Abstract
Trafficking of transcription factors between the cytoplasm and the nucleus is an essential aspect of signal transduction, which is particularly challenging in neurons due to their highly polarized structure. Disruption in the subcellular localization of many proteins, including transcription factors, is observed in affected neurons of human neurodegenerative diseases. In these diseases, there is also growing evidence supporting alterations in nuclear transport as potential mechanisms underlying the observed mislocalization of proteins. Oxidative stress, which plays a key pathogenic role in these diseases, has also been associated with significant alterations in nuclear transport. After providing an overview of the major nuclear import and export pathways and discussing the impact of oxidative injury on nuclear trafficking of proteins, this review synthesizes emerging evidence for altered nuclear transport as a possible mechanism in the pathogenesis of neurodegenerative diseases. Potential strategies to overcome such deficits are also discussed.
Keywords: Nuclear transport, nuclear pore complex, oxidative stress, neurodegeneration, Parkinson's disease, Alzheimer's disease, amyotrophic lateral sclerosis, polyglutamine diseases
1. Introduction
Neurons, with their highly polarized biology, face unique challenges in intracellular trafficking of signaling proteins. In response to both physiologic and pathologic stimuli, signals need to traverse dendrites or axon to affect transcriptional responses in the nucleus–the efficient regulation of which is crucial for neuronal plasticity and survival [1]. Specifically, bidirectional trafficking of proteins such as transcription factors between the cytoplasm and the nucleus is a crucial aspect of signal transduction essential for the proper regulation of transcription.
Disruption of efficient nucleocytoplasmic transport can significantly impair neuronal function and lead to neurodegeneration. This review will focus on emerging evidence that suggests altered nuclear transport as a possible mechanism involved in the pathogenesis of neurodegenerative disorders and briefly discuss potential strategies to overcome such deficits. A discussion of the current evidence in the literature of altered nuclear transport in response to oxidative stress, which is thought to play a significant role in the pathogenesis of many neurodegenerative diseases, is also provided.
2. Nucleocytoplasmic transport
2.1 Overview of the classical nuclear protein import and export cycle
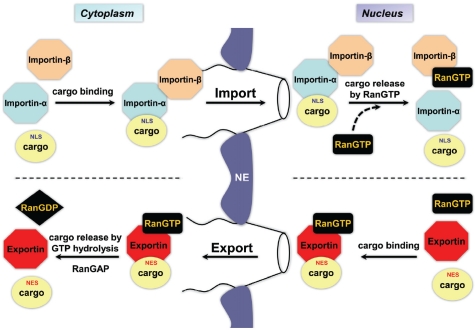
The transport of macromolecules across the nuclear membrane has been extensively studied [2-6]. Nuclear transport of proteins occurs through channels in the nuclear envelope formed by nuclear pore complexes (NPCs). NPCs are large supra molecular assemblies composed of multiple copies of approximately 30 proteins called nucleoporins [3, 7, 8]. Small proteins (< 40 kDa) do not require active transport and can passively equilibrate across the nuclear envelope. However, larger proteins require carrier proteins of the evolutionarily conserved karyopherin-β family, such as importins (for nuclear import) and exportins (for nuclear export), for active transport into and out of the nucleus [3]. Cargo proteins containing a nuclear localization signal (NLS) are targeted for nuclear import. There are different types of NLSs, but the best characterized is the classical NLS, which is composed of one or two stretches of positively-charged amino acids [9]. Cargo proteins bearing a classical NLS interact with importin-β through the adaptor protein importin-α (Figure 1). The interaction between importin-β and the nucleoporins making up the NPC facilitates the translocation of the cargo-importin complex into the nucleoplasm. In the nucleus, RanGTP (small G protein Ran bound to GTP) binds to importin-β to induce the dissociation of the complex, releasing the cargo protein. RanGTP-bound importin-β, or importin-α in complex with RanGTP and the exportin CAS (cellular apoptosis susceptibility protein), are then recycled back to the cytoplasm where they are released upon GTP hydrolysis catalyzed by Ran GTPase activating protein (RanGAP).
Figure 1.
Simplified model of classical nuclear import and nuclear export. Import through the nuclear pore complex of cargo containing a classical NLS occurs via importin-α / importin-β heterodimer. Nuclear export of cargo containing a NES occurs with the help of exportins. As noted in the diagram, the gradient of RanGTP and RanGDP across the nucleus is an essential component of this process. See text for details. NE = nuclear envelope, NLS = nuclear localization signal, NES = nuclear export signal. Adapted from [120].
Protein export from the nucleus is mediated by exportins (e.g. exportin 1 / Crm1) that complex with RanGTP and cargo containing a nuclear export signal (NES). Cargo release to the cytoplasm occurs upon GTP hydrolysis with the help of RanGAP (Figure 1). RanGDP is recycled back to the nucleus with the help of the carrier, nuclear transport factor-2 (NTF2). The nucleotide state of Ran is regulated by two factors - RanGAP and Ran guanine nucleotide-exchange factor (RanGEF). These Ran regulators are highly compartmentalized (RanGAP in the cytoplasm and RanGEF to the nucleus) - resulting in RanGTP being concentrated in the nucleus. This gradient is essential in establishing the directionality of the transport process. In addition to the classic pathway, evidence of alternative nuclear transport pathways, such as those independent of Ran and importins, have recently emerged in the field [3, 10].
2.2 Additional roles of nuclear transport factors in neurons
While most studies of nuclear import have utilized non-neuronal cells, the vast cellular distances separating axon terminals from the nucleus of neurons pose additional challenges for transduction of target-derived signals that classically promote neuron survival. Indeed, recent evidence is beginning to highlight new roles for many nuclear transport factors in overcoming the spatiotemporal challenges of neurons. Early work in Aplysia neurons showed that NLS-targeted proteins injected into distal growth cones were retrogradely transported along the axon into the nucleus with the help of the microtubule network [11, 12]. Interestingly, Hanz et al. found importin-α protein constitutively associated with the motor protein dynein (involved in retrograde trafficking along microtubules) in the axons of peripheral neurons [13]. In response to a nerve lesion, importin-β protein levels increase by local translation of axonal mRNA, leading to the formation of an importin-α/ importin-β/dynein complex that traffics cargo retrogradely. Furthermore, Ran and its associated effectors have been shown to regulate the formation of importin signaling complexes [14]. In response to lesion, axonal RanBP1 (Ran-binding protein 1) protein levels increase from local translation of mRNA and axonal RanGAP is recruited, which leads to GTP hydrolysis and the dissociation of axonal Ran from importins. This allows the newly synthesized importin-β to form a complex with importin-α and dynein. This complex provides the lesioned neuron an efficient way of transmitting retrograde injury signals from distal neurites to the nucleus. Although NLS proteins are targeted to this complex, proteins lacking classical NLS sequences, such as ERK1/2 can also utilize importins in a vimentin-dependent manner for retrograde transport in lesioned nerves [15]. Importins have also been recently implicated in regulating neuronal differentiation [16], axon guidance [17], and long-term synaptic plasticity [18].
3. Effects of oxidative stress on nuclear transport
3.1 Oxidant signaling
The subcellular localization of proteins, such as transcription factors, is a key mechanism in regulating transcription under both basal and stimulus-induced conditions. Many signals, such as phosphorylation / dephosphorylation, acetylation / deacetylation, and oxidative modification, can alter the cytoplasmic to nuclear ratios of proteins - often by regulating interactions with cytoplasmic anchors and masking or exposing nuclear import and export signals [3, 19-24]. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) protein provides an excellent example of a protein whose subcellular localization is regulated by such modifications [24]. Under basal conditions, Nrf2 is sequestered in the cytoplasm by its association with Keap1 and is targeted for proteasomal degradation. However, exposure to reactive oxygen species (ROS) or electrophiles leads to oxidation/modification of sulfhydryl groups in Keap1 as well as post-translational modification of Nrf2 (via activated protein kinases, PKC and MAPK) - ultimately causing dissociation of Nrf2 from the Keap1 inhibitory complex and allowing it to translocate into the nucleus [25-29]. Interestingly, other stimuli that alter activation of protein kinases, such as serum deprivation, have also been shown to affect the subcellular distribution of Nrf2 [30]. Nrf2 is just one such example of a protein whose subcellular localization is regulated by numerous mechanisms.
The signaling events that occur upon exposure to ROS have been well studied [31-34]. Such signaling is essential to activating cellular defense mechanisms to protect against oxidative injury. Examples of proteins involved in ROS signaling include Nrf2, NF-kB, p53, FOXO, ERK1/2, and JNK - which either directly or indirectly regulate gene transcription [21, 34]. Such signaling is especially important in the brain as it is particularly prone to oxidative damage due to its high level of aerobic respiration (∼20% of the resting total body oxygen) combined with lower levels/activities of antioxidants, such as glutathione, superoxide dismutases (SOD), catalase, and vitamin E [35-38]. In addition, elevated levels of iron in regions such as the substantia nigra can contribute to the production of the highly reactive hydroxyl radical (OH-) via Fenton chemistry [39, 40]. Dopamine can undergo autoxidation to form ROS and quinones/semiquinones, each of which can derivatize proteins [36, 41]. Mitochondria also play a major role in producing cellular ROS and hence impairment in mitochondrial respiratory chain function, such as decreased complex I activity as observed in PD patient brains, can further increase ROS production [42, 43].
3.2 Nuclear transport alterations in response to oxidative stress
The oxidative stress hypothesis proposes an imbalance in cellular levels of ROS, which due to severe or prolonged exposures overwhelms the cellular capacity to elicit an antioxidant response - leading to cellular damage and ultimately cell death. In addition to oxidative modification to proteins and lipids, damage to DNA and subsequent impairment in expression of genes may also contribute to cellular degeneration. Interestingly, impaired function of transcription factors can affect the success of antioxidant responses to stress. There is increasing evidence in the literature of disruption in various mechanisms of nuclear transport in response to oxidative stressors in various models [44-52]. Given the important role of nuclear transport of signaling proteins on the regulation of gene expression, alterations in this process can have significant consequences for the cell. The following sections discuss some key findings concerning the effects of oxidative stress on nuclear transport. Although the models utilized by these studies are non-neuronal, the findings are very consistent across the various models and research groups - suggesting that they are likely to be relevant to neuron cell biology as well.
3.2.1 Effects on nuclear import
As previously discussed, proteins over 40 KDa are actively transported through the nuclear pore complex across the nuclear envelope. Hence, the nuclear envelope is an essential barrier separating the nuclear compartment from the cytoplasmic compartment. In response to a high-degree of oxidative stress induced by diethyl maleate (DEM) (5mM, 4hrs) or hydrogen peroxide (H2O2) (20mM, 1hr), there is a breakdown of the nuclear envelope in HeLa cells as indicated by increased levels of β-galactosidase (normally restricted to the cytoplasm) in the nucleus and access of lamin B immunoglobulins to the nuclear lamina in cells treated with digitonin, which permeabilizes the plasma membrane but not the nuclear membrane [44]. The changes in nuclear membrane integrity observed at these high doses of oxidative stressors may be a consequence of cell death. None-theless, lower concentrations of these agents have been shown to impair nuclear transport without affecting the structural integrity of the nuclear envelope [47, 50-52]. Earlier studies looking at the effects of oxidative stress on nuclear transport showed significant alterations in classical nuclear import. In S. cerevisiase, acute treatments with H2O2 (2mM, 10min) and DEM (2mM, 2hrs) led to the inhibition of nuclear import of NLS-GFP [45]. This study also found that the small GTPase Gsp1p / Ran, a key player in classical import/export cycle, redistributed to the cytoplasm - suggesting perturbation of the Ran gradient as a mechanism explaining impaired classical import. Pierce and coworkers showed in semi-permeabilized aortic smooth muscle cells that H2O2 concentration as low as 100μM (1hr) led to impaired import of the fluorescent import substrate BODIPY-BSA-NLS, also increasing the levels of Ran in the cytoplasm [46]. This study utilized an in vitro import assay that takes advantage of the ability of the detergent digitonin to permeabilize membranes rich in cholesterol (e.g. plasma membrane) while leaving other membranes (e.g. nuclear membrane) intact [53, 54]. The nuclear envelope remains in a state competent for studying nuclear import of exogenously added substrates under various experimental conditions. Pierce and coworkers found that treatment of the nuclear import cocktail with H2O2 but not the permeabilized cells themselves led to the nuclear import defect - suggesting the involvement of soluble cytosolic factor(s). The group provides evidence that activation of extracellular signal-regulated kinase-2 (ERK2) in response to H2O2 is involved in inhibiting nuclear import in this model system.
Subsequently, Kodiha et al. carried out a detailed study on the effects of H2O2 on nuclear transport factors [47]. Utilizing HeLa cells, they found significant reduction in nuclear distribution of NLS-GFP but not of GFP, which is small enough to passively enter, at H2O2 doses that do not affect the integrity of the nuclear envelope (assessed by localization of GFP-β-gal and inability of lamin B immunoglobulin to access the nuclear lamina). Interestingly, as opposed to the study by Pierce and coworkers, treatment with an inhibitor of MEK/ERK1/2 signaling (PD98059) did not reverse the nuclear import inhibition. Kodiha et al. also report various alterations in Ran: (1) increased in the cytoplasm, consistent with other reports, (2) increased degradation, (3) reduced efficiency in recycling back to the nucleus, and (4) reduced ratio of RanGTP to RanGDP. The authors also report alterations in other soluble nuclear transport factors: (1) decreased levels of importin-β in the cytoplasm and at the nuclear periphery, (2) relocation of Nup153, an important component of the NPC that regulates functions such as nuclear pore basket formation, (3) reduced docking of importin-α/importin-β/cargo complex at the nuclear envelope, and (4) increased degradation of importin-β and Nup153. These effects are somewhat selective as the subcellular localization of Hsc70 and lamin B and the proteolysis of nucleoporin p62 are not altered. Given the important roles of these transport factors in nuclear transport, such alterations are expected to have significant consequences for nuclear transport of cargo in stressed cells. Miyamoto et al. also showed that HeLa cells treated with H2O2 (200M, 30min) show increased importin-α in the nucleus [48]. This group showed that introducing exogenous recombinant importin-α, but not importin-β, in stressed cells partially reduced the nuclear import defect of NLS-GFP. They also found a breakdown of the Ran gradient across the nucleus. The results of this study suggest that impaired nuclear export of importin -α leads to its retention in the nucleus. In addition to using H2O2 as an oxidative stressor, a few studies have also utilized DEM to induce oxidative stress. DEM reduces glutathione levels, leading to an increase in ROS levels [55, 56]. HeLa cells treated with DEM exhibited a significant impairment in nuclear import of NLS-GFP and GR-GFP at DEM concentrations (2mM, 4hrs) that did not affect the structural integrity of the nuclear envelope [50]. Interestingly, no breakdown in the Ran gradient was observed at this concentration of DEM, however at higher doses (5mM) a gradient collapse was observed. The authors found that importin-α and CAS levels were increased in the nucleus along with Nup153, Nup88, and Nup50. An increase in large complex formation of importin-α/Nup153/ Nup88 in response to stress is suggested as a mechanism that leads to their increased localization in the nucleus. Depletion of cytoplasmic importin-α would reduce the efficacy of importin -dependent nuclear import.
3.2.2 Effects on nuclear export
Given the significance of the Ran gradient across the nucleus for the import/export cycle and the importance of nucleoporins, such as Nup153, in maintaining the integrity of the NPC, alterations in their levels or localizations may have significant consequences for nuclear export as well. Crampton et al. carried out a detailed study on the effects of DEM (2mM, 4hrs) on nuclear export in HeLa cells [52]. Using a NES-tagged substrate, the authors found that DEM caused its increased levels in the nucleus. Nup358, involved in Crm1-dependent nuclear export, was found to be reduced at the nuclear envelope whereas Crm1 and other nucleoporins involved in nuclear export (e.g. Nup214 and Nup62) were increased at the nuclear membrane. Oxidative stress also caused altered interactions between Crm1 and various nucleoporins and also between Ran and Crm1. Furthermore, the exit of Crm1 out of the nucleus was reduced in response to stress. In all, these results strongly suggest a significant alteration of nuclear export in DEM-stressed HeLa cells.
3.2.3 Possible mechanisms involved in nuclear transport defects
The specific mechanisms involved in the various alterations in levels and localization of nuclear transport factors still remain to be determined. A few studies have tried to address this issue. A key finding in some studies seems to be the collapse of the Ran-GTP gradient across the nucleus in response to oxidative stress, at least under high, acute toxicity paradigms. Work from Yasuda et al. have suggested that a decrease in the cellular ATP levels in response to H2O2-stress in HeLa cells causes a collapse of the Ran gradient [49]. ATP-depleting agents were able to reproduce this effect and introduction of exogenous ATP in stressed cells was able to restore the Ran gradient. Furthermore, as suggested by Pierce and coworkers in their study of aortic smooth muscle cells stressed with H2O2, a recent study by the Stochaj group examined the signaling events that impact classical nuclear import [51]. Utilizing DEM as their model of oxidative injury, they found that inhibition of MEK/ERK1/2 signaling (PD98059) reduced the impairment in nuclear import. Furthermore, they also found that stress-induced changes in the level and localization of some transport factors, importin-α and various nucleoporins, were sensitive to alterations in MEK and/or PI3K signaling cascades. However, their earlier paper suggested that MEK signaling is not involved in the inhibition of nuclear import seen with H2O2 [47].
Hence, the role of these signaling cascades in explaining the nuclear import defects in other model systems is unclear.
Alterations in post-translational modifications of various nuclear transport factors have also been reported [51, 52]. In response to DEM-induced oxidative stress, an increased phos-phorylation of importin-α, CAS, Nup88, and Nup153 as well as increased O-glycosylation of Nup153 was observed [51]. Furthermore, similar stress caused increased phosphorylation of Nup98, Nup62, and Nup214 as well as increased O-glycosylation of Nup62 and Nup214 [52]. The consequences of such modifications are unknown; however it is possible that they can affect protein stability and/or interactions with key proteins - leading to their altered levels, localization or function.
4. Neurodegeneration and nuclear transport
4.1 Oxidative stress and altered gene expression in neurodegeneration
Neurodegenerative disorders, such as Parkinson's disease (PD), Alzheimer's disease (AD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) are associated with specific alterations in gene expression in affected neurons [57-59]. A common change among the various disorders includes an up-regulation of genes involved in cellular stress responses, such as oxidative stress, which may reflect compensatory changes that nevertheless fail to rescue the neurons in the long run. Analysis of post -mortem brains of various neurodegenerative disorders shows a significant amount of oxidative damage. There are increases in markers of lipid oxidation, such as malondialdehyde and 4-hydroxy-2-nonenal, compared to age-matched controls [60-65]. In addition, there is an increase in oxidative damage of proteins, such as carbonyl modifications as well as cross-linking and fragmentation [62-67]. Oxidative damage to DNA is also observed in degenerating brains as evidenced by an increase in 8-oxodeoxyguanine levels in nuclear and mitochondrial DNA compared to age-matched controls [62-65, 68-70].
Evidence of the primary role of oxidative stress in the pathogenesis of many neurodegenerative diseases also comes from genetic and environmental models [63-65]. For example, in the case of PD, mutations or deficiency of proteins such as α-synuclein, Parkin, PINK1, DJ-1, and LRRK2, have been shown to increase susceptibility of cells to oxidative stress-mediated cell death [71-73]. Parkinsonian toxins such as, 6-hydroxydopamine (6OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, and paraquat, are extensively utilized models of PD involving mitochondrial dysfunction, generation of ROS and neuronal cell death [74].
4.2 Altered subcellular localization of proteins in neurodegeneration
As previously mentioned, a key mechanism in the regulation of many transcription factors is their subcellular distribution. Activated transcription factors undergo nuclear translocation where they can regulate gene expression. Impairments in the subcellular distribution of signaling proteins, including transcription factors, are documented in neurodegenerative diseases [75]. Examples of proteins that show such alterations in their subcellular localization include Nuclear factor-kappa B (NF-kB), ERK1/2, activating transcription factor 2 (ATF2), TAR DNA binding protein 43 (TDP-43), p53, sma/mothers against decapentaplegic (Smad), E2 promoter binding factor 1 (E2F1), Nrf2, and cyclic AMP response element-binding protein (CREB) [75]. Specifically, analysis of post-mortem brains show increased cytoplasmic ATF2 levels in AD [76], cytoplasmic aggregates of pCREB and lack of nuclear pCREB in PD [77], reduced nuclear localization of Nrf2 in AD [78], and increased cytoplasmic:nuclear ratios of TDP-43 in fronto-temporal lobar degeneration (FTLD) and ALS [79]. Other and more detailed examples of protein mislocalization observed in human neurodegenerative disease tissues were previously reviewed [75].
There are likely to be numerous mechanisms that could explain the altered subcellular distributions of proteins in degenerating neurons. A key mechanism for which there is increasing evidence is impairment in nucleocytoplasmic transport. The role of NPCs and nuclear transport has been investigated in a number of human diseases, such as neoplasms, viral infections, primary biliary cirrhosis, and triple A syndrome [80, 81]. Recently, their role in the pathogenesis of neurodegenerative disorders has begun to be elucidated. Aging, an independent risk factor for many neurodegenerative disorders, has also been associated with impairments in nuclear transport [82-85]. What follows below is a discussion of the current evidence in the literature that suggests an alteration in nuclear transport as a potential common mechanism of neuronal degeneration in neurodegenerative disorders.
4.2.1 Parkinson's Disease (PD)
Toxin models have been instrumental in understanding the pathologic mechanisms that are involved in neuronal degeneration seen in PD. 6 -hydroxydopamine (6OHDA) is an analog of catecholamine neurotransmitters that is widely used to model PD-relevant oxidative stress. 6OHDA is taken up into cells by dopamine and norepi-nephrine reuptake transporters where it demonstrates an early autoxidation phase of ROS production and also elicits a delayed phase of mitochondrial ROS production [86]. Although used as an exogenous neurotoxin, 6OHDA can be formed from dopamine oxidation in vivo, with elevated body fluid levels in patients treated with L-Dopa [87, 88].
Altered subcellular localization of pCREB and pERK1/2 has been observed in dopaminergic neurons of the substantia nigra in PD brains [77, 89]. Previous work from our lab has shown that 6OHDA also causes abnormal cytoplasmic accumulations of pERK1/2 in cultured cells [90], accompanied by decreased nuclear levels of GFP-ERK2 [91]. As discussed above, MEK/ ERK1/2 signaling may contribute to oxidative impairment of nuclear import in non-neuronal models. As ERK1/2 activation contributes to cell death in the 6OHDA as well as MPP+ models [93, 94], the potential effects of ERK1/2 on nuclear trafficking remain to be determined in these contexts.
There are also decreases in the nuclear to cytoplasmic ratios of pCREB in response to 6OHDA-induced oxidative stress in SH-SY5Y cells, a human neuroblastoma-derived dopaminergic cell line, and in primary midbrain neurons [77]. CREB-regulated gene transcription, which is important for axon growth and neuronal survival, was also repressed in 6OHDA treated cells. Impaired gene transcription in response to 6OHDA-stress was reversed by cAMP treatment, which bypasses the requirement for nuclear import [92]. This suggests that impairment in nucleocytoplasmic trafficking of CREB could result in the impaired gene expression that is observed in 6OHDA-stressed cells.
4.2.2 Alzheimer's Disease (AD)
Early ultrastructural studies comparing normal and AD brains revealed significant nuclear changes in both tangle-bearing and nearby neurons lacking paired helical filaments. These include irregularities in the nuclear membrane and aggregation of nuclear pores [95, 96]. These studies suggest that the interface between the cytoplasm and the nucleus is altered in AD. A recent study by Sheffield et al. further examined in detail the effects on NPC in neurons with and without tangles in control and AD cases [97]. In both CA1 and CA4 hippocampal neurons of AD patients, there was a significant increase in nuclear irregularity compared to control as assessed by nucleoporin p62 staining. Furthermore, although not statistically significant, the irregularity was more frequently observed in tangle-bearing neurons. These changes were not associated with markers of apoptosis (active caspase-3 and TUNEL), suggesting that this effect is not a result of the apoptotic process. Interestingly, the authors also found that NTF2, a key transport factor for nucleocytoplasmic trafficking, accumulated in the cytoplasm of some hippocampal neurons (regardless of the presence of tangles) in AD cases but not in control cases. This finding suggests a possible impairment in nucleocytoplasmic trafficking in affected neurons of AD.
Zhu and colleagues examined the localization of importin-α1 in AD cases and found that it localized to Hirano bodies, which are rod-shaped inclusions that contain actin and actin-associated proteins, and not in neurofibrilary tangles or amyloid-β plaques in hippocampal CA1 neurons, compared to the diffuse cytoplasmic localization of importin-α1 in age-matched controls [98]. Interestingly, the authors did not find importin-α1 localization in Lewy bodies of PD or Pick bodies in Pick disease, suggesting some specificity of this effect to AD. Importin-α is a key player in nucleocytoplasmic transport and the mislocalization of this protein, which is also observed upon oxidative injury as previously discussed, can have significant consequences for cell survival. The Jordan-Sciutto lab examined Nrf2 nuclear localization in AD versus PD cases, finding that hippocampal neurons in AD show cytoplasmic Nrf2 accumulation with loss of nuclear staining, which was not seen in the hippocampus or midbrain of PD cases [78].
4.2.3 Amyotrophic Lateral Sclerosis (ALS)
Recently, there has been a substantial amount of evidence that suggests alterations in nuclear transport as one of the key mechanisms involved in neuronal degeneration in ALS. Kusaka and coworkers have carried out a couple of detailed studies examining nuclear membrane integrity and localization of nuclear transport factors in ALS [99, 100]. In a common transgenic mouse model of ALS (G93A mutation in superoxide dismutase 1 (SOD1)), the authors observed decreased immunoreactivity for importin-α and importin-β in the nucleus and an increase in the cytoplasm for a subset of anterior horn cells compared to control mice [100]. Strong reactivity was also observed for Lewy body-like hyaline inclusions (LBHIs). Histone H1, which exhibits importin-β-dependent nuclear transport, also showed increased cytoplasmic levels in affected neurons. It was observed that with disease progression, importin-β levels began to accumulate in the cytoplasm, while decreasing in the nucleus of the anterior horn cells. Importantly, these findings were not associated with the apoptotic process as assessed by caspase-3 staining. These results suggest alterations in nuclear transport in affected neurons.
The localization of beta-catenin, which exhibits importin-β-independent nuclear transport, was also examined in this study [100]. The results showed increased levels in the cytoplasm and decreased levels in the nucleus in affected anterior horn cells compared to control. Hence, in addition to effects on importins, it is possible that abnormal function of NPCs also account for the mislocalization of other proteins observed in degenerating neurons. Kusaka and coworkers examined NPCs in degenerating neurons of ALS [99], analyzing nuclear integrity by staining for nucleoporin p62. In contrast to control cells, which showed smooth nuclear contours, anterior horn cells of the transgenic mice showed irregular, tortuous nuclear contours with ruffled edges. With disease progression, the percentage of cells showing irregular nuclear contours increased. This effect was seen even in pre-symptomatic stages and was not associated with apoptosis. Furthermore, analysis of both familial (fALS) and sporadic ALS (sALS) patient cases showed irregular nuclear contours as well. The affected neurons in sALS also lacked importin-β immunoreactivity in the nucleus, which is consistent with the results seen in the mouse model of ALS [100]. These data from mice model of ALS as well as post-mortem brain show significant alterations in the nuclear transport machinery and hence strongly suggests impaired nuclear transport as an important mechanism in anterior horn cell degeneration.
Mutations in the fused in sarcoma (FUS) gene are associated with familial ALS. FUS undergoes nucleocytoplasmic shuttling and regulates functions such as transcription and mRNA splicing [101]. Interestingly, some of the fALS-associated mutations in FUS are localized to the c-terminal NLS [102, 103]. Recent studies have shown that these mutations are sufficient to impair the nuclear localization of FUS and therefore explain its increased levels in the cytoplasm where it is recruited into stress granules, which are thought to play a key role in disease pathogenesis [102, 103]. The degree of impairment in nuclear import was also found to correlate with the age of disease onset [102]. These studies provide evidence of a specific protein whose impaired nuclear transport has significant consequences for cell survival and disease pathogenesis.
4.2.4 Huntington's (HD) and other Polyglutamine Diseases
The Huntingtin (Htt) protein has been shown to be important for functions such as transcription and intracellular transport [104]. Since one of the hallmarks of HD, as well as other polyglutamine diseases, is increased levels of the polyglutamine-expanded proteins in the nucleus, alterations in transcription is thought to be a key factor in the toxicity of these mutant proteins. How levels of mutant Htt increase in the nucleus are still unknown although a number of studies have suggested possible mechanisms [105]. One such possibility includes reduction in the nuclear export of Htt. Li and coworkers showed that polyglutamine expansion of N-terminal Htt fragments reduces its interaction with a nuclear pore protein, Tpr, which is involved in its nuclear export [106]. Polyglutamine expansion of other proteins, such as ataxin-3 and ataxin-7 (which are linked to spinocerebellar ataxias type 3 and 7, respectively), have been shown to reduce their rate of nuclear export, hence explaining in part their increased nuclear localizations [107, 108]. Furthermore, live-cell imaging studies examining dynamics of polyglutamine-expanded proteins, including various ataxins and Htt, show that these proteins have reduced dynamics - suggesting that they may also have reduced rates of nucleocytoplasmic transport [107, 108].
The Htt protein contains many HEAT repeat domains, which are also found in importin-β and are essential for binding to FG-repeat nucleoporins [109, 110]. Hence, it has been suggested that Htt may also function as a nuclear transport factor. Recent work form Kennedy and coworkers has shown that Htt promotes the synaptic-to-nuclear transport of NF-kB and that polyglutamine expansion of Htt impairs this function [111]. Whether Htt truly functions like importin-β by carrying other NLS-containing cargo from the synapse to the nucleus is yet to be determined. In addition, cell culture and transgenic mice models of polyglutamine-expanded Htt show distortions in the nuclear envelope as well as an increase in the clustering of nuclear pores [112, 113]. Deformed nuclear envelopes have also been observed in a cellular model of spinocerebellar ataxia type 1 and 3 [114, 115]. Although these structural changes are interesting, whether or not they cause functional consequences for nuclear transport is not known.
In Dentatorubral-pallidoluysian atrophy (DRPLA), which results from polyglutamine-expansion of atrophin-1, abnormal phosphorylation of the nuclear membrane has been noted in dentate nucleus neurons, as determined by increased staining with anti-phosphoserine antibody [116]. Analysis of affected cerebellar granule cells showed nuclear membrane indentations amongst other changes in nuclear morphology [117]. Given that alterations in the nuclear membrane morphology are seen in many of the polyglutamine diseases, it is possible that altered NPC function and nuclear transport may be a common mechanism of neuronal degeneration resulting from the toxicity of polyglutamine-expanded proteins.
4.2.5 Other neurodegenerative disorders
There are examples of altered nuclear transport in other neurodegenerative diseases as well. In Friedreich's ataxia (FRDA), an impaired nuclear translocation of Nrf2 in response to oxidative stimulus was seen in cultured fibroblasts from patients with FRDA compared to control fibroblasts [118]. Reduced expression of Nrf2-targeted genes, which are important for the antioxidant defense of cells, was also observed. An immortalized neuronal cell culture model of FRDA recapitulated these findings [118]. In FTLD as well as ALS, the cytoplasmic localization of TDP-43 is thought to play a key role in neuronal degeneration. Rogelj and coworkers first showed that knockdown of importin-β1 or CAS resulted in cytoplasmic accumulation of TDP-43 in various cell lines and primary neuronal cultures [119]. Interestingly, they found reduced levels of CAS and also importin-α2 in brains of patients with TDP-43-positive FTLD. These results suggest that perhaps impaired nuclear import may result in increased levels of TDP-43 in the cytoplasm of affected neurons in FTLD.
5. Concluding remarks
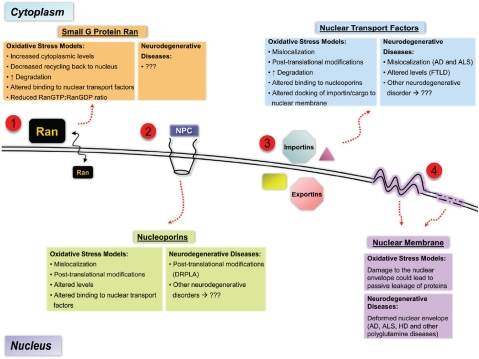
Examination of data from both human tissue and disease model studies relating to several neurodegenerative diseases shows a common theme implicating alterations in nucleocytoplasmic transport (Figure 2). Much of the evidence presented comes from static analysis of degenerating neurons. To elucidate the specific mechanisms involved in such impairments requires extensive study in model systems, and likely require kinetic studies as well as molecular manipulations. Oxidative stress has been shown to significantly alter nucleocytoplasmic transport in a variety of non-neuronal model systems (Figure 2), but it is unknown whether such mechanisms are also observed in neurons, which are quite susceptible to oxidative stress. Hence, examining nucleocytoplasmic transport in well-designed oxidative models of neuronal injury is a very exciting avenue for future studies in the field of neurodegeneration.
Figure 2.
Summary of the major findings of alterations in nuclear transport in models of oxidative stress and in neurodegenerative diseases. (1) Increased cytoplasmic distribution of Ran is a common finding in many of the studies of oxidative injury, however nothing is known about its distribution in affected neurons of neurodegenerative diseases. Other changes in Ran are also observed in oxidative stress models (see text). (2) Nucleoporins, which make up the NPC, also show changes in response to oxidative injury such as mislocalization, altered levels, and post-translational modifications. Some evidence of post-translational modification of nuclear envelope proteins exists in DRPLA, however nothing is known in other neurodegenerative diseases. (3) Nuclear transport factors, such as importins and exportins, show significant changes in their distributions, levels, and interactions in response to oxidative stress. Some evidence of the same is also found in various neurodegenerative diseases, such as AD, ALS, and FTLD. (4) Overt damage to the nuclear envelope is observed at high concentrations of oxidative stressors, which likely is an end -stage effect of cell death. However, interesting structural changes (e.g. irregular contours) in the nuclear envelope have been observed in many neurodegenerative diseases. The functional consequences of such changes are not known. NPC = nuclear pore complex, AD = Alzheimer's disease, ALS = Amyotrophic lateral sclerosis, HD = Huntington's disease, FTLD = Frontotemporal lobar degeneration, DRPLA = Dentatorubral-pallidoluysian atrophy.
In many of the cases, particularly acute toxicity models, the observed nucleocytoplasmic trafficking impairment appears to arise from global mechanisms that likely affect the transport of many different types of cargo. These include breakdown of the Ran gradient or bioenergetic depletion. Such indications may suggest targeting nuclear transport in general as a possible therapeutic strategy. There are, however, numerous contraindications to such an approach, at least in the context of neurodegenerative diseases. First, there are not many agents that can selectively target the NPCs and nuclear transport on a more general level [80]. Furthermore, altering the global nuclear transport process would have too many nonspecific effects given how common this process is for cellular function. It would most likely result in cellular toxicity. Therefore, such agents may be more suited for cancer therapeutics and for other disorders where there is abnormal cellular growth.
A better strategy for neurodegenerative disorders might be to target specific proteins whose mislocalization is associated with neuronal degeneration. For example, degenerating substantia nigra neurons in PD showed preserved Nrf2 nuclear localization [78] but decreased nuclear localization of factors involved in trophic signaling [77, 90], suggesting selective alterations in the nuclear transport of subsets of transcription factor. Targeting the activation and/or subcellular localization of specific pathways would minimize potential toxicity resulting from nonselective effects of modulating global nuclear transport. For diseases where key proteins are mislocalized, such as pCREB in PD, TDP-43 in ALS and FTLD, and Nrf2 in AD and FRDA, targeting their localization to the correct subcellular compartment could help delay or even reverse neuronal degeneration. For diseases where mutant proteins have a toxic gain of function, such as the polyglutamine-expanded proteins in the nucleus, perhaps preventing their accumulation in these compartments is the strategy of choice. Pharmacological agents for the above-mentioned strategies are limited, however, and therefore future studies are needed to focus on the development of agents that can specifically accomplish these goals.
Acknowledgments
This work is supported in part by R01 AG026389. VPP has also been supported in part by T32NS007433 and T32GM008208.
References
- 1.Bito H, Takemoto-Kimura S. Ca(2+)/CREB/ CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. doi: 10.1016/s0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- 2.Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16:319–330. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Sorokin AV, Kim ER, Ovchinnikov LP. Nu-cleocytoplasmic transport of proteins. Biochemistry (Mosc) 2007;72:1439–1457. doi: 10.1134/s0006297907130032. [DOI] [PubMed] [Google Scholar]
- 4.Weis K. Regulating access to the genome: nu-cleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 5.Otis KO, Thompson KR, Martin KC. Importin -mediated nuclear transport in neurons. Curr Opin Neurobiol. 2006;16:329–335. doi: 10.1016/j.conb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 7.Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagstaff KM, Jans DA. Importins and beyond: non-conventional nuclear transport mechanisms. Traffic. 2009;10:1188–1198. doi: 10.1111/j.1600-0854.2009.00937.x. [DOI] [PubMed] [Google Scholar]
- 11.Ambron RT, Schmied R, Huang CC, Smedman M. A signal sequence mediates the retrograde transport of proteins from the axon periphery to the cell body and then into the nucleus. J Neurosci. 1992;12:2813–2818. doi: 10.1523/JNEUROSCI.12-07-02813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmied R, Huang CC, Zhang XP, Ambron DA, Ambron RT. Endogenous axoplasmic proteins and proteins containing nuclear localization signal sequences use the retrograde ax-onal transport/nuclear import pathway in Aplysia neurons. J Neurosci. 1993;13:4064–4071. doi: 10.1523/JNEUROSCI.13-09-04064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 14.Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss JL, Fainzilber M. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- 17.Kumar JP, Wilkie GS, Tekotte H, Moses K, Davis I. Perturbing nuclear transport in Droso-phila eye imaginal discs causes specific cell adhesion and axon guidance defects. Dev Biol. 2001;240:315–325. doi: 10.1006/dbio.2001.0468. [DOI] [PubMed] [Google Scholar]
- 18.Thompson KR, Otis KO, Chen DY, Zhao Y, O'Dell TJ, Martin KC. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Harreman MT, Kline TM, Milford HG, Harben MB, Hodel AE, Corbett AH. Regulation of nuclear import by phosphorylation adjacent to nuclear localization signals. J Biol Chem. 2004;279:20613–20621. doi: 10.1074/jbc.M401720200. [DOI] [PubMed] [Google Scholar]
- 21.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 22.Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004;259:208–217. discussion 218-225. [PubMed] [Google Scholar]
- 23.Spilianakis C, Papamatheakis J, Kretsovali A. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol Cell Biol. 2000;20:8489–8498. doi: 10.1128/mcb.20.22.8489-8498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zipper LM, Mulcahy RT. The Keap1 BTB/ POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 28.Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AN. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- 29.Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]
- 30.Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 31.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JM, Crack PJ. Impact of oxidative stress on neuronal survival. Clin Exp Pharmacol Physiol. 2004;31:397–406. doi: 10.1111/j.1440-1681.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 33.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 34.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 35.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 36.Chinta SJ, Andersen JK. Redox imbalance in Parkinson's disease. Biochim Biophys Acta. 2008;1780:1362–1367. doi: 10.1016/j.bbagen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 38.Cooper AJL. Glutathione in the brain: disorders of glutathione metabolism. In: Rosenberg RN, Prusiner SB, DiMauro S, Barchi RL, Kunk LM, editors. The Molecular and Genetic Basis of Neurological Disease. Boston: Butter-worth-Heinemann; 1997. pp. 1195–1230. [Google Scholar]
- 39.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 40.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82-83:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- 41.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- 42.Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez L, Kodiha M, Stochaj U. Monitoring the disruption of nuclear envelopes in inter-phase cells with GFP-beta-galactosidase. J Biomol Tech. 2005;16:235–238. [PMC free article] [PubMed] [Google Scholar]
- 45.Stochaj U, Rassadi R, Chiu J. Stress-mediated inhibition of the classical nuclear protein import pathway and nuclear accumulation of the small GTPase Gsp1p. FASEB J. 2000;14:2130–2132. doi: 10.1096/fj.99-0751fje. [DOI] [PubMed] [Google Scholar]
- 46.Czubryt MP, Austria JA, Pierce GN. Hydrogen peroxide inhibition of nuclear protein import is mediated by the mitogen-activated protein kinase, ERK2. J Cell Biol. 2000;148:7–16. doi: 10.1083/jcb.148.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodiha M, Chu A, Matusiewicz N, Stochaj U. Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ. 2004;11:862–874. doi: 10.1038/sj.cdd.4401432. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto Y, Saiwaki T, Yamashita J, Yasuda Y, Kotera I, Shibata S, Shigeta M, Hiraoka Y, Haraguchi T, Yoneda Y. Cellular stresses induce the nuclear accumulation of importin alpha and cause a conventional nuclear import block. J Cell Biol. 2004;165:617–623. doi: 10.1083/jcb.200312008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasuda Y, Miyamoto Y, Saiwaki T, Yoneda Y. Mechanism of the stress-induced collapse of the Ran distribution. Exp Cell Res. 2006;312:512–520. doi: 10.1016/j.yexcr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Kodiha M, Tran D, Qian C, Morogan A, Presley JF, Brown CM, Stochaj U. Oxidative stress mislocalizes and retains transport factor importin-alpha and nucleoporins Nup153 and Nup88 in nuclei where they generate high molecular mass complexes. Biochim Biophys Acta. 2008;1783:405–418. doi: 10.1016/j.bbamcr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Kodiha M, Tran D, Morogan A, Qian C, Stochaj U. Dissecting the signaling events that impact classical nuclear import and target nuclear transport factors. PLoS One. 2009;4:e8420. doi: 10.1371/journal.pone.0008420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crampton N, Kodiha M, Shrivastava S, Umar R, Stochaj U. Oxidative stress inhibits nuclear protein export by multiple mechanisms that target FG nucleoporins and Crm1. Mol Biol Cell. 2009;20:5106–5116. doi: 10.1091/mbc.E09-05-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adam SA, Sterne-Marr R, Gerace L. In vitro nuclear protein import using permeabilized mammalian cells. Methods Cell Biol. 1991;35:469–482. doi: 10.1016/s0091-679x(08)60584-1. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Xiao N, DeFranco DB. Use of digitonin-permeabilized cells in studies of steroid receptor subnuclear trafficking. Methods. 1999;19:403–409. doi: 10.1006/meth.1999.0876. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz E, Siow RC, Bartlett SR, Jenner AM, Sato H, Bannai S, Mann GE. Vitamin C inhibits di-ethylmaleate-induced L-cystine transport in human vascular smooth muscle cells. Free Radic Biol Med. 2003;34:103–110. doi: 10.1016/s0891-5849(02)01192-9. [DOI] [PubMed] [Google Scholar]
- 56.Molteni SN, Fassio A, Ciriolo MR, Filomeni G, Pasqualetto E, Fagioli C, Sitia R. Glutathione limits Ero1-dependent oxidation in the endoplasmic reticulum. J Biol Chem. 2004;279:32667–32673. doi: 10.1074/jbc.M404992200. [DOI] [PubMed] [Google Scholar]
- 57.Courtney E, Kornfeld S, Janitz K, Janitz M. Transcriptome profiling in neurodegenerative disease. J Neurosci Methods. 2010;193:189–202. doi: 10.1016/j.jneumeth.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Malaspina A, Kaushik N, de Belleroche J. Differential expression of 14 genes in amyotro-phic lateral sclerosis spinal cord detected using gridded cDNA arrays. J Neurochem. 2001;77:132–145. doi: 10.1046/j.1471-4159.2001.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka F, Niwa J, Ishigaki S, Katsuno M, Waza M, Yamamoto M, Doyu M, Sobue G. Gene expression profiling toward understanding of ALS pathogenesis. Ann N Y Acad Sci. 2006;1086:1–10. doi: 10.1196/annals.1377.011. [DOI] [PubMed] [Google Scholar]
- 60.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 61.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 64.Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 65.Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 66.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 67.Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 68.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 69.Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K. Oxidative damage in nucleic acids and Parkinson's disease. J Neurosci Res. 2007;85:919–934. doi: 10.1002/jnr.21191. [DOI] [PubMed] [Google Scholar]
- 70.Shimura-Miura H, Hattori N, Kang D, Miyako K, Nakabeppu Y, Mizuno Y. Increased 8-oxo-dGTPase in the mitochondria of substantia nigral neurons in Parkinson's disease. Ann Neurol. 1999;46:920–924. [PubMed] [Google Scholar]
- 71.Tan EK, Skipper LM. Pathogenic mutations in Parkinson disease. Hum Mutat. 2007;28:641–653. doi: 10.1002/humu.20507. [DOI] [PubMed] [Google Scholar]
- 72.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liou AK, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–124. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic Biol Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chu CT, Plowey ED, Wang Y, Patel V, Jordan-Sciutto KL. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol. 2007;66:873–883. doi: 10.1097/nen.0b013e318156a3d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamada T, Yoshiyama Y, Kawaguchi N. Expression of activating transcription factor-2 (ATF-2), one of the cyclic AMP response element (CRE) binding proteins, in Alzheimer disease and non-neurological brain tissues. Brain Res. 1997;749:329–334. doi: 10.1016/S0006-8993(96)01356-X. [DOI] [PubMed] [Google Scholar]
- 77.Chalovich EM, Zhu JH, Caltagarone J, Bowser R, Chu CT. Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J Biol Chem. 2006;281:17870–17881. doi: 10.1074/jbc.M602632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 80.Chahine MN, Pierce GN. Therapeutic targeting of nuclear protein import in pathological cell conditions. Pharmacol Rev. 2009;61:358–372. doi: 10.1124/pr.108.000620. [DOI] [PubMed] [Google Scholar]
- 81.Cronshaw JM, Matunis MJ. The nuclear pore complex: disease associations and functional correlations. Trends Endocrinol Metab. 2004;15:34–39. doi: 10.1016/j.tem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Pujol G, Soderqvist H, Radu A. Age-associated reduction of nuclear protein import in human fibroblasts. Biochem Biophys Res Commun. 2002;294:354–358. doi: 10.1016/S0006-291X(02)00492-8. [DOI] [PubMed] [Google Scholar]
- 83.Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 84.D'Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hetzer MW. The role of the nuclear pore complex in aging of post-mitotic cells. Aging (Albany NY) 2010;2:74–75. doi: 10.18632/aging.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andrew R, Watson DG, Best SA, Midgley JM, Wenlong H, Petty RK. The determination of hydroxydopamines and other trace amines in the urine of parkinsonian patients and normal controls. Neurochem Res. 1993;18:1175–1177. doi: 10.1007/BF00978370. [DOI] [PubMed] [Google Scholar]
- 88.Linert W, Jameson GN. Redox reactions of neurotransmitters possibly involved in the progression of Parkinson's Disease. J Inorg Biochem. 2000;79:319–326. doi: 10.1016/s0162-0134(99)00238-x. [DOI] [PubMed] [Google Scholar]
- 89.Ferrer I, Blanco R, Carmona M, Puig B, Barrachina M, Gomez C, Ambrosio S. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/ JNK), and p38 kinase expression in Parkinson's disease and Dementia with Lewy bodies. J Neural Transm. 2001;108:1383–1396. doi: 10.1007/s007020100015. [DOI] [PubMed] [Google Scholar]
- 90.Zhu JH, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am J Pathol. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson's disease. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevenson AS, Cartin L, Wellman TL, Dick MH, Nelson MT, Lounsbury KM. Membrane depolarization mediates phosphorylation and nuclear translocation of CREB in vascular smooth muscle cells. Exp Cell Res. 2001;263:118–130. doi: 10.1006/excr.2000.5107. [DOI] [PubMed] [Google Scholar]
- 93.Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Metuzals J, Robitaille Y, Houghton S, Gauthier S, Leblanc R. Paired helical filaments and the cytoplasmic-nuclear interface in Alzheimer's disease. J Neurocytol. 1988;17:827–833. doi: 10.1007/BF01216709. [DOI] [PubMed] [Google Scholar]
- 96.Mirra SS, Wood JG, Pollock NJ, Bakay RA, Binder LI, Ellisman MH. New observations on the fine structure of Alzheimer's disease. Journal of Neuropathology & Experimental Neurology. 1987;46:377. [Google Scholar]
- 97.Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS. Nuclear pore complex proteins in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:45–54. doi: 10.1097/01.jnen.0000195939.40410.08. [DOI] [PubMed] [Google Scholar]
- 98.Lee HG, Ueda M, Miyamoto Y, Yoneda Y, Perry G, Smith MA, Zhu X. Aberrant localization of importin alpha1 in hippocampal neurons in Alzheimer disease. Brain Res. 2006;1124:1–4. doi: 10.1016/j.brainres.2006.09.084. [DOI] [PubMed] [Google Scholar]
- 99.Kinoshita Y, Ito H, Hirano A, Fujita K, Wate R, Nakamura M, Kaneko S, Nakano S, Kusaka H. Nuclear contour irregularity and abnormal transporter protein distribution in anterior horn cells in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2009;68:1184–1192. doi: 10.1097/NEN.0b013e3181bc3bec. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Ito H, Wate R, Ohnishi S, Nakano S, Kusaka H. Altered distributions of nucleo-cytoplasmic transport-related proteins in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol. 2006;112:673–680. doi: 10.1007/s00401-006-0130-4. [DOI] [PubMed] [Google Scholar]
- 101.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, Neumann M, Haass C. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann Neurol. 2011;69:152–162. doi: 10.1002/ana.22246. [DOI] [PubMed] [Google Scholar]
- 104.Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C, Krishna G, Davies JE, Ttofi E, Underwood BR, Rubinsztein DC. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- 105.Truant R, Atwal RS, Burtnik A. Nucleocytoplasmic trafficking and transcription effects of huntingtin in Huntington's disease. Prog Neurobiol. 2007;83:211–227. doi: 10.1016/j.pneurobio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Cornett J, Cao F, Wang CE, Ross CA, Bates GP, Li SH, Li XJ. Polyglutamine expansion of huntingtin impairs its nuclear export. Nat Genet. 2005;37:198–204. doi: 10.1038/ng1503. [DOI] [PubMed] [Google Scholar]
- 107.Taylor J, Grote SK, Xia J, Vandelft M, Graczyk J, Ellerby LM, La Spada AR, Truant R. Ataxin-7 can export from the nucleus via a conserved exportin-dependent signal. J Biol Chem. 2006;281:2730–2739. doi: 10.1074/jbc.M506751200. [DOI] [PubMed] [Google Scholar]
- 108.Chai Y, Shao J, Miller VM, Williams A, Paulson HL. Live-cell imaging reveals divergent intracellular dynamics of polyglutamine disease proteins and supports a sequestration model of pathogenesis. Proc Natl Acad Sci U S A. 2002;99:9310–9315. doi: 10.1073/pnas.152101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J Biol Chem. 2002;277:50597–50606. doi: 10.1074/jbc.M209037200. [DOI] [PubMed] [Google Scholar]
- 110.Takano H, Gusella JF. The predominantly HEAT-like motif structure of huntingtin and its association and coincident nuclear entry with dorsal, an NF-kB/Rel/dorsal family transcription factor. BMC Neurosci. 2002;3:15. doi: 10.1186/1471-2202-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marcora E, Kennedy MB. The Huntington's disease mutation impairs Huntingtin's role in the transport of NF-kappaB from the synapse to the nucleus. Hum Mol Genet. 2010;19:4373–4384. doi: 10.1093/hmg/ddq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chapple JP, Bros-Facer V, Butler R, Gallo JM. Focal distortion of the nuclear envelope by huntingtin aggregates revealed by lamin immunostaining. Neurosci Lett. 2008;447:172–174. doi: 10.1016/j.neulet.2008.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 114.Rich T, Varadaraj A. Ataxin-1 fusion partners alter polyQ lethality and aggregation. PLoS One. 2007;2:e1014. doi: 10.1371/journal.pone.0001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evert BO, Wullner U, Schulz JB, Weller M, Groscurth P, Trottier Y, Brice A, Klockgether T. High level expression of expanded full-length ataxin-3 in vitro causes cell death and formation of intranuclear inclusions in neuronal cells. Hum Mol Genet. 1999;8:1169–1176. doi: 10.1093/hmg/8.7.1169. [DOI] [PubMed] [Google Scholar]
- 116.Yazawa I. Aberrant phosphorylation of dentatorubral-pallidoluysian atrophy (DRPLA) protein complex in brain tissue. Biochem J. 2000;351(Pt 3):587–593. [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi H, Egawa S, Piao YS, Hayashi S, Yamada M, Shimohata T, Oyanagi K, Tsuji S. Neuronal nuclear alterations in dentatoru-bral-pallidoluysian atrophy: ultrastructural and morphometric studies of the cerebellar granule cells. Brain Res. 2001;919:12–19. doi: 10.1016/s0006-8993(01)02986-9. [DOI] [PubMed] [Google Scholar]
- 118.Paupe V, Dassa EP, Goncalves S, Auchere F, Lonn M, Holmgren A, Rustin P. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS One. 2009;4:e4253. doi: 10.1371/journal.pone.0004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nishimura AL, Zupunski V, Troakes C, Kathe C, Fratta P, Howell M, Gallo JM, Hortobagyi T, Shaw CE, Rogelj B. Nuclear import impairment causes cytoplasmic trans-activation response DNA-binding protein accumulation and is associated with frontotemporal lobar degeneration. Brain. 2010;133:1763–1771. doi: 10.1093/brain/awq111. [DOI] [PubMed] [Google Scholar]
- 120.Gorlich D. Transport into and out of the cell nucleus. EMBO J. 1998;17:2721–2727. doi: 10.1093/emboj/17.10.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]