Abstract
We introduced the mechanosome hypothesis in 2003 as a heuristic model for investigating mechanotransduction in bone (Pavalko et al., J Cell Biochem, 2003, 88(1):104–112). This model suggested specific approaches for investigating how mechanical information is conveyed from the membrane of the sensor bone cell to the target genes and how this transmitted information from the membrane is converted into changes in transcription. The key concepts underlying the mechanosome hypothesis are that load-induced deformation of bone deforms the sensor cell membrane; embedded in the membrane are the focal adhesion and cadherin–catenin complexes, which in turn are physically connected to the chromatin via a solid-state scaffold. The physical stimulation of the membrane launches multiprotein complexes (mechanosomes) from the adhesion platforms while concomitantly tugging target genes into position for contact with the incoming mechanosomes, the carriers of the mechanical information to the nucleus. The mechanosome is comprised of an adhesion-associated protein and a nucleocytoplasmic shuttling transcription factor. Upon arrival at the target gene, mechanosomes alter DNA conformation and thus influence the interactions between trans-acting proteins along the gene, changing gene activity. Here, we update significant progress related to the mechanosome concept since publication of our original hypothesis. The launching of adhesion- and cytoskeletal-associated proteins into the nucleus toward target genes appears to be a common mechanism for regulating cell response to changes in its mechanical microenvironment.
Keywords: Bone, Mechanotransduction, Adhesion, Cytoskeleton, Integrin, Cadherin, FAK, β-Catenin
Introduction
The Origins of the Rationale Underlying the Mechanosome Hypothesis
We introduced the mechanosome hypothesis [1] as a heuristic model for investigating mechanotransduction in bone. This model suggests specific approaches for investigating how mechanical information is conveyed from the membrane of the sensor bone cell to the target genes and how this transmitted information from the membrane is converted into changes in transcription.
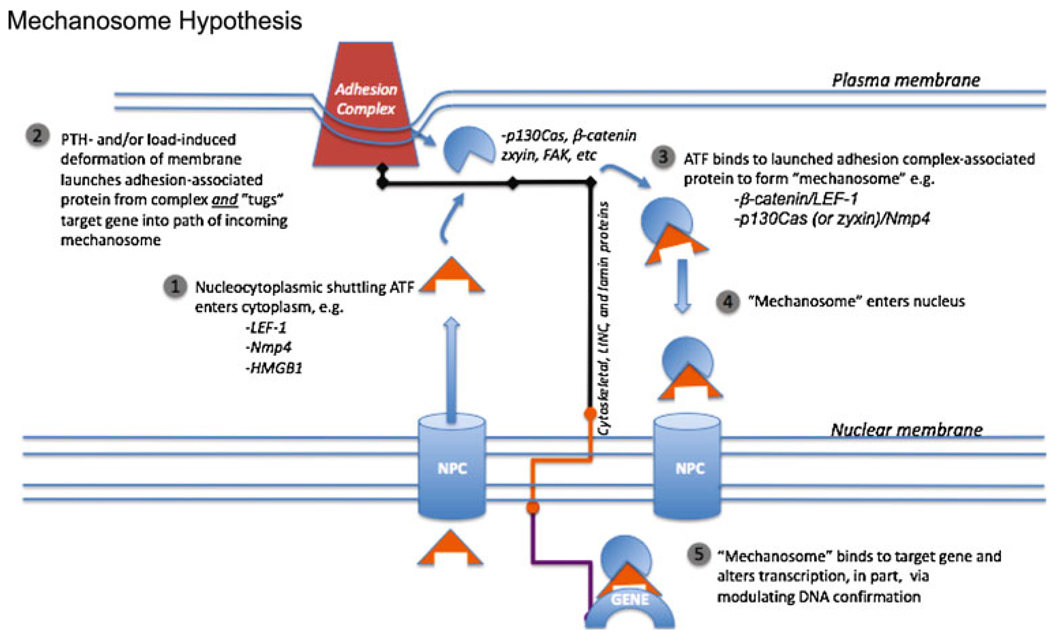
The key concepts underlying the mechanosome hypothesis are that load-induced deformation of bone deforms the sensor cell membrane; embedded in the membrane are the focal adhesion and cadherin–catenin complexes, which in turn are physically connected to the chromatin via a solid-state scaffold. The physical stimulation of the membrane launches multiprotein complexes (mechanosomes) from the adhesion platforms while concomitantly tugging target genes into position for contact with the incoming mechanosomes, the carriers of the mechanical information to the nucleus. The mechanosome is comprised of an adhesion-associated protein and a nucleocytoplasmic shuttling transcription factor. Upon arrival at the target gene, mechanosomes alter DNA conformation and thus influence the interactions between trans-acting proteins along the gene, changing gene activity. “Bending bone ultimately bends genes” (see Fig. 1) [1].
Fig. 1.
The mechanosome hypothesis. Bending the bone from mechanical load or challenging bone tissue with parathyroid hormone (PTH) deforms the bone cell membrane and “launches” adhesion-associated molecules from adhesion complexes. These adhesion-associated molecules then complex with nucleocytoplasmic shuttling architectural transcription factors (ATFs). This mechanosome complex enters the nucleus and binds to a target gene that has been pulled into the path of the oncoming mechanosome by the solid-state scaffold comprised of cytoskeletal and associated proteins, LINC proteins, and lamins. The action of the ATF alters the DNA conformation of the target gene, thus altering the interaction between other transcription factors along the gene’s regulatory regions and changing its activity
The conceptual framework for the idea that mechanosomes are launched from adhesion platforms that move genes into their target range via the cell’s solid-state scaffolding was initially based on the observations that (1) twisting integrins moves chromatin and changes gene expression [2, 3]; (2) LEF-1 (lymphoid enhancer factor-1, a nucleocytoplasmic shuttling transcription factor, escorts β-catenin, a cadherin-associated protein, into the nucleus to its target gene [4, 5]; and (3) LEF-1 is an “architectural transcription factor” (ATF) and regulates gene expression by bending DNA [6, 7]. Together, these observations provide the proof-of-concept steps necessary for translating cell deformation into molecular torque and ultimately alterations in phenotype.
We proposed that β-catenin/LEF-1 is the archetypal mechanosome comprised of an adhesion-associated protein and an ATF and that the load-induced release of β-catenin triggers LEF-1-mediated bending of the target gene [1]. Soon thereafter, we demonstrated that fluid shear stress indeed drives β-catenin into the osteoblast nucleus [8]. Also at this time, the critical significance of the Wnt/β-catenin signaling pathway in the regulation of bone formation was demonstrated by a number of laboratories [9–11], and Robinson and colleagues [12] showed that this signaling pathway is responsive to load in vivo (see below for more details). We also proposed that the putative interaction between Nmp4/CIZ (nuclear matrix protein 4/cas interacting zinc finger protein, hereafter referred to as Nmp4 for simplicity) and p130Cas [13] qualifies as a mechanosome [1] because like LEF-1, Nmp4 is a nucleocytoplasmic shuttling ATF [13–15] and p130Cas association with focal adhesions had been established [16–18]. However, data supporting the presence of p130Cas in the nucleus have not been as compelling [19, 20] other than as the 31-kD caspase-generated cleavage product produced during apoptosis [21]. Interestingly, a FAK/p130Cas complex at the focal adhesions launches JNK into the nucleus as part of a matrix survival-signaling pathway [22] but whether a similar load-induced pathway involves FAK/p130Cas/Nmp4 remains to be determined.
In an early review on bone mechanotransduction, Chen and Ingber [23] speculated that the components of the osteoblast cytoskeleton act as “tensile guy wires” anchoring the nucleus in place and transferring mechanical signals generated from ECM deformation to individual genes. Nevertheless, the specific physical connections between the cell adhesion complexes and the target genes were not known. One of us proposed that nuclear matrix ATFs might act as the final link between the cell’s solid-state scaffolding and the DNA, thus bending the gene in response to the mechanical signals coming down these guy wires [24].
The Mechanosome Revisited: Expansion, Revision and Support
Both Load and PTH Launch Mechanosomes
A significant revision to our original hypothesis is the expansion of the mechanosome’s function to include mediating bone cell response to parathyroid hormone (PTH) [25]. Like mechanical load, intermittent PTH induces bone growth in the mature skeleton [26] and activates many of the same diffusion-controlled and solid-state signaling pathways in the bone cell [20, 27–32]. Intermittent PTH is the only FDA-approved drug for enhancing bone growth in the osteoporotic skeleton [33], and the synergistic or additive action of PTH and load on bone anabolism [34–37] holds promise for enhancing the cost-effectiveness of this hormone [25, 38]. Finally, the relationship between PTH and load within the context of bone may transcend synergistic growth, but instead may be interdependent since load-induced bone formation is abrogated in rats after the removal of their parathyroid gland and the magnitude of the PTH-induced response is attenuated under hind limb suspension [39, 40].
Both PTH and mechanical load stabilize cytosolic β-catenin and thus launch the β-catenin/LEF-1 mechanosome into the osteoblast nucleus [8, 41–45]. A recent study has demonstrated that PTH activation of β-catenin involves recruitment of the requisite adapter protein Dishevelled by the PTH1R receptor [41], which may result in the phosphorylation and inhibition of GSK3, although this remains to be established. Interestingly, load-induced inhibition of GSK3 and enhancement of osteoblastogenesis are independent of Wnt association with its receptor complex [43, 44].
Both mechanical load and hormone impact the expression, activation, and/or organization of focal adhesions and focal adhesion-associated proteins but the functional significance of these interactions is only beginning to emerge [46–50]. For example, focal adhesion kinase (FAK), a non-receptor tyrosine kinase associated with these complexes, supports oscillatory fluid flow-induced activation of ERK 1/2, c-fos, COX-2, and PGE2 in osteoblasts, all of these pathways are direct or indirect contributors to the anabolic response [47]. PTH activates RhoA in osteoblasts [49], a member of the family of GTPases that play an as yet fully specified role in bone cell adhesion and motility.
The physical impact of mechanical load (shear stress) or PTH on the bone cell is profound. Briefly, both agonists significantly alter bone cell cytoskeletal and nuclear organization, often effecting a noticeable change in osteoblast shape; there are numerous studies showing that connective tissue cell morphology has a direct effect on transcription and mRNA stability, particularly type I collagen (see [25] and references therein). Duncan and colleagues elegantly demonstrated the interaction between PTH, shear stress, and cytoskeletal organization using UMR 106-01 osteoblast-like cells and pharmacological agents that interfere with actin polymerization and depolymerization [28]. Cytochalasin D-induced depolymerization of actin augmented the peak shear stress-induced increase in cytosolic calcium as did a 30-min pretreatment with PTH; conversely, stabilization of actin with phalloidin prevented the PTH-enhanced increase in cytosolic calcium in response to shear [28]. Inhibition of the mechanosensitive, cation-selective channel with gadolinium significantly blocked the peak cytosolic calcium response in cells pretreated with either PTH or cytochalasin D. These data indicate that PTH may reduce the mechanical threshold of osteoblasts via the alteration of actin organization [28]; additionally, it identifies part of the mechanism underlying the synergistic effect of PTH and load on bone.
Our contention that PTH/load-induced alteration of the bone cell cytoskeletal and nucleoskeletal architectures mediate the “tugging” of target genes into the pathway of incoming mechansomes is indeed on the surface somewhat fanciful; however, a direct link between focal adhesions and DNA has recently been mapped since we first proposed this aspect of our mechanism. The LINC (linker of nucleus and cytoskeleton) complex physically connects the focal adhesion to DNA (reviewed in [25] and references therein) and provides the pathway that mediates integrin “tugging” of chromatin first demonstrated by Ingber and colleagues [3]. However, our updated version of the mechanosome hypothesis is still vague on the specifics by which target genes are pulled into transcription factories that receive the incoming mechanosomes. Indeed, we have shown that an intact cytoskeleton is not required for all mechanically induced responses in bone cells [51].
Other shared bone cell signaling pathways between PTH and mechanical load include increases in cytosolic calcium, cAMP, and activation of the MAPK/ERK pathway but how these second messenger cascades influence the launching of mechanosomes remains to be determined [28, 37, 52–56]. Both PTH and load stimulate c-fos expression [32, 57], and this may be a key immediate early step in initiating the anabolic program and critical to their synergistic effect on bone growth.
Nmp4/p130Cas: the “STOP” Mechanosome
Our original hypothesis envisioned a few protein complexes launching from focal adhesions directly to target genes. However, we now posit a large network comprised of “GO” mechanosomes and at least one “STOP” mechanosome, specifically Nmp4/p130Cas [25].
Studies from the research group of Maska Noda and our own laboratories suggest that Nmp4 is a general inhibitor of bone anabolism by acting as a repressive trans-acting protein and as a signaling molecule capable of antagonizing the launching of the β-catenin/LEF-1 “GO” mechanosome [15, 58–63]. Nmp4-knockout (KO) mice exhibited a modest increase in baseline bone mineral density and bone mineral content when compared to wild-type (WT) mice [59, 61]. However, the null mice manifested a strikingly enhanced response to anabolic PTH; specifically, daily injections of hormone for 7 weeks resulted in significantly augmented increases in femoral trabecular bone compared to WT [59]. Additionally, the PTH-stimulated increase in cortical bone was not impaired in the null mice. The Nmp4-KO mice showed more robust increases in trabecular bone volume/total volume (BV/TV), connectivity density, (Conn D), trabecular number (Tb N), and trabecular thickness (Tb Th). Additionally, the null mice exhibited a striking hormone-induced decrease in the structural model index (SMI), which characterizes the rod- to plate-shaped transition of the trabecular bone, when compared to the WT animals [59]. Conversely, the Nmp4-KO mice are resistant to bone loss induced by unloading [60]. Unloading by hindlimb suspension for 2 weeks resulted in a decrease in femoral BV/TV and femoral cortical thickness and area in WT mice but no loss was observed in the null mice. Bone histomorphometric analysis revealed that unloading suppressed trabecular and cortical mineralization apposition rate (MAR), mineralizing surface/bone surface (MS/BS), and bone formation rate (BFR) in the WT mice but not in the Nmp4-KO mice [60]. The formation of mineralized nodules in the bone marrow cultures obtained from WT mice subjected to unloading was attenuated but this suppression was not observed in cultures derived from the null animals [60]. Finally, there appeared to be no significant differences in osteoclast behavior between the two genotypes under the unloading conditions [60].
Nmp4 binds to the minor groove of AT-rich DNA [13–15], bends DNA like an ATF [13], and suppresses PTH-stimulated transcription induction [62]. There is a conserved 5′-regulatory region of the matrix metalloproteinase-13 (MMP13) in the mouse, rat, and human genes that governs PTH-stimulated transcription induction [64]. The rat PTH-response regulatory region spans nucleotides (nt) −148 to −38 and supports binding of numerous transcription factors, including Runx2, c-Fos/c-Jun, Ets-1, and Nmp4, the latter binding to the AT-rich sequence spanning (−119/−110 nt). Introduction of a null-binding mutation of this Nmp4 consensus sequence within an Mmp13 promoter-reporter construct containing the first 1329 nt of the 5′-regulatory region enhanced PTH induction in UMR 106-01 osteoblast-like cells [62]. These data support our contention that at least part of the suppressive action of the Nmp4 STOP mechanosome is mediated by this protein’s capacity for directly repressing transcription.
Nmp4 antagonizes shear stress-induced β-catenin nuclear translocation in osteoblasts [58], which suggests a second pathway for functioning as a STOP mechanosome by interfering with the launching of the GO β-catenin/LEF-1 mechanosome. Subjecting WT and Nmp4-KO calvarial osteoblasts to oscillatory fluid shear stress enhanced the increase in null-cell nuclear β-catenin when compared to the WT osteoblasts. Additionally, the absence of Nmp4 augmented shear-stimulated increases in the activities of ERK, Akt, and GSK3β. Finally, the shear-mediated increase in cyclin D1 expression, a target gene for β-catenin, was enhanced in the null cells [58].
Nmp4 also antagonizes Smad-mediated gene transcription in bone cells [63], perhaps another example of this STOP mechanosome interfering with nucleocytoplasmic traffic. The Smad proteins shuttle between the cytoplasm and nucleus. Receptor-induced Smad phosphorylation results in their accumulation in the nucleus where these DNA-binding trans-acting proteins regulate target gene activity [65]. Noda and colleagues determined that overexpression of Nmp4 in the murine osteoblast cell line MC3T3-E1 blocked BMP-induced Smad1 and Smad5 activation of a luciferase reporter construct containing a BMP-specific Smad-binding element (12× GCCG elements) linked to the type X collagen promoter [63]. The investigators noted that co-transfection of Nmp4 and Smad5 expression vectors inhibited the full-length Smad5-mediated BMP2 signaling but also inhibited the Smad1 MH2 domain-mediated signaling (the functional domain that does not bind to the DNA consensus sequence). They concluded that the suppressive action of Nmp4 could occur prior to DNA binding of the R-Smads [63], i.e. Nmp4 might have interfered with activated Smad trajectory into the nucleus.
Whether the putative interaction between Nmp4 and p130Cas is significant to repressing the launching of the β-catenin/LEF-1 mechanosome or interfering with Smad activity remains to be determined but the role of p130Cas as a focal adhesion-associated cell mechanosensor has been established since the publication of our original paper on mechanosomes [66–69]. Upon mechanical stretch of the cell, p130Cas undergoes a conformational change that promotes its phosphorylation by Src family kinases, which supports integrin-mediated signaling [66]. Perhaps the load- and possibly PTH-induced activation of p130Cas acts to sequester some Nmp4 in the cytoplasm where it then suppresses the stabilization of β-catenin.
Nmp4 is a highly conserved protein [15]; however, the human ortholog ZNF384 binds directly to zyxin, not p130Cas, and in turn zyxin binds to p130Cas [70]. Like p130Cas, zyxin associates at high traction force focal adhesions and is required for load-induced actin polymerization at these sites and disassociates during a relaxation in stretch [71, 72]. Mechanical load-induced zyxin nuclear translocation in smooth muscle cells is critical for the regulation of specific load-responsive target genes [72]. Zyxin binds to the homeodomain transcriptional repressor Xanf1/Hesx1 and attenuates its transcriptional repressive activity during Xenopus embryogenesis [73], thus providing another proof-of-concept that mechano-sensitive adhesion-associated molecules can directly alter gene transcription.
β-Catenin/LEF-1: Identification of a “GO” Mechanosome
Since we initially proposed the load-induced activation of β-catenin/LEF-1 signaling as an archetypal example of a load-bearing mechanosome [1], there has been considerable experimental support for this idea from both in vitro and in vivo studies. A key piece of supporting data came with the confirmation that fluid shear stress (FSS) actually promoted the load-induced movement of β-catenin to the nucleus and that nuclear translocation was coupled to activation of LEF-1 in cultured bone cells [8]. These experiments demonstrated that 1 h of laminar FSS (10 dynes/cm2)-induced translocation of β-catenin from the cytoplasm to the nucleus and activated an LEF-1/TCF reporter gene. Related studies have shown that dynamic mechanical strain of bone cells causes accumulation of β-catenin in the cytoplasm and translocation to the nucleus [44]. Analysis of potential upstream regulators of β-catenin identified two potential mechanisms through which FSS promotes β-catenin translocation [8, 74]. First, FSS induced a transient, but significant, increase in the phosphorylation of both GSK-3β and Akt. Second, FSS reduced the amount of β-catenin that was physically associated with N-cadherin at the cell membrane. This later finding suggested that sequestration of β-catenin by cadherins was decreased in osteoblasts subjected to FSS and provided experimental support for the idea that cadherins serve as launching platforms for β-catenin following mechanical stimulation.
Functional analysis of how β-catenin transduced mechanically stimulated signals in osteoblasts revealed two novel observations. First, endogenous, nuclear β-catenin purified from osteoblasts formed a complex with a TCF-binding element in the cyclooxygenase-2 (Cox-2) promoter, and second, overexpression of either a constitutively active β-catenin molecule or inhibition of GSK-3β activity increased basal expression of cyclooxygenase-2 (Cox-2) protein [8]. These data revealed for the first time that FSS modulates the activity of both GSK-3β and β-catenin and that launching of β-catenin/LEF-1 from a membrane-associated pool to the nucleus altered target gene expression. Together, these studies support a model in which mechanically induced GSK3β inactivation and activation of Akt promotes mobilization of β-catenin from a membrane-sequestered pool that accumulates in the cytoplasm and subsequently translocates to the nucleus where it regulates the expression of mechanically responsive genes.
The integral membrane protein caveolin-1 may represent another mechanism through which β-catenin can be sequestered at the plasma membrane until it receives an appropriate “launch” signal [75]. In vivo evidence supporting such a functional role for caveolin-1 sequestration of β-catenin comes from caveolin-1 knockout mice. These animals, which have a high bone mass phenotype [76] possibly due to increased β-catenin accumulation in the cytoplasm and decreased accumulation in the nucleus, may be unable to properly regulate β-catenin/LEF-1 mechanosomes. More direct support of a role of β-catenin/LEF-1 signaling in bone was subsequently confirmed in vivo [12]. These studies showed an increased expression of Wnt/β-catenin target genes in osteoblasts derived from the tibia of high bone mass LRP5 G171V transgenic mice. When tibias from LRP5 G171V transgenic mice were subjected to mechanical loading, the expression of Wnt/β-catenin target genes was increased compared to non-transgenic mice. Loading resulted in the increased expression of multiple Wnt/β-catenin target genes including Wnt10B, SFRP1, cyclin D1, FzD2, WISP2, and connexin 43. While target gene expression increased following loading of both wild-type and high bone mass LRP5 G171V transgenic mice, the transcriptional response was further increased in the transgenic mice. These in vivo results were further corroborated by in vitro mechanical loading experiments in which MC3T3-E1 osteoblastic cells that were subjected to 3400 microstrain alone for 5 h exhibited increased expression of Wnt10B, SFRP1, cyclin D1, FzD2, WISP2, and connexin 43. Thus, both in vitro and in vivo studies support the hypothesis that mechanical loading promotes β-catenin signaling as part of the normal physiological response to load.
Targeted deletion of β-catenin from osteocytes in mice resulted in decreased bone mass as a result of progressive bone loss in the appendicular and axial skeleton [77]. Both cortical bone and cancellous bone mass were reduced. Interestingly, this low bone mass phenotype exhibited normal osteoblast function and osteocyte number but an increased osteoclast number and activity. The conditional knockout mice also showed reduced osteoprotegerin (OPG) levels consistent with the enhanced osteoclast number and activity.
Estrogen receptor signaling may also impact mechanosome responses involving β-catenin. In osteoblastic ROS 17/2.8 cells and primary osteoblast cultures, mechanical strain increased nuclear accumulation of activation of β-catenin and TCF/LEF reporter activity as expected [78]. This response was blocked, however, by estrogen receptor (ER) modulators and was diminished in cultures of primary osteoblasts that were isolated from mice that lacked ERα expression. The mechanical stimulation not only promoted osteogenic processes, but inhibited differentiation of multipotent osteoblastic precursors such as mesenchymal stem cells (MSC) from differentiating into adipocytes [43]. This effect by mechanical regulation of differentiation potential was also dependent on β-catenin. When MSCs were cultured in media that normally promotes adipogenesis, mechanical loading inhibited GSK3β activity and induced β-catenin signaling to limit adipogenesis and enhance osteogenic differentiation.
Although as indicated previously, PTH stimulates the launching of the β-catenin/LEF-1 mechanosome into the nucleus and regulates the expression of numerous components of the Wnt signaling pathway [41, 79], it is not clear as to whether the activation of the Wnt receptors are required for PTH-induced bone formation. Mice lacking the Wnt co-receptor lipoprotein-related protein-5 (LRP5) exhibited no defect in PTH-induced bone formation [80]; additionally, transgenic mice overexpressing osteoblast Dickkopf-1 (Dkk1), a soluble inhibitor of Wnt/β-catenin signaling via its binding to LRP5, also showed no attenuation in intermittent PTH-induced bone formation [81]. However, in another report, these same Dkk1 transgenic mice failed to gain bone under conditions inducing hyperparathyroidism, whereas their WT littermates did exhibit increased bone formation under this regimen [42]. Recent evidence indicates that Smad3 is required for PTH-induced launching of β-catenin/LEF-1 [79, 82], which suggests a potential pathway for side-stepping activation via Wnt co-receptors.
Potential Mechanomes: HMGB1/RAGE, FAK/Pyk2, ILK, NF-KB
HMGB1 is a ubiquitously expressed nucleocytoplasmic shuttling ATF that shares a DNA-binding domain with LEF-1 [83]. As a nuclear protein HMGB1 mediates transcription, chromatin remodeling, DNA repair, and recombination [84]. However, unlike LEF-1 and Nmp4, it can exit the cell via secretion, necrosis, or apoptosis and acts as a potent pro-inflammatory cytokine in the extracellular environment through its interaction with the receptor for advanced glycation end productions (RAGE) or the toll-like receptors TLR4 and TLR2 [85].
The emerging role of HMGB1 in bone remodeling is not surprising [86–90] since as a cytokine, it typically acts as an alarmin, an endogenously released molecule that alerts the innate immune system to the need for tissue repair [91], and bone remodeling is a form of tissue repair. RANKL stimulates HMGB1 release from macrophages [86] and PTH attenuates the release of HMGB1 from osteoblasts by repressing apoptosis [87]. HMGB1, via its interaction with RAGE, enhances osteoclastogenesis at subthreshold concentrations of RANKL [86], acts as a chemotactic agent to both osteoblasts and osteoclasts, and regulates endochondral ossification [89].
The PTH-induced increase in femoral trabecular bone is strikingly attenuated in Rage-KO mice compared to WT animals but hormone-stimulated bone formation is not diminished in the vertebra of the null mice [92]; that the femur is a load-bearing bone and the vertebra is relatively under-loaded in rodents raises the question as to whether the HMGB1/RAGE signaling axis requires load to drive PTH-stimulated bone formation. The capacity of RAGE to act as an adhesion receptor strengthens its candidacy as a member of the putative mechanosome network. RAGE mediates adherence and spreading of alveolar epithelial cells to type IV collagen-coated surfaces and to a lesser extent, type I collagen [93]. Endothelial expressed RAGE and ICAM-1 cooperatively mediate leukocyte adhesion during acute trauma-induced inflammation [94]. We are currently evaluating the potential role of RAGE in osteoblast and osteoclast adhesion.
Like zyxin, there are a number of mechano-sensitive, focal adhesion-associated proteins that are potential candidates for membership into the mechanosome network but all require further study before any definitive conclusions can be made. Of particular interest are focal adhesion kinase (FAK), proline-rich kinase 2 (Pyk2), and integrin-linked kinase (ILK). Early evidence implicated a particularly important role for FAK in response to FSS in endothelial cells [95, 96]. FAK signaling is controlled by autophosphorylation at tyrosine 397 (Tyr397). Once activated, FAK associates with and activates the tyrosine kinase c-Src. This FAK-c-Src association renders c-Src capable of phosphorylating two additional focal adhesion-associated proteins, paxillin and p130Cas. This complex functions as a molecular scaffold for recruitment of other adaptors and signaling intermediates.
Although FAK is not required for osteoblast differentiation, it is needed for bone regeneration in adult mice [97]; consistent with these data, we have recently shown that FAK is required for osteoblast response to shear stress [47]. Briefly, we disabled FAK in osteoblasts using a variety of methods and observed that numerous responses to oscillatory fluid flow including extracellular signal-related kinase 1/2 phosphorylation, upregulation of c-fos, cyclooxygenase-2, and osteopontin expression, and release of prostaglandin E(2) were all compromised in the absence of this focal adhesion-associated protein [47].
Although FAK’s role in osteoblast mechanotransduction is emerging, whether it translocates to the nucleus as a mechanosome is not known and only recently has FAK been observed in the nucleus in some cells under different circumstances. During mouse development, nuclear FAK performs a scaffolding role that ultimately mediates enhanced p53 degradation promoting cell survival; Pky2 has a similar role [98, 99]. In muscle cells, FAK associates with methyl-CpG-binding domain protein 2 (MBD2) resulting in the remodeling of heterochromatin, thus directly or indirectly mediating the regulation of myogenin expression and muscle differentiation [100, 101]. Although Pyk2 exhibited a shear stress-induced redistribution in the osteoblast [102] and depolarization of hippocampal slices induced a rapid and transient nuclear accumulation of PYK2 [103], the role in the nucleus in any cell is not known.
ILK, a nucleocytoplasmic shuttling protein, is involved in various cellular processes including proliferation, adhesion and migration, and osteoblast differentiation [104, 105]. This protein has been proposed to function as a sensor of mechanical stretch in cardiac tissue [106], in fibroblasts [107], and in smooth muscle [108]. Its role in the nucleus remains to be fully characterized but recent evidence suggests it can act as a transcriptional repressor to CNDSR3, a scaffold protein [109, 110]. But whether ILK binds to a nucleocytoplasmic shuttling ATF or plays a significant role in bone cell mechanotransduction remains to be determined.
Nuclear factor-kappa B (NF-κB) translocation to the osteoblast nucleus suppresses bone formation [111]. NF-κB nuclear translocation is activated by FSS in osteoblasts [112] and requires FAK [46]. Similarly, FAK mediates NF-κB signaling in endothelial cells in response to FSS [113] and in cardiac myocytes in response to mechanical strain [114]. The precise details of how FAK and NF-κB may interact as part of a “STOP” mechanosome complex remain to be determined.
Bone Mechanobiology and the Mechanosome Concept
Great progress has been made in recent years in identifying key cellular and molecular mechanisms that regulate the response of bone to mechanical loading. Many of these findings are consistent with the mechanosome hypothesis. For example, Bonewald’s group has focused attention on the key role played by osteocytes that are embedded deep within the mineralized bone matrix in detection of mechanical signals, reviewed in [115]. While osteoblasts secrete new bone matrix proteins, it is the osteocytes that are thought to be critical for translating signals induced by mechanical loading into the appropriate biochemical signals that properly regulate bone remodeling. Going forward, it will be important to recognize the distinct differences between how osteoblasts and osteocytes detect and respond to mechanical signals given their distinct functions. This may be particularly important when considering the ways in which Wnt/β-catenin/LEF-1 signaling occurs in response to mechanical loading. This pathway may play a distinct role in regulating osteoblasts differentiation, proliferation, and matrix synthesis compared to the osteocytes that may use this pathway primarily to transmit appropriate signals in response to mechanical loading to bone lining cells on surfaces of bone. For example, Wnt/β-catenin/LEF-1 signaling in osteocytes may play an important role, along with prostaglandin signaling, in downregulating expression molecules such as sclerostin (Sost) and Dkk1, which inhibit induction of new bone remodeling. It will be interesting to learn how these cell types may differ in the way mechanosomes function to control these distinct responses to similar mechanical signals.
Another area of exciting progress in bone mechanobiology is the increased recognition of the role of the primary cilium as a mechanosensor during skeletal development and in adult bone homeostasis, reviewed in [116]. Primary cilia are microtubule-based organelles that grow from the basal body and extend from the cell surface where they are positioned to act as sensors of extracellular signals. How signals are detected by this structure and passed to established mechanotransduction pathways is not known. Could primary cilia serve as launching platforms for previously unrecognized mechanosome complexes? A recent report suggests that signaling via the primary cilium involves activation of adenylyl cyclase 6 and cAMP as second messengers [117].
A recent report [118] highlights a potentially novel mechanosome protein that may link activation of the primary cilia to mechanotransduction in bone; this is the zinc finger protein of the cerebellum (Zic1). A comparison of gene expression in human bone samples from sites that experience high and low levels of mechanical stress, lumbar spine and iliac crest, respectively, revealed that Zic1 was significantly upregulated in the lumbar spine compared to the iliac crest. Interestingly, data mining by this group identified proteins involved with primary cilia, PATCH1 and GLI-Kruppel family members Gli1 and Gli3, as potential mechanosome-like proteins associated with Zic1. Immunolocalization studies confirmed that Zic1 is in both the cytosol and nucleus of the MLO-Y4 osteocytic cells, MC3T3-E1 osteoblast-like cells, and in primary osteoblast cultures. Application of oscillatory FSS to MLO-Y4 cells resulted in translocation of Zic1 from the cytoplasm to the nucleus. Also of interest, silencing the Zic1 gene blocked FSS-induced LEF-1/TCF activation. Thus, Zic1, PATCH1, Gli1, and Gli3 may represent a novel mechanosome involved in mechanotransduction via the primary cilium and/or part of a pathway that promotes Wnt/β-catenin signaling in response to FSS in osteocytes.
A persistent concern in the field of bone mechanobiology has been that the strain levels induced by normal skeletal loading, such as walking, are not sufficient to stimulate intracellular signaling in osteocytes or osteoblasts. Weinbaum, Shaffler and colleagues have proposed a mechanism by which amplification of tissue level strains occur at the cellular level [119]. This quantitative model of signal propagation postulates that extensions of the osteocyte membrane can attach directly to the canalicular wall at discrete locations via the integrin receptors. Indeed, rapid fixation and immunolocalization techniques revealed that these attachment sites contained β3 integrins at their tips [120]. Their model predicts that small tensile forces in the tissue in the low pico-Newton range produce axial strains up to two orders of magnitude greater at the cellular level via these integrin attachments. These focally amplified small tissue-level strains would likely be sufficient to directly stimulate mechanosome activity in osteocytes.
Summary
Seven years ago, we proposed that the deformation of bone distorted the bone cell membrane, which ultimately tugged and twisted the target gene DNA, thus altering its activity, i.e. “bending bone ultimately bends genes” [1]. We speculated that the formation of mechanosomes, the transient formation of complexes comprised of adhesion-associated molecules and nucleocytoplasmic shuttling ATFs, transferred information from the membrane to the nucleus. Furthermore, we proposed that β-catenin/LEF-1 and Nmp4/p130Cas were the archetypal mechanosomes.
Since the publication of that hypothesis, load-induced nuclear translocation of β-catenin/LEF-1 is accepted as a significant component of bone mechanotransduction, and Nmp4 has been characterized as a general inhibitor of bone anabolism. The launching of adhesion- and cytoskeletal-associated proteins into the nucleus toward target genes appears to be a common mechanism for regulating cell response to changes in its mechanical microenvironment.
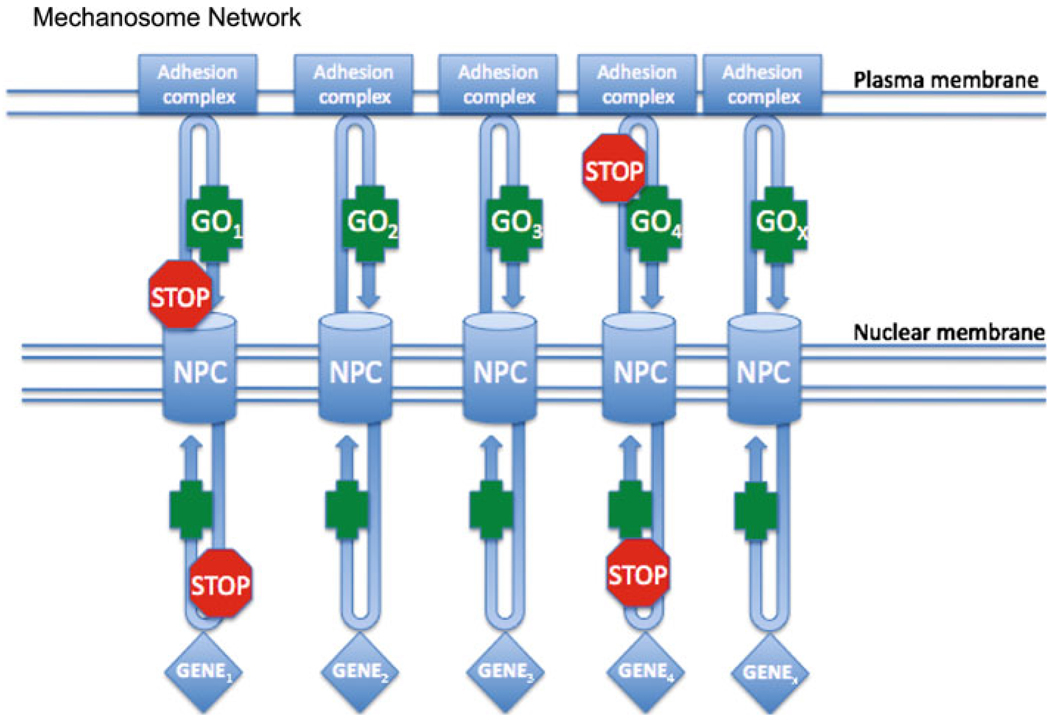
Further investigation is required to validate the details of these proposed pathways and to integrate the mechanosome hypothesis with the myriad of other mechano- and PTH-sensitive focal adhesion proteins into a cohesive network (see Fig. 2). Additionally, whether mechanosomes launch from the primary cilium is of interest.
Fig. 2.
The mechanosome network. Nucleocytoplasmic shuttling transcription factors cycle between the cytoplasm and nucleus. PTH- and/or load-induced deformation of the membrane launches adhesion-associated molecules from the adhesion complexes. The association of some of these adhesion proteins with the transcription factors for an armed “GO” mechanosome that drives bone formation. Other complexes form “STOP” mechanosomes that can attenuate “GO” mechanosome activity in the cytoplasm or nucleus
Contributor Information
Joseph P. Bidwell, Email: jbidwell@iupui.edu, Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Fredrick M. Pavalko, Email: fpavalko@iupui.edu, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
References
- 1.Pavalko FM, et al. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88(1):104–112. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, et al. Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. Am J Physiol Cell Physiol. 2001;280(6):C1475–C1484. doi: 10.1152/ajpcell.2001.280.6.C1475. [DOI] [PubMed] [Google Scholar]
- 3.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K, Hay ED. New evidence that nuclear import of endogenous beta-catenin is LEF-1 dependent, while LEF-1 independent import of exogenous beta-catenin leads to nuclear abnormalities. Cell Biol Int. 2001;25(11):1149–1161. doi: 10.1006/cbir.2001.0799. [DOI] [PubMed] [Google Scholar]
- 5.Behrens J, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 6.Giese K, Grosschedl R. LEF-1 contains an activation domain that stimulates transcription only in a specific context of factor-binding sites. EMBO J. 1993;12(12):4667–4676. doi: 10.1002/j.1460-2075.1993.tb06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giese K, Cox J, Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- 8.Norvell SM, et al. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75(5):396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 9.Jackson A, et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36(4):585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Tamamura Y, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280(19):19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama H, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson JA, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 13.Nakamoto T, et al. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20(5):1649–1658. doi: 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez M, et al. PTH-responsive osteoblast nuclear matrix architectural transcription factor binds to the rat type I collagen promoter. J Cell Biochem. 1998;69(3):336–352. doi: 10.1002/(sici)1097-4644(19980601)69:3<336::aid-jcb11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Thunyakitpisal P, et al. Cloning and functional analysis of a family of nuclear matrix transcription factors (NP/NMP4) that regulate type I collagen expression in osteoblasts. J Bone Miner Res. 2001;16(1):10–23. doi: 10.1359/jbmr.2001.16.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Nojima Y, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270(25):15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 17.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270(38):22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 18.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92(23):10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai R, et al. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13(16):3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charoonpatrapong-Panyayong K, et al. Nmp4/CIZ contributes to fluid shear stress induced MMP-13 gene induction in osteoblasts. J Cell Biochem. 2007;102(5):1202–1213. doi: 10.1002/jcb.21349. [DOI] [PubMed] [Google Scholar]
- 21.Kim W, et al. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J Biol Chem. 2004;279(9):8333–8342. doi: 10.1074/jbc.M312026200. [DOI] [PubMed] [Google Scholar]
- 22.Almeida EA, et al. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149(3):741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CS, Ingber DE. Tensegrity and mechanoregulation: from skeleton to cytoskeleton. Osteoarthritis Cartilage. 1999;7(1):81–94. doi: 10.1053/joca.1998.0164. [DOI] [PubMed] [Google Scholar]
- 24.Bidwell JP, et al. Nuclear matrix proteins and osteoblast gene expression. J Bone Miner Res. 1998;13(2):155–167. doi: 10.1359/jbmr.1998.13.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Childress P, Robling AG, Bidwell JP. Nmp4/CIZ: Road block at the intersection of PTH and load. Bone. 2010 doi: 10.1016/j.bone.2009.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goltzman D. Studies on the mechanisms of the skeletal anabolic action of endogenous and exogenous parathyroid hormone. Arch Biochem Biophys. 2008;473(2):218–224. doi: 10.1016/j.abb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YL, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am J Physiol Cell Physiol. 2009;296(6):C1391–C1399. doi: 10.1152/ajpcell.00549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21(11):1729–1737. doi: 10.1359/jbmr.060722. [DOI] [PubMed] [Google Scholar]
- 29.Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88(3):446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- 30.Ryder KD, Duncan RL. Parathyroid hormone modulates the response of osteoblast-like cells to mechanical stimulation. Calcif Tissue Int. 2000;67(3):241–246. doi: 10.1007/s002230001115. [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi A, et al. Parathyroid hormone-activated volume-sensitive calcium influx pathways in mechanically loaded osteocytes. J Biol Chem. 2000;275(5):3335–3342. doi: 10.1074/jbc.275.5.3335. [DOI] [PubMed] [Google Scholar]
- 32.Chow JW, et al. Role for parathyroid hormone in mechanical responsiveness of rat bone. Am J Physiol. 1998;274(1 Pt 1):E146–E154. doi: 10.1152/ajpendo.1998.274.1.E146. [DOI] [PubMed] [Google Scholar]
- 33.Lane NE, Silverman SL. Anabolic therapies. Curr Osteoporos Rep. 2010;8(1):23–27. doi: 10.1007/s11914-010-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts MD, Santner TJ, Hart RT. Local bone formation due to combined mechanical loading and intermittent hPTH-(1–34) treatment and its correlation to mechanical signal distributions. J Biomech. 2009;42(15):2431–2438. doi: 10.1016/j.jbiomech.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Sugiyama T, et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone. 2008;43(2):238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Kim CH, et al. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res. 2003;18(12):2116–2125. doi: 10.1359/jbmr.2003.18.12.2116. [DOI] [PubMed] [Google Scholar]
- 37.Li J, et al. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology. 2003;144(4):1226–1233. doi: 10.1210/en.2002-220821. [DOI] [PubMed] [Google Scholar]
- 38.Warden SJ, Fuchs RK. Are “exercise pills” the answer to the growing problem of physical inactivity? Br J Sports Med. 2008;42(11):562–563. doi: 10.1136/bjsm.2008.053512. [DOI] [PubMed] [Google Scholar]
- 39.Turner CH. Bone strength: current concepts. Ann NY Acad Sci. 2006;1068:429–446. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- 40.Turner RT, et al. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol. 2006;101(3):881–886. doi: 10.1152/japplphysiol.01622.2005. [DOI] [PubMed] [Google Scholar]
- 41.Romero G, et al. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285(19):14756–14763. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo J, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161–171. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen B, et al. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284(50):34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Case N, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283(43):29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni NH, et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95(6):1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 46.Young SR, et al. Activation of NF-kappaB by fluid shear stress, but not TNF-alpha, requires focal adhesion kinase in osteoblasts. Bone. 2010;47(1):74–82. doi: 10.1016/j.bone.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young SR, et al. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24(3):411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee DY, et al. Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by alphavbeta3 and beta1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J Biol Chem. 2010;285(1):30–42. doi: 10.1074/jbc.M109.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kazmers NH, et al. Rho GTPase signaling and PTH 3–34, but not PTH 1–34, maintain the actin cytoskeleton and antagonize bisphosphonate effects in mouse osteoblastic MC3T3-E1 cells. Bone. 2009;45(1):52–60. doi: 10.1016/j.bone.2009.03.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaiser E, et al. Parathyroid hormone (1–34) regulates integrin expression in vivo in rat osteoblasts. J Cell Biochem. 2001;83(4):617–630. doi: 10.1002/jcb.1256. [DOI] [PubMed] [Google Scholar]
- 51.Norvell SM, et al. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96(3):957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- 52.Lee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Curr Opin Nephrol Hypertens. 2009;18(4):298–302. doi: 10.1097/MNH.0b013e32832c2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehrotra M, et al. Fluid flow induces Rankl expression in primary murine calvarial osteoblasts. J Cell Biochem. 2006;98(5):1271–1283. doi: 10.1002/jcb.20864. [DOI] [PubMed] [Google Scholar]
- 54.Cherian PP, et al. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 55.Cahir McFarland ED, Izumi KM, Mosialos G. Epstein-barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene. 1999;18(49):6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- 56.Carvalho RS, et al. Stimulation of signal transduction pathways in osteoblasts by mechanical strain potentiated by parathyroid hormone. J Bone Miner Res. 1994;9(7):999–1011. doi: 10.1002/jbmr.5650090707. [DOI] [PubMed] [Google Scholar]
- 57.Onyia JE, et al. In vivo, human parathyroid hormone fragment (hPTH 1–34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone. 1995;17(5):479–484. doi: 10.1016/8756-3282(95)00332-2. [DOI] [PubMed] [Google Scholar]
- 58.Yang Z, et al. Nmp4/CIZ inhibits mechanically induced beta-catenin signaling activity in osteoblasts. J Cell Physiol. 2010;223(2):435–441. doi: 10.1002/jcp.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robling AG, et al. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol. 2009;219(3):734–743. doi: 10.1002/jcp.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hino K, et al. Deficiency of CIZ, a nucleocytoplasmic shuttling protein, prevents unloading-induced bone loss through the enhancement of osteoblastic bone formation in vivo. Bone. 2007;40(4):852–860. doi: 10.1016/j.bone.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 61.Morinobu M, et al. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201(6):961–970. doi: 10.1084/jem.20041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah R, et al. Nmp4/CIZ regulation of matrix metalloproteinase 13 (MMP-13) response to parathyroid hormone in osteoblasts. Am J Physiol Endocrinol Metab. 2004;287(2):E289–E296. doi: 10.1152/ajpendo.00517.2003. [DOI] [PubMed] [Google Scholar]
- 63.Shen ZJ, et al. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J Biol Chem. 2002;277(33):29840–29846. doi: 10.1074/jbc.M203157200. [DOI] [PubMed] [Google Scholar]
- 64.Selvamurugan N, et al. Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem. 1998;273(17):10647–10657. doi: 10.1074/jbc.273.17.10647. [DOI] [PubMed] [Google Scholar]
- 65.Ross S, Hill CS. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40(3):383–408. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geiger B. A role for p130Cas in mechanotransduction. Cell. 2006;127(5):879–881. doi: 10.1016/j.cell.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 68.Zaidel-Bar R, Kam Z, Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J Cell Sci. 2005;118(Pt 17):3997–4007. doi: 10.1242/jcs.02523. [DOI] [PubMed] [Google Scholar]
- 69.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7(5):709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 70.Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4-zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312(7):1194–1204. doi: 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121(Pt 17):2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 72.Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension. 2004;43(4):726–730. doi: 10.1161/01.HYP.0000119189.82659.52. [DOI] [PubMed] [Google Scholar]
- 73.Martynova NY, et al. The LIM-domain protein Zyxin binds the homeodomain factor Xanf1/Hesx1 and modulates its activity in the anterior neural plate of Xenopus laevis embryo. Dev Dyn. 2008;237(3):736–749. doi: 10.1002/dvdy.21471. [DOI] [PubMed] [Google Scholar]
- 74.Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4(4):e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galbiati F, et al. Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem. 2000;275(30):23368–23377. doi: 10.1074/jbc.M002020200. [DOI] [PubMed] [Google Scholar]
- 76.Rubin J, et al. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res. 2007;22(9):1408–1418. doi: 10.1359/jbmr.070601. [DOI] [PubMed] [Google Scholar]
- 77.Kramer I, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30(12):3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Armstrong VJ, et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282(28):20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 79.Tobimatsu T, et al. Parathyroid hormone increases beta-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147(5):2583–2590. doi: 10.1210/en.2005-1627. [DOI] [PubMed] [Google Scholar]
- 80.Sawakami K, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 81.Yao GQ, et al. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab. 2010 doi: 10.1007/s00774-010-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inoue Y, et al. Role of Smad3, acting independently of transforming growth factor-beta, in the early induction of Wnt-beta-catenin signaling by parathyroid hormone in mouse osteoblastic cells. J Cell Biochem. 2009;108(1):285–294. doi: 10.1002/jcb.22252. [DOI] [PubMed] [Google Scholar]
- 83.Giese K, Amsterdam A, Grosschedl R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 1991;5(12B):2567–2578. doi: 10.1101/gad.5.12b.2567. [DOI] [PubMed] [Google Scholar]
- 84.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799(1–2):101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010;1799(1–2):149–156. doi: 10.1016/j.bbagrm.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Z, et al. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res. 2008;23(7):1084–1096. doi: 10.1359/JBMR.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J, et al. HMGB1 is a bone-active cytokine. J Cell Physiol. 2008;214(3):730–739. doi: 10.1002/jcp.21268. [DOI] [PubMed] [Google Scholar]
- 88.Bidwell JP, Yang J, Robling AG. Is HMGB1 an osteocyte alarmin? J Cell Biochem. 2008;103(6):1671–1680. doi: 10.1002/jcb.21572. [DOI] [PubMed] [Google Scholar]
- 89.Taniguchi N, et al. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol Cell Biol. 2007;27(16):5650–5663. doi: 10.1128/MCB.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charoonpatrapong K, et al. HMGB1 expression and release by bone cells. J Cell Physiol. 2006;207(2):480–490. doi: 10.1002/jcp.20577. [DOI] [PubMed] [Google Scholar]
- 91.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 92.Philip BK, et al. RAGE supports parathyroid hormone-induced gains in femoral trabecular bone. Am J Physiol Endocrinol Metab. 2009;298(3):E714–E725. doi: 10.1152/ajpendo.00564.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demling N, et al. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323(3):475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 94.Frommhold D, et al. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood. 2010;116(5):841–849. doi: 10.1182/blood-2009-09-244293. [DOI] [PubMed] [Google Scholar]
- 95.Li S, et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem. 1997;272(48):30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi M, et al. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vasc Res. 1997;34(3):212–219. doi: 10.1159/000159225. [DOI] [PubMed] [Google Scholar]
- 97.Kim JB, et al. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone. 2007;41(1):39–51. doi: 10.1016/j.bone.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim ST, et al. Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J Biol Chem. 2010;285(3):1743–1753. doi: 10.1074/jbc.M109.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lim ST, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mei L, Xiong WC. FAK interaction with MBD2: A link from cell adhesion to nuclear chromatin remodeling? Cell Adh Migr. 2009;4(1):77–80. doi: 10.4161/cam.4.1.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo SW, et al. Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. EMBO J. 2009;28(17):2568–2582. doi: 10.1038/emboj.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guignandon A, et al. Cyclic strain promotes shuttling of PYK2/Hic-5 complex from focal contacts in osteoblast-like cells. Biochem Biophys Res Commun. 2006;343(2):407–414. doi: 10.1016/j.bbrc.2006.02.162. [DOI] [PubMed] [Google Scholar]
- 103.Faure C, et al. Calcineurin is essential for depolarization-induced nuclear translocation and tyrosine phosphorylation of PYK2 in neurons. J Cell Sci. 2007;120(Pt 17):3034–3044. doi: 10.1242/jcs.009613. [DOI] [PubMed] [Google Scholar]
- 104.Su JL, et al. CYR61 regulates BMP-2-dependent osteoblast differentiation through {alpha}v{beta}3 integrin/ILK/ERK pathway. J Biol Chem. 2010 doi: 10.1074/jbc.M109.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakrieko KA, et al. Modulation of integrin-linked kinase nucleo-cytoplasmic shuttling by ILKAP and CRM1. Cell Cycle. 2008;7(14):2157–2166. doi: 10.4161/cc.7.14.6241. [DOI] [PubMed] [Google Scholar]
- 106.Bendig G, et al. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 2006;20(17):2361–2372. doi: 10.1101/gad.1448306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maier S, et al. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim Biophys Acta. 2008;1783(6):1150–1162. doi: 10.1016/j.bbamcr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 108.Huang S, et al. Modulation of microvascular smooth muscle adhesion and mechanotransduction by integrin-linked kinase. Microcirculation. 2010;17(2):113–127. doi: 10.1111/j.1549-8719.2009.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ziera T, et al. Cnksr3 is a direct mineralocorticoid receptor target gene and plays a key role in the regulation of the epithelial sodium channel. FASEB J. 2009;23(11):3936–3946. doi: 10.1096/fj.09-134759. [DOI] [PubMed] [Google Scholar]
- 110.Acconcia F, et al. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci USA. 2007;104(16):6782–6787. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang J, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15(6):682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen NX, et al. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33(3):399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 113.Petzold T, et al. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am J Physiol Cell Physiol. 2009;297(4):C814–C822. doi: 10.1152/ajpcell.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crosara-Alberto DP, Inoue RY, Costa CR. FAK signalling mediates NF-kappaB activation by mechanical stress in cardiac myocytes. Clin Chim Acta. 2009;403(1–2):81–86. doi: 10.1016/j.cca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 115.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Temiyasathit S, Jacobs CR. Osteocyte primary cilium and its role in bone mechanotransduction. Ann N Y Acad Sci. 2010;1192(1):422–428. doi: 10.1111/j.1749-6632.2009.05243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwon RY, et al. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24(8):2859–2868. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kalogeropoulos M, et al. Zic1 transcription factor in bone: neural developmental protein regulates mechanotransduction in osteocytes. FASEB J. 2010;24(8):2893–2903. doi: 10.1096/fj.09-148908. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, et al. Strain amplification and integrin based signaling in osteocytes. J Musculoskelet Neuronal Interact. 2008;8(4):332–334. [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, et al. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci USA. 2007;104(40):15941–15946. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]