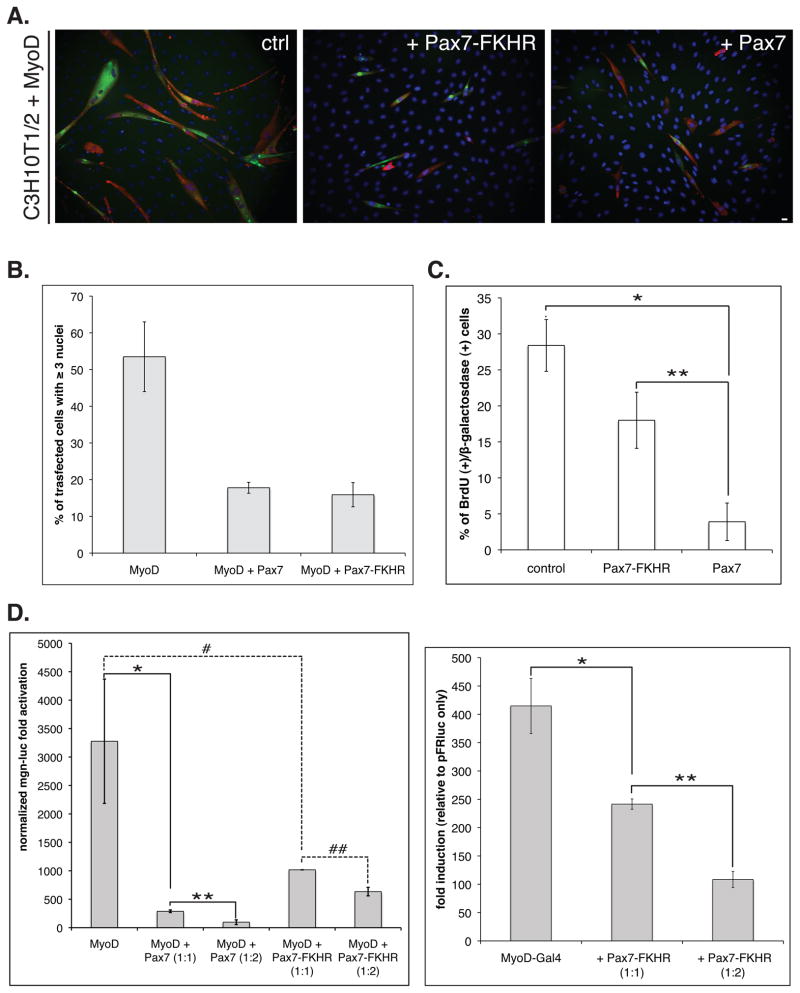
Pax7 effectively represses muscle differentiation of myogenic progenitors via regulation of MyoD activity [Olguin et al. 2007]. Since ARMS is resistant to myogenic differentiation, we first asked if Pax7-FKHR expression could block MyoD-dependent myogenic conversion of C3H10T1/2 cells [Lassar et al. 1986;Davis et al. 1987]. Upon forced expression of MyoD, C3H10T1/2 cells commit to myogenesis 48h after induction of differentiation, as measured by myogenin (not shown) and myosin heavy chain (MyHC) expression, detected by indirect immunofluorescence (Figure 1A). As predicted, myogenic conversion was markedly impaired upon Pax7-FKHR co-expression and is comparable to the repression of myogenesis observed when Pax7 is co-expressed with MyoD (Figure 1A). Quantification of multinucleated myotube formation, another hallmark of myogenesis, also indicates that Pax7 and Pax7-FKHR repress muscle differentiation to a similar extent, as either reduced myotube formation by 50% (defined as ≥3 nuclei/transfected cell; Figure 1B). We have previously demonstrated that transient Pax7 over-expression leads to a temporal cell cycle withdrawal [Olguin and Olwin 2004]. However, we found that over expression of Pax7-FKHR impacted cell cycle in less significant manner than Pax7 as shown by a 2h BrdU pulse (Figure 1C). This observation is consistent with recently described effects of Pax3-FKHR on the myoblast cell cycle [Graf Finckenstein et al. 2008].
FIGURE 1. Pax7-FKHR represses MyoD transcriptional activity.
A. MyoD ability to direct C3H10T1/2 cells into the myogenic lineage (left panel) is efficiently inhibited by Pax7-FKHR (middle panel) co-expression, analogous to the previously described effect for Pax7 (right panel). Myogenic conversion was evaluated trough the co-expression of myosin heavy chain (MyHC, green) and β-galactosidase (transfection marker, red) in transfected cells. Cell nuclei were identified by DAPI staining (blue). Scale bar: 12 μm. B. Myogenic differentiation was quantified as the formation of multinucleated cells (≥3 nuclei/ β-galactosidase positive cell), revealing a >3 fold reduction in cell fusion upon Pax7 or Pax7-FKHR co-expression. A and B: MyoD to Pax7/Pax7-FKR molar ratio= 1:2. A and B are representative of at least three independent experiments. Error bars= standard deviation. C. Pax7-FKHR exhibits a less marked inhibitory effect on cell cycle compared to Pax7, measured by BrdU incorporation in asynchronous populations of C3H10T1/2 cells. Mean values are representative of at least two independent experiments. Error bars= standard deviation. *P < 0.001; **P < 0.01. D. Right panel: Pax7-FKHR represses MyoD transcriptional activation (>3 fold reduction) of the 4RTK reporter gene during myogenic conversion of C3H10T1/2 cells. Note that under same conditions, Pax7 represses MyoD activity over 10 fold. MyoD to Pax7/Pax7-FKR molar ratio= 1:2. *, **P < 0.01; # P < 0.05; ## P < 0.01. Left panel: Pax7-FKHR co-expression represses transcriptional activation mediated by the Gal4-MyoD fusion protein in a dose dependent manner. Mean values are representative of at least three independent experiments. Error bars= standard deviation. *P < 0.01; **P < 0.001.
To better understand the mechanisms involved, we asked if repression of myogenesis by Pax7-FKHR affected MyoD-dependent transcription. First, we investigated the effect of Pax7-FKHR expression on the MyoD induction of a luciferase reporter gene under the control of the myogenin proximal promoter (myogenin-luc) [Olguin et al. 2007], a key MyoD target and member of the MyoD family of muscle regulatory factors (MRFs). MyoD activity was reduced ~3.2 fold by Pax7-FKHR (Figure 1D, left panel), which appears to be sufficient to block terminal differentiation (see Figures 1A and B), although is 3-fold lower than the repressive activity observed for Pax7 (~10 fold decrease in MyoD activity). Interestingly, we observed a dose dependent reduction in the induction of a Gal4 reporter gene (pFRluc) by MyoD fused to Gal4-DNA binding domain (Gal4-MyoD), when co-expressed with Pax7-FKHR (Figure 1D, right panel). Thus, Pax7-FKHR inhibition appears to target MyoD in different promoter contexts. Together, these results suggest that Pax7-FKHR blocks myogenesis via inhibition of MyoD transcriptional activity, analogous to what has been described for Pax7 [Olguin et al. 2007].
MyoD specifically enhances Pax7-FKHR transcriptional activity
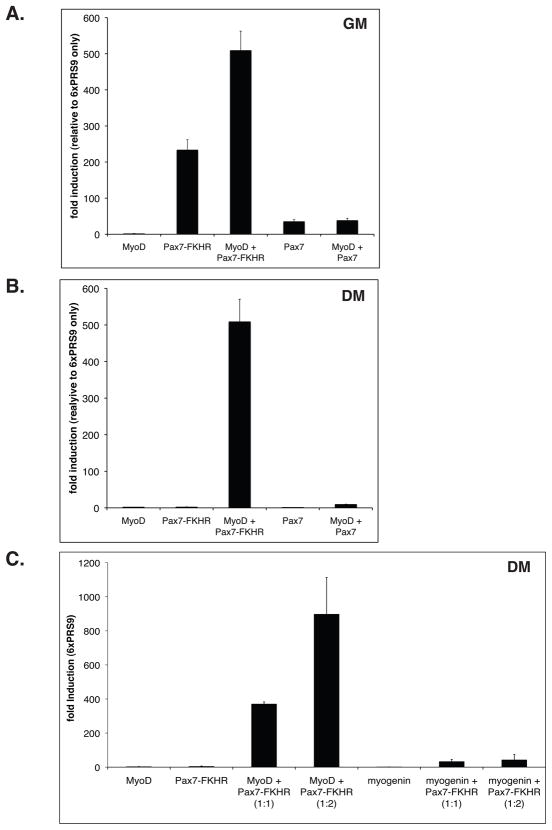
Pax-FKHR proteins induce MyoD expression in different cell lines [Khan et al. 1999;Tomescu et al. 2004;Davicioni et al. 2006;Ebauer et al. 2007;Graf Finckenstein et al. 2008;Cao et al. 2010]. Since the cellular origin of ARMS has not been established, therefore is not clear if MyoD expression is a direct consequence of Pax-FKHR activity in vivo. Thus, it remains to be determined if MyoD expression is functionally relevant for Pax-FKHR function in ARMS. Therefore, we evaluated if Pax7-FKHR transcriptional activity is affected by MyoD co-expression. For this, we measured Pax7-FKHR activity in C3H10T1/2 cells in the presence or the absence of MyoD. In proliferating cultures, Pax7-FKHR induction of a Pax3/Pax7 reporter gene (6xPRS9-luc) is approximately 6.5-fold greater than that observed for Pax7 (Figure 2A). Unexpectedly, Pax7-FKHR activity increased >2 fold upon MyoD co-expression (Figure 2A). This increase appears specific for Pax7-FKHR, since MyoD did not similarly affect Pax7 transcriptional activity (Figure 2A). When Pax7 or Pax7-FKHR function was tested in cells induced to differentiate, reporter gene induction was virtually undetectable (Figure 2B). However, co-expression of MyoD induced Pax7-FKHR activity >500 fold over background levels, while Pax7 activity was minimally affected (Figure 2B). Thus, unlike Pax7, Pax7-FKHR transcriptional activity is dramatically enhanced by MyoD co-expression in proliferating or differentiating cell cultures.
FIGURE 2. Pax7-FKHR transcriptional activity is specifically enhanced by MyoD expression.
A. Pax7-FKHR-dependent activation of the Pax3/7 reporter gene 6xPRS9-luc, is increased ~2 fold upon co-expression with MyoD in C3H10T1/2 cells maintained in myoblast proliferation medium (GM). Note that MyoD has no detectable effect on Pax7 transcriptional activity. B. Pax7-FKHR activity is reduced close undetectable levels when transfected cells are maintained in myogenic differentiation medium (DM). However, robust Pax7-FKHR activity is observed upon MyoD co-expression (> 70 fold activation over Pax7-FKHR alone, Figure 2A). Pax7 activity is only mildly affected by MyoD. A-B, mean values are representative of at least three independent experiments. Error bars= standard deviation. C. Myogenin cannot recapitulate enhancement of Pax7-FKHR transcriptional activity in differentiating cells. As shown in B, MyoD co-expression is sufficient to maintain maximum Pax7-FKHR activity in myogenic differentiation cultures (compare with Figure 2B). Mean values where calculated from three independent experiments. Error bars= standard error of the mean.
Is the induction of Pax7-FKHR transcriptional activity specific to MyoD or are other MRFs are able to mimic this effect? To test this, we asked if myogenin (an MRF involved in terminal differentiation) could enhance Pax7-FKHR transcriptional activity. Noticeably, MyoD-dependent enhancement of Pax7-FKHR activity cannot be recapitulated by myogenin (Figure 2C). Moreover, changes in the myogenin-to-Pax7-FKHR molar ratio had no detectable effect on the reporter gene activity (Figure 2C).
Together, these results provide support for the concept that Pax7-FKHR transcriptional activity is specifically enhanced in MyoD expressing cells.
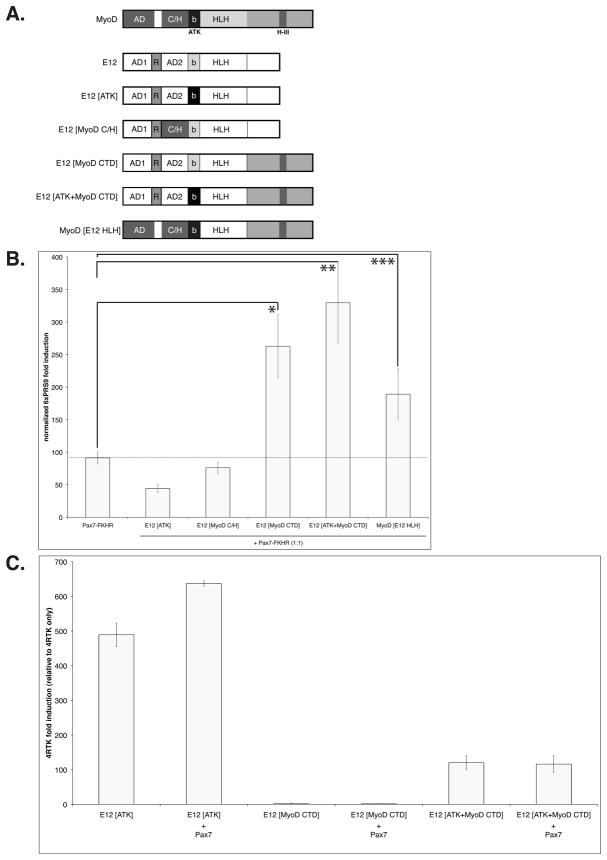
Pax7/MyoD and Pax7-FKHR/MyoD functional interactions require different MyoD domains
The differential effects of MyoD on Pax7 and Pax7-FKHR transcriptional activity, suggest that distinct MyoD/Pax7 and MyoD/Pax7-FKHR functional interactions may take place. To test this idea, we asked if sensitivity to Pax7 repression [Olguin et al. 2007] and the enhancement of Pax7-FKHR activity here described, involved distinct MyoD protein domains. For this we used a battery of MyoD mutant proteins (Figure 3A), where key functional domains have been swapped between MyoD and the ubiquitous HLH protein E12 (Figure 3A) that dimerizes with MyoD to activate transcription [Tapscott 2005]. Upon co-expression, we evaluated i) enhancement of Pax7-FKHR function (6xPRS9-luc reporter gene induction), and ii) the transcriptional activity of the E12/MyoD mutants (4RTK-luc reporter gene induction). As shown in Figure 3A, E12 proteins bearing MyoD sequences required for muscle specific promoter recognition (E12[ATK]) [Huang et al. 1998] and transcription (E12[MyoD C/H]) are unable to up-regulate Pax7-FKHR above control values (Pax7-FKHR only). Interestingly, E12/MyoD swap mutants containing the MyoD C-terminus domain (MyoD CTD) increased Pax7-FKHR transcriptional activity >2 fold (Figure 3B). The magnitude of this induction is comparable with that observed upon co-expression of wild type MyoD (see Figure 2C). Both MyoD C/H and CTD (containg the helix III region) are critical for MyoD function [Gerber et al. 1997;Bergstrom and Tapscott 2001;Berkes et al. 2004] and are not conserved in myogenin. Intriguingly, induction of Pax7-FKHR function does not correlate with the ability of the MyoD mutants to induce transcription. For example, E12[ATK] protein induces a robust stimulation of a myogenic reporter gene, yet has no effect on Pax7-FKHR activity (compare Figure 3B and C). A similar effect was observed with the E12[MyoD C/H] mutant (data not shown). Conversely, mutants proteins containing the MyoD CTD have reduced or non-detectable transcriptional activity when compared to E12[ATK] (E12[MyoD CTD] and E12[ATK+MyoD CTD], respectively), but both MyoD mutant proteins enhanced Pax7-FKHR activity similarly (Figure 3C). Supporting the concept that Pax7 and Pax7-FKHR may establish distinct functional interactions with MyoD, we observed no Pax7-mediated repression on the activity of either E12/MyoD swap mutants containing the MyoD CTD region (Figure 3C). Moreover, Pax7 efficiently repressed the activity of the E12[MyoD C/H] mutant protein, which has no detectable effect on Pax7-FKHR activity (data not shown).
FIGURE 3. Enhancement of Pax7-FKHR activity and susceptibility to Pax7 repression, correlate with distinct MyoD protein domains.
A. Diagrams representing functional regions in full-length MyoD, E12, and E12/MyoD swap domains mutants, respectively. B. Co-expression of an E12 protein with intrinsic myogenic activity [Huang et al. 1998] does not enhance the transcriptional activity of Pax7-FKHR. E12 proteins bearing the MyoD C-terminus region (MyoD CTD) can enhance Pax7-FKHR activity >2.5 fold. As control, MyoD-mutant protein carrying the E12 HLH domain enhances Pax7-FKHR activity ~2 fold. Dashed line indicates Pax7-FKHR maximum activity when expressed alone in C3H10T1/2 cells maintained in myoblast proliferation conditions. *, **P < 0.01; ***P < 0.05. C. Presence of the MyoD CTD does not render MyoD-mutants sensitive to Pax7-dependent repression. Moreover, transcriptional activity of E12/MyoD swap domain mutants does not correlate with their ability to enhance Pax7-FKHR function (compare to the effect of each mutant in Figure 3A).
Although our results show that both Pax7 and Pax7-FKHR are capable of inhibiting MyoD function, the regulation of Pax7 and Pax7-FKHR by MyoD appear distinct and utilize different MyoD domains; illustrating the complex relationships involved in the control of cell fate in ARMS.
DISCUSSION
Alveolar rhabdomyosarcoma are characterized by the expression of Pax3-FKHR or Pax7-FKHR fusion proteins arising from t(2;13)(q35;q14) and t(1;13)(p36;q14) chromosomal translocations, respectively [Barr 2001]. Several studies have focused on identifying a Pax-FKHR transcriptional signature that could explain ARMS origin and/or progression [Khan et al. 1999;Tomescu et al. 2004;Davicioni et al. 2006;Ebauer et al. 2007;Cao et al. 2010]. Interestingly, these and other studies revealed that Pax3-FKHR protein induces the expression of key myogenic regulators such as MyoD, yet paradoxically suppresses terminal differentiation via regulation of MyoD activity [Graf Finckenstein et al. 2008]. Since the cellular origin of ARMS has not been established, is unclear if MyoD expression results from Pax-FKHR activity in vivo or if MyoD could be relevant for Pax-FKHR function.
Here we present evidence demonstrating that Pax7-FKHR represses myogenesis by negatively regulating MyoD activity. As we have described previously for Pax7, regulation of MyoD by Pax7-FKHR appears to be independent of the promoter context, suggesting that both Pax7 and Pax7-FKHR can regulate MyoD protein [Olguin et al. 2007]. Although the effects of Pax7 or Pax7-FKHR over-expression on MyoD activity appear similar, MyoD has radically different effects on Pax7 and Pax7-FKHR function. Although MyoD does not detectably affect Pax7-dependent transcription, Pax7-FKHR activity was increased two fold in the presence of MyoD. This effect was dramatically enhanced when cells were induced to differentiate. Regulation of Pax7-FKHR activity maps to a MyoD C-terminus region containing the helix III domain. This domain is not conserved in myogenin and may explain why this closely related MRFs lacks an affect on Pax7-FKHR transcriptional activity. The MyoD Helix III domain is critical for MyoD-induced transcription and lineage commitment and is involved in mediating interactions with protein complexes containing the homeodomain protein Pbx [Berkes et al. 2004]. It is believed that Pbx containing complexes mark critical muscle promoters, targeting the binding of MyoD to inactive promoters facilitating chromatin remodeling and promoter activation [Berkes et al. 2004;Berkes and Tapscott 2005]. Thus, is tempting to speculate that Pax7-FKHR could be forming de novo chromatin-remodeling complexes with proteins that normally interact with MyoD.
Sequence homology has favored the idea of functional redundancy between Pax3 and Pax7. However, their respective expression patterns [Horst et al. 2006;Kassar-Duchossoy et al. 2005;Montarras et al. 2005;Relaix et al. 2005], differential regulation of their activity [Boutet et al. 2007;Kuang et al. 2006] and different clinical outcomes of Pax3-FKHR and Pax7-FKHR expressing ARMS [Sorensen et al. 2002], strongly suggest they are not functionally redundant. Similarly, our data suggest that critical functional differences exist between Pax7 and Pax7-FKHR. We have shown that Pax7 activity is down-regulated upon induction of muscle differentiation, concomitant with a decrease in Pax7 protein stability [Olguin et al. 2007]. In contrast, Pax7-FKHR function is enhanced in cellular conditions that mimic a muscle differentiation environment. Moreover, MyoD domains distinct from those involved in Pax7 functional interaction appear to be critical for Pax7-FKHR activity. Our current efforts are focused on determining the underlying mechanisms, and the precise role of MyoD in this phenomenon. It would be interesting to determine how our findings relate to regulation of embryonal rhabdomyosarcoma (ERMS). EMRS is normally not associated with expression of Pax-FKHR proteins, however Pax7 is abnormally up regulated. Moreover, MyoD is also expressed in ERMS but exhibits poor transcriptional activity due in part to a competitive mechanism requiring the inhibitory protein MyoR/musculin [Yang et al. 2009]. In both scenarios, the cell origin may be a committed muscle precursor, such as an activated satellite cell. Finally, our observations suggest that transcriptionally inactive MyoD may likely play a critical role in the molecular regulation ARMS, identifying a cooperation between MyoD and Pax-FKHR proteins that could be a relevant pharmacological target for future rhabdomyosarcoma therapies.
Acknowledgments
This work was supported by Internal Grant from P. Catholic University of Chile (VRAID #20/2009 and #17/2010; to HO) and NIH AR039467 and NIH AR049446 (to BO).
References
- Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;20:5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- Bennicelli JL, Advani S, Schafer BW, Barr FG. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene. 1999;18:4348–4356. doi: 10.1038/sj.onc.1202812. [DOI] [PubMed] [Google Scholar]
- Bennicelli JL, Edwards RH, Barr FG. Mechanism for transcriptional gain of function resulting from chromosomal translocation in alveolar rhabdomyosarcoma. Proc Natl Acad Sci U S A. 1996;93:5455–5459. doi: 10.1073/pnas.93.11.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennicelli JL, Fredericks WJ, Wilson RB, Rauscher FJ3, Barr FG. Wild type PAX3 protein and the PAX3-FKHR fusion protein of alveolar rhabdomyosarcoma contain potent, structurally distinct transcriptional activation domains. Oncogene. 1995;11:119–130. [PubMed] [Google Scholar]
- Bergstrom DA, Tapscott SJ. Molecular distinction between specification and differentiation in the myogenic basic helix-loop-helix transcription factor family. Mol Cell Biol. 2001;21:2404–2412. doi: 10.1128/MCB.21.7.2404-2412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14:465–477. doi: 10.1016/s1097-2765(04)00260-6. [DOI] [PubMed] [Google Scholar]
- Boutet SC, Disatnik MH, Chan LS, Iori K, Rando TA. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- Cao L, Yu Y, Bilke S, Walker RL, Mayeenuddin LH, Azorsa DO, Yang F, Pineda M, Helman LJ, Meltzer PS. Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer. Cancer Res. 2010;70:6497–6508. doi: 10.1158/0008-5472.CAN-10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davicioni E, Finckenstein FG, Shahbazian V, Buckley JD, Triche TJ, Anderson MJ. Identification of a PAX-FKHR gene expression signature that defines molecular classes and determines the prognosis of alveolar rhabdomyosarcomas. Cancer Res. 2006;66:6936–6946. doi: 10.1158/0008-5472.CAN-05-4578. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Barr FG. Fusion genes resulting from alternative chromosomal translocations are overexpressed by gene-specific mechanisms in alveolar rhabdomyosarcoma. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1997;94:8047–8051. doi: 10.1073/pnas.94.15.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Ebauer M, Wachtel M, Niggli FK, Schäfer BW. Comparative expression profiling identifies an in vivo target gene signature with TFAP2B as a mediator of the survival function of PAX3/FKHR. Oncogene. 2007;26:7267–7281. doi: 10.1038/sj.onc.1210525. [DOI] [PubMed] [Google Scholar]
- Fredericks WJ, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr FG, Rauscher FJ3. The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol. 1995;15:1522–1535. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber AN, Klesert TR, Bergstrom DA, Tapscott SJ. Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 1997;11:436–450. doi: 10.1101/gad.11.4.436. [DOI] [PubMed] [Google Scholar]
- Graf Finckenstein F, Shahbazian V, Davicioni E, Ren YX, Anderson MJ. PAX-FKHR function as pangenes by simultaneously inducing and inhibiting myogenesis. Oncogene. 2008;27:2004–2014. doi: 10.1038/sj.onc.1210835. [DOI] [PubMed] [Google Scholar]
- Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin Cell Dev Biol. 2005;16:575–584. doi: 10.1016/j.semcdb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Horst D, Ustanina S, Sergi C, Mikuz G, Juergens H, Braun T, Vorobyov E. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol. 2006;50:47–54. doi: 10.1387/ijdb.052111dh. [DOI] [PubMed] [Google Scholar]
- Huang J, Weintraub H, Kedes L. Intramolecular regulation of MyoD activation domain conformation and function. Molecular and Cellular Biology. 1998;18:5478–5484. doi: 10.1128/mcb.18.9.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J, Bittner ML, Saal LH, Teichmann U, Azorsa DO, Gooden GC, Pavan WJ, Trent JM, Meltzer PS. cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1999;96:13264–13269. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986;47:649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177:769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7- dependent population of skeletal muscle progenitor cells. Nature. 2005 doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, Bridge JA, Crist WM, Triche TJ, Barr FG. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children's oncology group. J Clin Oncol. 2002;20:2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Thayer MJ, Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–1453. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- Tomescu O, Xia SJ, Strezlecki D, Bennicelli JL, Ginsberg J, Pawel B, Barr FG. Inducible short-term and stable long-term cell culture systems reveal that the PAX3-FKHR fusion oncoprotein regulates CXCR4, PAX3, and PAX7 expression. Lab Invest. 2004;84:1060–1070. doi: 10.1038/labinvest.3700125. [DOI] [PubMed] [Google Scholar]
- Yang Z, MacQuarrie KL, Analau E, Tyler AE, Dilworth FJ, Cao Y, Diede SJ, Tapscott SJ. MyoD and E-protein heterodimers switch rhabdomyosarcoma cells from an arrested myoblast phase to a differentiated state. Genes Dev. 2009;23:694–707. doi: 10.1101/gad.1765109. [DOI] [PMC free article] [PubMed] [Google Scholar]