Abstract
Aims
Withdrawal symptoms have been linked to a propensity for relapse to drug abuse. Inasmuch as this association applies to methamphetamine (MA) abuse, an understanding of the course of MA withdrawal symptoms may help to direct treatment for MA dependence. Previous studies of symptoms manifested during abstinence from MA have been limited in size and scope. We asked (i) whether debilitating psychological and/or physical symptoms appear during the first several weeks of MA abstinence, (ii) how craving for MA evolves and (iii) whether psychiatric symptoms (e.g. depression, psychosis) persist beyond a month of abstinence.
Design
A study of MA-dependent participants, who initiated and maintained abstinence from the drug for up to 5 weeks, compared to a matched healthy comparison group.
Setting
In-patient research hospital ward (MA-dependent subjects) and out-patient (comparison subjects).
Participants
Fifty-six MA-dependent and eighty-nine comparison subjects.
Measurements
Rater-assessed MA withdrawal questionnaire and self-report assessment of craving (MA-dependent subjects) and self-report assessment of psychiatric symptoms (both groups).
Findings
At study entry, MA-dependent subjects exhibited a wide range in severity of depressive symptoms, with the average score at a mild–moderate level of severity. Symptoms of psychosis were also prevalent. While depressive and psychotic symptoms largely resolved within a week of abstinence, craving did not decrease significantly from the time of initiating abstinence until the second week, and then continued at a reduced level to the fifth week.
Conclusions
Depressive and psychotic symptoms accompany acute withdrawal from methamphetamine but resolve within 1 week. Craving is also present and lasts at least 5 weeks.
Keywords: Craving, depression, methamphetamine, psychosis, stimulant, withdrawal
INTRODUCTION
Methamphetamine (MA) abuse is a substantial global problem, with as many as 50 million users world-wide [1,2]. The US National Survey on Drug Use and Health demonstrated that the prevalence of use was approximately 0.5% nation-wide, with use being more endemic in the western US [3]. MA addiction produces serious medical complications, and imposes costly social burdens [1,4]. In addition, MA dependence is resistant to treatment, with psychosocial interventions providing help to only some affected individuals [5,6], and no medications proven to be effective in treating this disorder [7,8].
Physiological dependence, associated withdrawal-related symptoms and craving are thought to reinforce continued drug-taking, including MA self-administration [9–11]. An understanding of MA withdrawal may therefore inform the development of strategies for relapse prevention. Amphetamine withdrawal is well represented in the clinical literature, including the development of validated rating scales, which have contributed to defining the symptom cluster recognized in the DSM IV-TR [12–14]. The DSM IV-TR identifies dysphoric mood as the main symptom for amphetamine (including MA) withdrawal, and requires at least two of the following additional symptoms for a positive diagnosis: fatigue, insomnia or hypersomnia, increased appetite, psychomotor agitation or retardation and vivid, unpleasant dreams [15,16].
MA differs from its parent compound, amphetamine, by N-methylation, which increases its lipid solubility and permeability of the blood–brain barrier [17,18]. These physical properties lead potentially to increased potency and toxicity [17,18]. Two studies of MA withdrawal have been conducted [19,20] with small sample sizes. They focused primarily upon depressive symptoms, craving and changes in appetite and sleep. In one of these studies, MA addicts (n = 21), who were seeking treatment, were assessed over 3 weeks, using a DSM-III-derived amphetamine withdrawal questionnaire [19]. A withdrawal syndrome, with an acute phase of 7–10 days followed by a subacute phase of up to 3 weeks, was described. The acute phase featured hyperphagia, MA craving and depression-related symptoms, during which symptom severity declined from a high initial peak that generally occurred ~24 hours after stopping MA use. In the subacute phase, symptoms were mild and stable for another 2 weeks. In the other withdrawal study, the MA-dependent participants (n = 19) were not seeking treatment [20]. It found that depressive symptoms, which were generally mild, varied considerably in intensity and duration, and resolved within the first 2 weeks of abstinence.
While these studies have provided intriguing preliminary information regarding the symptoms of MA withdrawal, more extensive studies were warranted. Until now the association between psychotic symptoms and MA withdrawal and abstinence has not been well characterized. Although psychotic symptoms are not a diagnostic feature of MA withdrawal, they are often prominent during early abstinence [21].
Here we describe the time–course of psychological and physical symptoms reported by in-patient, abstinent MA-dependent research volunteers (MA subjects; n = 56) during a period of up to 5 weeks of continuous, monitored abstinence. We also compared this sample of MA participants to a matched sample of healthy control (HC) participants, using self-report ratings of dysphoria/depressive and psychotic symptoms. The aims of this study were: (i) to determine whether MA withdrawal symptoms result typically in debilitating psychological and physical symptoms; (ii) to evaluate craving for MA; and (iii) to assess psychiatric symptoms, such as depression and psychosis, in the transition from active MA abuse to abstinence of up to 5 weeks.
METHODS
Participants
Fifty-six MA and 89 HC participants took part in this study after receiving a detailed explanation and giving written informed consent, as approved by the UCLA Institutional Review Board. Participants were recruited by means of print, radio and internet advertisements. Participants in both groups were excluded if they had major medical illness, including human immunodeficiency virus (HIV) seropositivity, as determined by medical history, physical examination and laboratory assay. The MA participants were not seeking treatment, and were not dependent upon other substances (besides nicotine). However, they met criteria for MA dependence, and were active users of MA (urine toxicology screen positive for MA) at study entry. HC participants had no history of substance use disorders (besides nicotine dependence) and had negative urine tests throughout the study. Light alcohol use (equivalent to 7.5 drinks per week) was not an exclusion criterion. Participants were excluded in both groups if they met criteria for other current Axis I diagnoses based on the Structured Clinical Interview for DSM-IV disorders (SCID-IV), or any history of recent psychotropic medication use. HC participants were studied on an out-patient basis. All participants were allowed to smoke tobacco ad libitum to avoid the possible confounding effects of nicotine withdrawal. All participants were compensated for their time in the study.
In-patient milieu
MA participants resided at the UCLA General Clinical Research Center (GCRC) for the duration of the studies, and were monitored via daily urine and alcohol breath testing to confirm abstinence. They were housed individually, with care taken to minimize contact with any other participants enrolled concurrently. MA- or drug-related cues, personal effects and media (e.g. movies) were proscripted during the course of the study. Adjunctive medications without remarkable psychopharmacological activity were provided for physical symptoms (i.e. acetaminophen for pain and aluminum/magnesium hydroxide for dyspepsia), but medications were not provided for other complaints (e.g. insomnia, anxiety, fatigue). MA participants were studied in a series of neuroimaging studies that lasted either 2 or 5 weeks, and involved magnetic resonance imaging and positron emission tomography scanning, the results of which have been published previously [22–29]. All questionnaires were given at the same time each day (~9:00 a.m.). Most MA participants identified smoking as the primary route of MA administration.
Methamphetamine Withdrawal Questionnaire (MAQW)
We used the MAWQ, adapted and expanded for MA-dependent populations from the Amphetamine Withdrawal Questionnaire [13] by a member of our team (R.R.). This instrument differed from the parent instrument by substantially expanding the range of possible MA withdrawal-associated symptoms: these included many symptoms such as joint pain, red/itchy eyes, headache and anger, which have been seen anecdotally in clinical populations seeking treatment for MA dependence (Table 2). The MAWQ is a 30-item, rater-scored instrument using a four-point Likert-type scale that reviews drug withdrawal-related symptom clusters, separated into functional, emotional, physical and additional symptoms, along with a review of vital signs (see Table 2). The length of abstinence for MA participants was based upon self-reported last use of MA, which was corroborated with urine toxicology screening information at study entry, to control for varying lengths of pre-study abstinence among participants. All MA participants reported MA use within the last 7 days prior to admission to the hospital ward. Individuals with discrepant self-reported last use and urine toxicology test results were excluded from the analysis.
Table 2.
Effect of duration of abstinence from methamphetamine (MA) on Methamphetamine Withdrawal Questionnaire (MAWQ) subscales (n = 32).
| F | Effect of time (days 1–14) |
Study entry |
|
|---|---|---|---|
| P | Mean (SD) | ||
| Vital signs | |||
| Systolic blood pressure | 0.63 | 0.68 | 118.8 (16.8) |
| Diastolic blood pressure | 0.7 | 0.62 | 69.2 (12.2) |
| Pulse | 0.77 | 0.57 | 70.2 (10.7) |
| Physical symptoms | n (%) | ||
| Headaches | 0.36 | 0.98 | 8 (25) |
| Constipation | 0.46 | 0.96 | 2 (6) |
| Diarrhea | 1.31 | 0.2 | 4 (13) |
| Irregular or pounding heartbeat | 0.98 | 0.47 | 4 (13) |
| Muscle or joint pain | 1.24 | 0.25 | 9 (28) |
| Red/itching eyes | 4.33 | <0.001 | 10 (31) |
| Sweaty or clammy | 1.35 | 0.18 | 5 (16) |
| Emotional symptoms | |||
| Angry | 0.36 | 0.98 | 4 (13) |
| Anxious/nervous | 1.18 | 0.29 | 11 (34) |
| Craving | 0.67 | 0.79 | 22 (68) |
| Depressed | 1.22 | 0.26 | 8 (25) |
| Irritable | 0.8 | 0.66 | 6 (19) |
| No motivation | 2.8 | 0.001 | 12 (38) |
| Loss of interest or pleasure | 1.69 | 0.06 | 8 (25) |
| Restless | 0.54 | 0.90 | 13 (40) |
| Suicidal thoughts | 1.47 | 0.12 | 2 (6) |
| Functional symptoms | |||
| Increased appetite | 3.23 | <0.001 | 20 (63) |
| Decreased appetite | 0.51 | 0.92 | 4 (13) |
| Poor concentration | 1.02 | 0.43 | 6 (19) |
| Poor memory | 1.23 | 0.25 | 9 (28) |
| Sleep difficulties | 3.16 | <0.001 | 7 (22) |
| Tired or low energy | 5.36 | <0.001 | 18 (56) |
| Additional symptoms | |||
| ‘I am not feeling well’ | 1.22 | 0.26 | 6 (19) |
| ‘Sometimes it feels as though people are watching me or talking about me’ | 2.19 | 0.009 | 3 (9) |
| ‘Sometimes it feels like someone is touching me but no one is there’ | 0.83 | 0.63 | 1 (3) |
| ‘I often think about taking methamphetamine’ | 2.04 | 0.02 | 23 (72) |
| ‘My sexual pleasure is less than usual’ | 2.22 | 0.008 | 8 (25) |
| ‘Recently my interest in sex is less than usual’ | 1.26 | 0.24 | 7 (22) |
| ‘I am not able to deal with stress as well as usual’ | 1.39 | 0.16 | 7 (22) |
| ‘Sometimes I hear voices that no one else can hear’ | 0.87 | 0.58 | 1 (3) |
Effect of time of abstinence was analyzed by mixed-effects modeling over days 1–14 of abstinence from MA. The table presents the exact text of the instrument, which was rater-administered to MA-abusing participants. The instrument is scored on a four-point Likert-type scale from ‘none’ to ‘severe’ (physical, emotional, functional symptoms) or ‘none’ to ‘strongly agree’ (additional symptoms). P values reaching statistical significance (P < 0.05) are highlighted. SD: standard deviation.
Other procedures
All participants were assessed at study entry and weekly thereafter with the Brief Symptom Inventory (BSI [30]) and Beck Depression Inventory (BDI [31]). MA participants were also assessed at study entry and weekly with the Visual Analogue Scales (VAS [32]) for MA, alcohol, opioids and cocaine craving. Urine toxicology testing (Instant-View Urine Test kit, Alfa Scientific, Poway, CA, USA) was performed at intake and all subsequent procedure days for all participants, and daily while in-patients for the MA participants. The detection window for recent MA exposure for this kit is typically 48–72 hours (manufacturer’s information).
Data analysis
Data for repeated-measures instruments (MAWQ, BSI, BDI, VAS) were analyzed using linear mixed effects models; group comparisons used analyses of covariance (ANCOVAs) with post hoc Bonferroni correction, including demographic and behavioral variables which differed between study groups as covariates to adjust for potential bias. The association between craving for MA and BDI scores in MA subjects was determined using Pearson’s correlation.
RESULTS
Demographics
The groups did not differ significantly in age (P = 0.15) or gender (P = 0.16), but MA subjects had fewer years of education (P = 0.04) and lower maternal education than HC participants (P = 0.001; Table 1). Overall, there was a significant difference in ethnicity between groups (P = 0.001; Table 1), including: the HC group included a larger proportion of black and Asian individuals, while the MA group had a higher prevalence of Hispanic participants. More of the MA than the HC participants smoked cigarettes (P < 0.001; Table 1). While 56 MA participants provided data for at least one of the instruments used, n < 56 for individual analyses, demographic profiles of these subsets of the total number of MA participants did not differ significantly from the group as a whole.
Table 1.
Demographics of study participants.
| HC | MA | ||||
|---|---|---|---|---|---|
| No. of subjects | n = 89 | n = 56 | |||
| F | df | P | |||
| Age (years) | 31.7 (8.3) | 33.8 (8.2) | 2.4 | 111 | 0.15 |
| Years education | 14.8 (2.1) | 12.6 (1.3) | 4.4 | 107 | 0.04 |
| Years maternal education | 14.4 (2.7) | 12.7 (2.7) | 12.1 | 105 | 0.001 |
| χ2 | df | P | |||
| % Married | 20.2 | 5.4 | 8.0 | 3 | 0.05 |
| % Unemployed | 25.8 | 80.3 | 46.1 | 2 | <0.001 |
| Gender | |||||
| % Male | 50.6 | 62.5 | 2.0 | 1 | 0.16 |
| Ethnicity | 20.0 | 5 | 0.001 | ||
| % White | 66.3 | 53.6 | |||
| % Black | 12.4 | 1.8 | |||
| % Hispanic | 5.6 | 26.8 | |||
| % Asian | 10.1 | 3.6 | |||
| % Native American | 0.0 | 1.8 | |||
| % Other | 5.6 | 12.5 | |||
| % Parole/probation | 0.0 | 21.4 | 23.3 | 2 | <0.001 |
| Substance use | |||||
| % Smokers | 44.9 | 91.1 | 13.2 | 1 | <0.001 |
| Average years of MA use | NA | 11.1 (7.2) | |||
| Average days of use/30 days | NA | 20.4 (8.6) | |||
Continuous variables are listed as mean of each group with adjacent standard deviation in parentheses. Demographic variables were analyzed by two-way analysis of variance comparisons (continuous variables) or Pearson’s χ2 comparisons (categorical variables) to assess for statistical significances of differences in demographic profile information between study groups, as listed in Table 1. P values reaching statistical significance (<0.05) are shown in bold type. HC: healthy controls; MA: methamphetamine; NA: not applicable.
Methamphetamine withdrawal symptoms
In general, the MA withdrawal symptoms experienced by the participants were mild and well tolerated (Table 2). These included: mild paranoid ideation (‘… people are talking about me’), red/itchy eyes, sleep difficulties, lack of motivation, lack of energy, decreased sexual pleasure and increased appetite. Up to 70% of participants reported craving or ‘thinking about’ MA at study entry, which was the most frequent reported symptom, whereas only two of 32 participants reported any suicidal thoughts (Table 2). Any substantial changes which were observed during the course of the study occurred within the first 14 days of abstinence from MA.
Depressive symptoms
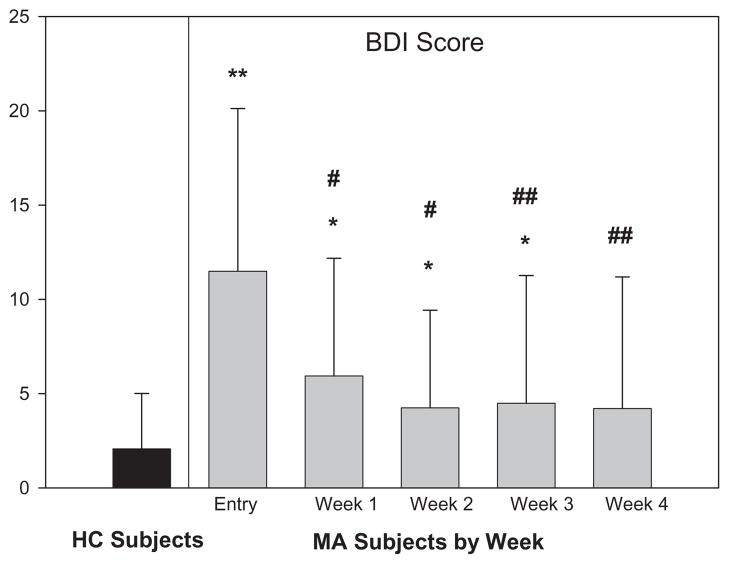
BDI scores in the HC group trended lower with time, but did not vary significantly (data not shown). At entry to the study, MA participants had a wide range of BDI scores, but the average was ~12, in the category of ‘mild–moderate’ depressive symptoms based upon experience with treatment-seeking psychiatric out-patients [31]. Depressive symptoms decreased substantially over the first 2 weeks of abstinence to stable low levels (P < 0.001 for effect of time; Fig. 1). During the first 3 weeks of abstinence, MA participants still had a significantly higher level of overall depressive symptoms than HC participants, even after controlling for demographic and behavioral variables that differ between study groups (P < 0.05; Fig. 1). MA participants’ BDI scores did not approach those of the HC participants until the end of 4 weeks of abstinence (P = 0.07; Fig. 1).
Figure 1.
Effect of duration of abstinence from methamphetamine (MA) on Beck Depression Inventory (BDI) score (n = 28), compared to healthy control (HC) subject sample (n = 89). Data represent mean BDI score at each time-point; error bars represent standard deviation of each value. The range of possible scores on the BDI is 0–69. *P < 0.05 for difference of MA versus HC sample; **P < 0.001 for difference of MA versus HC sample. One-way repeated-measures analysis of variance comparisons of BDI scores among MA subjects (within-group assessments) were conducted with post-hoc Bonferroni correction. #P < 0.05 for the difference between data at a later time-point compared with data at study entry. ##P < 0.001 for the difference between data at a later time-point compared with data at study entry
Craving for MA
MA participants at study entry exhibited elevated levels of craving for MA, which continued throughout the first week of abstinence (Fig. 2). Craving for MA dropped substantially with abstinence >2 weeks (P < 0.01 versus study entry), but still continued at a reduced level throughout 5 weeks of abstinence (Fig. 2).
Figure 2.
Effect of time in abstinence from methamphetamine (MA) on Visual Analogue Scale (VAS) craving for MA (n = 26). Data represent mean VAS scores at each time-point; error bars represent standard deviations of each value. ##P < 0.01 for each time-point versus study entry
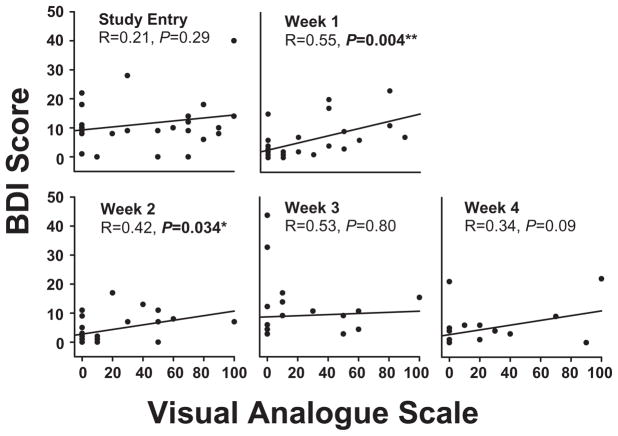
Craving for MA and depressive symptoms
Despite elevated levels of both depressive symptoms and craving for MA, there was no significant correlation between these two variables (P = 0.29; Fig. 3) in MA participants at study entry. However, by the end of the first week of abstinence (P = 0.004) to the end of the second week of abstinence (P = 0.03), a significant correlation between BDI and MA craving was established (Fig. 3). There were no correlations at later time-points of abstinence, such that by the end of weeks 3 (P = 0.8) and 4 (P = 0.09) no significant correlation between BDI score and MA craving was present (Fig. 3).
Figure 3.
Correlation of Beck Depression Inventory (BDI) and Visual Analogue Scale (VAS) during abstinence from methamphetamine (MA) (n = 26). The relationship between depressive symptoms (as measured by BDI) and levels of craving for MA (as measured by VAS) was investigated using Pearson’s product–moment correlation coefficient. Correlations between the two measures are presented in each figure. As demonstrated, BDI and VAS are significantly correlated days 7–14 of abstinence from MA. Study entry: days 0–1 of abstinence. Week 1: days 6–8 of abstinence; week 2: days 13–15 of abstinence; week 3: days 20–22 of abstinence; week 4: days 27–29 of abstinence
Psychiatric symptoms
BSI scores among HC participants did not change significantly over time. At study entry MA participants showed elevated levels of most psychopathology indices (Fig. 4), except for phobias (Fig. 4H). These elevated scores decreased significantly during the first week of abstinence, and reached a low baseline by the end of the second week (P < 0.004; Fig. 4). By the end of the second week of abstinence, all the BSI subscale scores for the MA participants had decreased to the level of the HC participants (Fig. 4), with the exception of the ‘additional symptoms’ subscale (Fig. 4E). The HC participants did not show significant changes over time on the BSI. The effect of duration of abstinence for MA subjects was significant (P < 0.001) when analyzed by linear mixed-effect modeling. A mixed between–within-subjects analysis of variance was conducted between MA subjects at each time-point and the HC subjects, assessing the impact of ethnicity, subject educational level, maternal educational level and cigarette smoking status differences among study participants, with post-hoc Bonferroni correction. The MA subjects scored higher than HC subjects at most time-points (Fig. 4).
Figure 4.
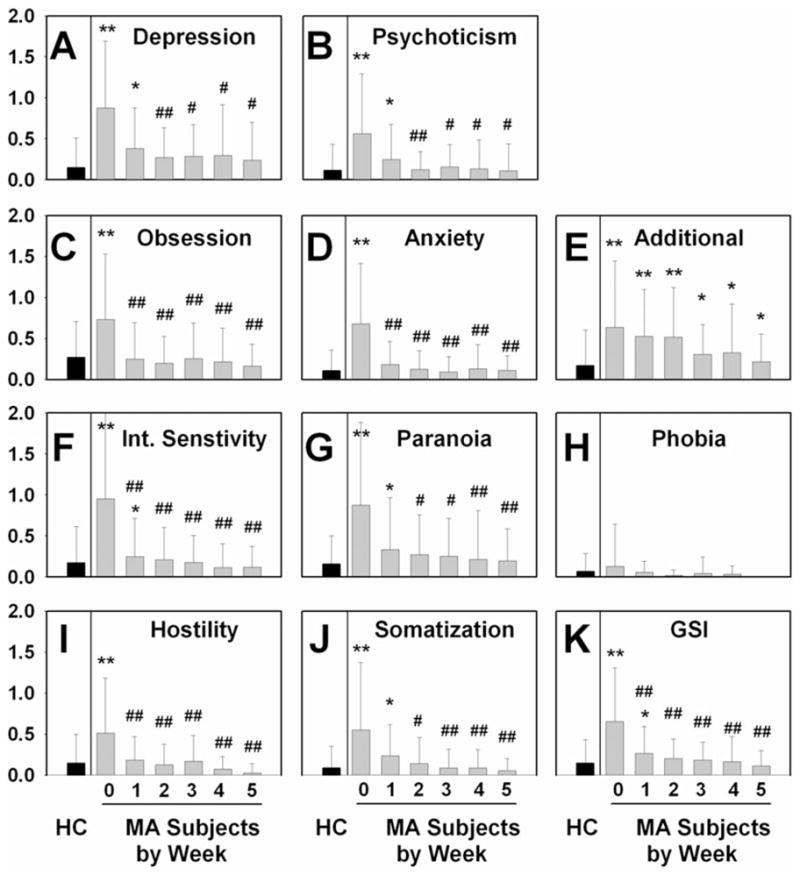
Effect of duration of abstinence from methamphetamine (MA) on Brief Symptom Inventory (BSI) scores (n = 25), compared to healthy control (HC) subject sample (n = 85). Data represent mean BSI subscale score at each time-point (0: study entry; 1–5: represents data collected at end of each week of hospitalization indicated); error bars represent standard deviation of each value. *P < 0.05 MA versus HC sample at time-point: **P < 0.001 MA versus HC sample at time-point. #P < 0.05 MA subjects: time-point versus study entry, ##P < 0.01 MA subjects: time-point versus study entry. (A) Depression subscale: effect of duration of abstinence, P = 0.001. (B) Psychoticism subscale: effect of duration of abstinence, P = 0.003. (C) Obsession subscale: effect of duration of abstinence, P < 0.001. (D) Anxiety subscale: effect of duration of abstinence, P < 0.001. (E) Additional symptoms subscale: effect of duration in abstinence, P = 0.131. (F) Interpersonal sensitivity subscale: effect of duration of abstinence, P < 0.001. (G) Paranoia subscale: effect of duration of abstinence, P = 0.003. (H) Phobia subscale: effect of duration of abstinence, P = 0.506. (I) Hostility subscale: effect of duration of abstinence, P < 0.001. (J) Somatization subscale: effect of duration of abstinence, P < 0.001. (K) Global symptoms index (GSI) subscale: effect of duration of abstinence, P < 0.001
DISCUSSION
Methamphetamine withdrawal symptoms
In this study, MA withdrawal symptoms (aside from craving for MA) were, on average, mild and resolved within 14 days of abstinence. In a previous study, MA withdrawal symptoms (increased appetite, craving for MA and depressive symptoms) decreased linearly from a peak in the first day of abstinence over 7–10 days [19]. The onset, appearance and subsequent decrease of ‘increased appetite’ observed in that study directly match the pattern that was also reported for the craving for MA [19] and is similar to the findings presented here. However, our survey of depressive symptoms based upon the MAWQ did not reveal a significant decrease over 1–2 weeks of abstinence.
Whereas the severity of MA withdrawal symptoms has not been linked previously to a propensity to relapse, a relationship between withdrawal severity and relapse has been well established for opiates, cocaine, nicotine and alcohol [33–36]. Therefore, the severity of MA withdrawal symptomatology (which varies considerably among MA-dependent individuals [20]) is likely to influence the ability of MA-dependent individuals to maintain abstinence (discussed in [8]).
Depressive symptoms in MA withdrawal and abstinence
Our findings on the time–course of BDI symptoms in MA abstinence replicated a prior report of acute (7–10 days) versus subacute (second and third weeks) depressive symptoms being a central hallmark of the MA withdrawal syndrome [19]. Our results also corroborate and expand upon previous research findings in 19 MA-dependent participants who were not seeking treatment [20]. That study also found a similar reduction in depressive symptoms as measured by BDI with continued abstinence up to 2 weeks [20]. Therefore, on average, MA-dependent individuals may be expected to have a reduction in depressive symptoms within 2 weeks of con-firmed abstinence.
Treatment-seeking MA users at greatest risk for developing major depressive disorder had a high BDI score at study entry and/or a past history of suicide attempts [37]. In addition, depressive symptoms after cessation of stimulant abuse have been associated with reduced retention in treatment [38]. In the context of previous reports from the literature, our findings indicated that some individuals continue to experience clinically notable depressive symptoms after 2 or more weeks of abstinence from MA, and may benefit from treatment for these symptoms.
Psychopathology and psychotic symptoms in MA withdrawal and abstinence
At study entry, BSI indices for the MA participants were similar to those of general adult out-patient treatment-seeking psychiatric patient populations [30]. Our finding that levels of psychopathology subsequently decreased substantially with a week or more of abstinence from MA complement data published recently showing a large reduction in BSI indices of psychopathology when measured over 3 weeks of abstinence from MA [39].
MA-induced psychotic symptoms are common among MA-dependent individuals who are not seeking treatment, with up to 60–80% reporting a life-time history of any psychotic symptoms [21]. In a study of 71 active MA-dependent participants who had experienced a recent episode of MA-associated psychosis, pathological levels of hostility were found to accompany psychotic symptoms in 27% of the most severe psychotic episodes, demonstrating further the association of hostility and psychotic symptoms [40]. MA-abusing individuals with more severe psychotic symptoms were found to require increased health service utilization and present with increased psychiatric burden [41]. Taken together, our results provide further evidence of the psychiatric burden that MA dependence imposes upon afflicted individuals, and also serves to inform clinicians that MA-abusing individuals are likely to have high levels of ongoing psychological distress and hostility that may influence adversely their interactions with treatment providers.
Craving for MA in withdrawal and abstinence
Our findings indicate that MA-dependent individuals may have difficulty maintaining abstinence for longer than a week due to continued craving for MA, which persists through several weeks of abstinence, and continues at a lower level throughout at least 5 weeks of abstinence. In this regard, a prospective study showed that levels of craving for MA in out-patient treatment-seeking participants predicted the likelihood to relapse on MA, with a craving score >20 out of 100 on the VAS leading to a doubling of the relative risk of relapse [42]. Therefore, continuous craving for MA in abstinence probably contributes to the high rate of relapse observed across treatment studies [7].
Our data on the correlation of depressive symptoms and craving for MA, when taken together with a review of the relevant literature, provide strong evidence that the most vulnerable time for relapse to MA is likely to be during days 7–14 of abstinence (Fig. 3 [38]). This assertion would be particularly valid for those subjects with significant depressive symptoms during this period of abstinence, as they are also likely to be experiencing high levels of craving for MA.
Limitations of current study
While the sample size for this study was larger than those of previously published studies of MA withdrawal [19,20], it is still relatively small, and limited to individuals who were not seeking treatment. Our findings therefore may not be generalizable to treatment seeking MA-dependent subjects, whose severity and/or time–course of withdrawal and abstinence-associated physical and psychiatric symptoms may be more severe (e.g. [43]). In addition, the fact that MA participants were studied as in-patients while the HC participants were studied as out-patients may confound interpretation of the results. Another limitation is that the current study did not extend beyond 5 weeks of continuous abstinence.
Finally, our results showed some discrepancies in psychiatric ratings on the investigator-scored MAWQ compared with self-report scales, with the MAWQ indicating lower symptom intensities than self-report ratings. This difference may reflect reluctance of the participants to provide accurate information when interacting with a rater than when responding more anonymously by written self-report. Similar discrepancies, suggesting greater reliability of self-report ratings than rater-administered scales, have also been found in clinical psychiatric populations [44]. We suggest that the rater-scored MAWQ may have greater utility in a treatment-seeking subject population, where the participant has already acknowledged distress by coming for treatment.
Implications for future research on treatments for MA dependence
Our sample of MA-dependent individuals who were not seeking treatment experienced moderate levels of depressive symptoms, psychotic symptoms and other psycho-pathological indices during the transition from active use to abstinence. This high level of psychiatric comorbidity seen in very early abstinence is likely to be a common experience among MA abusers, given the prevalence of a binge-type pattern of MA abuse [45], with the potential for repeated bouts of withdrawal. These symptoms decreased greatly within several weeks of continuous abstinence, and continued at low levels throughout 5 weeks of abstinence. Our data indicate further that MA abusers may have difficulty maintaining abstinence once initiated due to the presence of continued craving for MA throughout at least 5 weeks of abstinence. Therefore, we suggest that future research on treatments for MA abuse should target cravings experienced by some MA abusers in abstinence, which may improve the ability of treatment-seeking MA abusers to maintain sobriety and remain in treatment.
Acknowledgments
This study was supported by NIH grants P20 DA022539, RO1 DA020726, RO1 DA15179 (EDL) and MO1 RR 00865 (UCLA GCRC).
Footnotes
Declarations of interest
None.
References
- 1.Buxton JA, Dove NA. The burden and management of crystal meth use. Can Med Assoc J. 2008;178:1537–9. doi: 10.1503/cmaj.071234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Office on Drugs and Crime. World Drug Report. New York, NY: United Nations Publications; 2009. [Google Scholar]
- 3.United States Substance Abuse and Mental Health Administration. National Survey on Drug Use and Health. 2008 Available at: http://oas.samhsa.gov/nsduh.htm (accessed 2 April 2010). Archived at http://www.webcitation.org/5qVa2x4hE (15 June 2010)
- 4.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- 5.Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P. Treatment for amphetamine dependence and abuse. Cochrane Database Syst Rev. 2001;4:CD003022. doi: 10.1002/14651858.CD003022. [DOI] [PubMed] [Google Scholar]
- 6.Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:8–17. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 7.Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abuse. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoptaw SJ, Kao U, Heinzerling K, Ling W. Treatment for amphetamine withdrawal. Cochrane Database Syst Rev. 2009;2:CD003021. doi: 10.1002/14651858.CD003021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc Lond B. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Phil Trans R Soc Lond B. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton TF, De La Garza R, II, Kalechstein AD, Tziortzis D, Jacobsen CA. Theories of addiction: methamphetamine users’ explanations for continuing drug use and relapse. Am J Addict. 2009;18:294–300. doi: 10.1080/10550490902925920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossop MR, Bradley BP, Brewis RK. Amphetamine withdrawal and sleep disturbance. Drug Alcohol Depend. 1982;10:177–83. doi: 10.1016/0376-8716(82)90010-2. [DOI] [PubMed] [Google Scholar]
- 13.Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: I. Reliability, validity and factor structure of a measure. Aust NZ J Psychiatry. 1999;33:89–93. doi: 10.1046/j.1440-1614.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 14.McGregor C, Srisurapanont M, Mitchell A, Longo MC, Cahill S, White JM. Psychometric evaluation of the Amphetamine Cessation Symptom Assessment. J Subst Abuse Treat. 2008;34:443–9. doi: 10.1016/j.jsat.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.DSM-IV-T.R. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 16.Lago JA, Kosten TR. Stimulant withdrawal. Addiction. 1994;89:1477–81. doi: 10.1111/j.1360-0443.1994.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 17.Kish SJ. Pharmacologic mechanisms of crystal meth. Can Med Assoc J. 2008;178:1679–82. doi: 10.1503/cmaj.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–99. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 19.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time–course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–9. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 20.Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–55. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney JJ, III, Kalechstein AD, De La Garza R, II, Newton TF. Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependent participants. Am J Addict. 2008;17:83–98. doi: 10.1080/10550490701861201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 23.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci Nurs. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–8. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Voytek B, Berman SM, Hassid BD, Simon SL, Mandelkern MA, Brody AL, et al. Differences in regional brain metabolism associated with marijuana abuse in methamphetamine abusers. Synapse. 2005;57:113–5. doi: 10.1002/syn.20155. [DOI] [PubMed] [Google Scholar]
- 27.Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug Alcohol Depend. 2008;93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon S, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–44. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric out-patients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 33.Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–39. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- 34.Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- 35.Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MM, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2008;4:CD005031. doi: 10.1002/14651858.CD005031. [DOI] [PubMed] [Google Scholar]
- 36.Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Methamphetamine Treatment Project. Risk factors for suicide attempts in methamphetamine-dependent patients. Am J Addict. 2008;17:24–7. doi: 10.1080/10550490701756070. [DOI] [PubMed] [Google Scholar]
- 38.Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, et al. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict. 2008;17:218–23. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe C, Bush KR, Straits-Troster K, Meredith C, Romwall L, Rosenbaum G, et al. A comparison of methamphetamine-dependent inpatients childhood attention deficit hyperactivity disorder symptomatology. J Addict Dis. 2005;24:133–52. doi: 10.1300/J069v24n03_11. [DOI] [PubMed] [Google Scholar]
- 40.McKetin R, McLaren J, Lubman DI, Hides L. Hostility among methamphetamine users experiencing psychotic symptoms. Am J Addict. 2008;17:235–40. doi: 10.1080/10550490802019816. [DOI] [PubMed] [Google Scholar]
- 41.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Methamphetamine Treatment Project. Clinical course and outcomes of methamphetamine-dependent adults with psychosis. J Subst Abuse Treat. 2008;35:445–50. doi: 10.1016/j.jsat.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–76. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- 43.Rawson RA, Gonzales R, Marinelli-Casey P, Ang A. Methamphetamine dependence: a closer look at treatment response and clinical characteristics associated with route of administration in outpatient treatment. Am J Addict. 2007;16:291–9. doi: 10.1080/10550490701389864. [DOI] [PubMed] [Google Scholar]
- 44.Eichenberger A, Rössler W. Comparison of self-ratings and therapist ratings of outpatients’ psychosocial status. J Nerv Ment Dis. 2000;188:297–300. doi: 10.1097/00005053-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Halkitis PN, Shrem MT. Psychological differences between binge and chronic methamphetamine using gay and bisexual men. Addict Behav. 2006;31:549–52. doi: 10.1016/j.addbeh.2005.05.040. [DOI] [PubMed] [Google Scholar]