Abstract
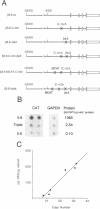
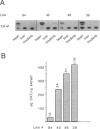
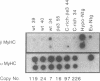
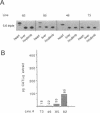
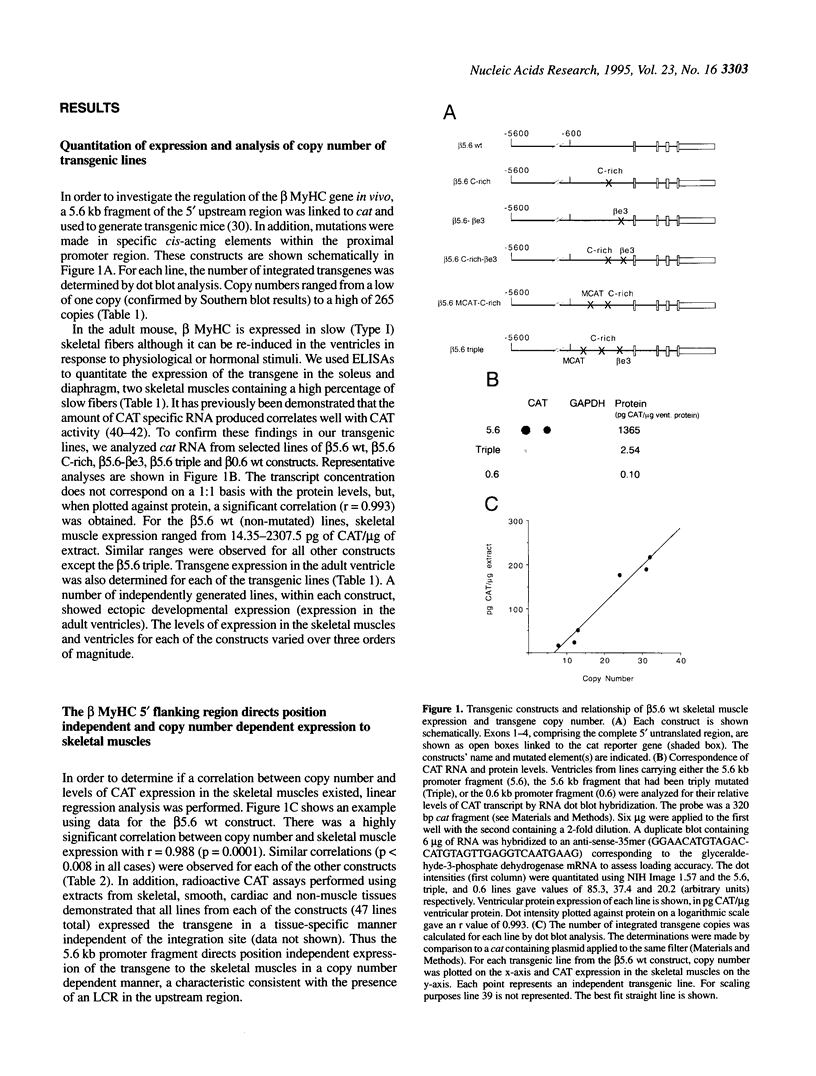
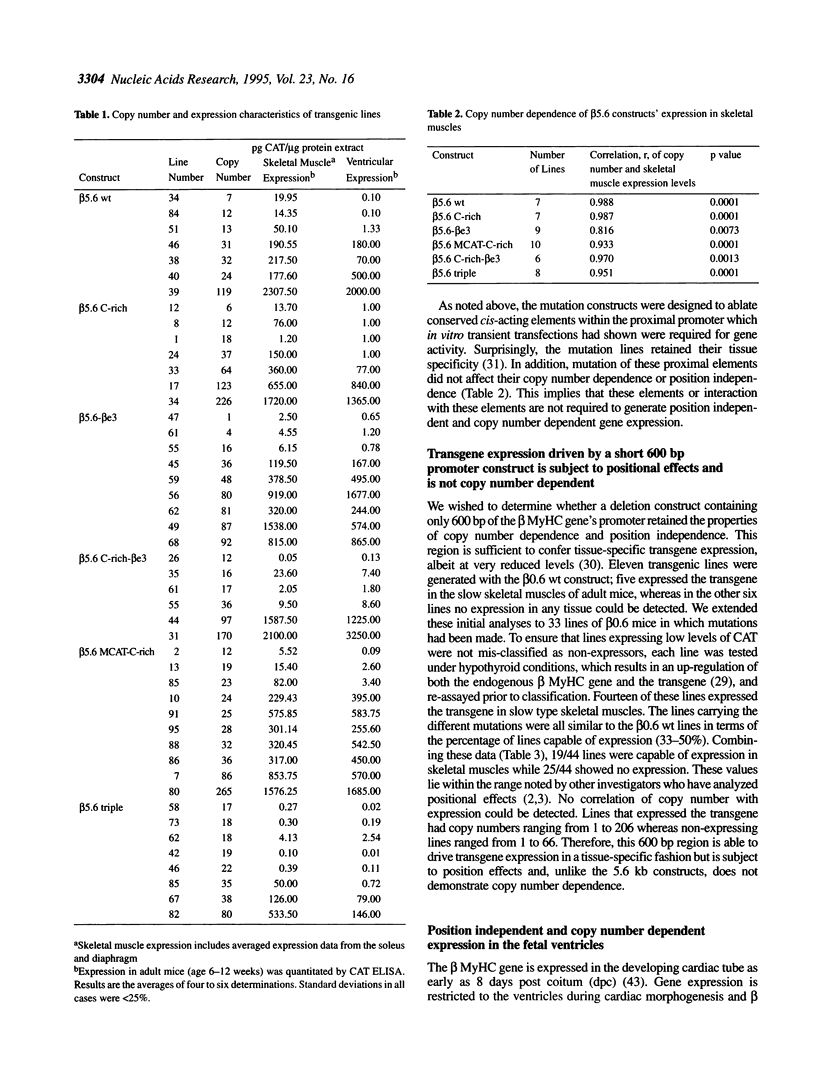
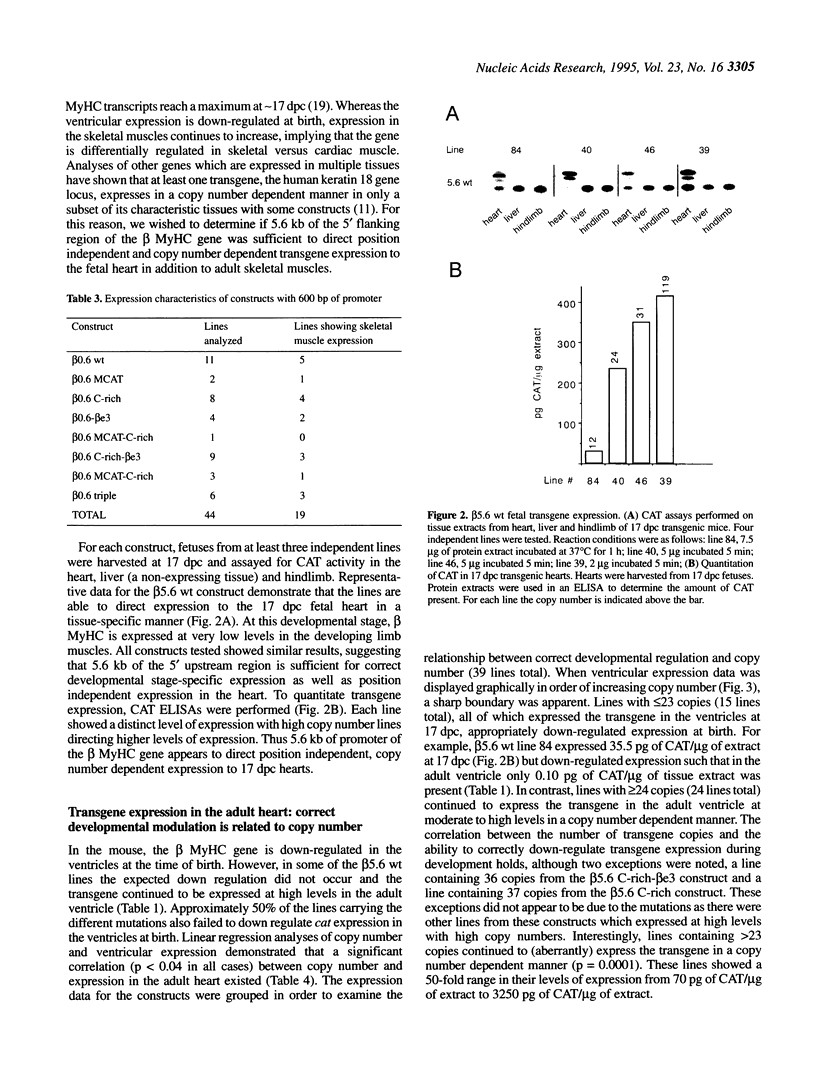
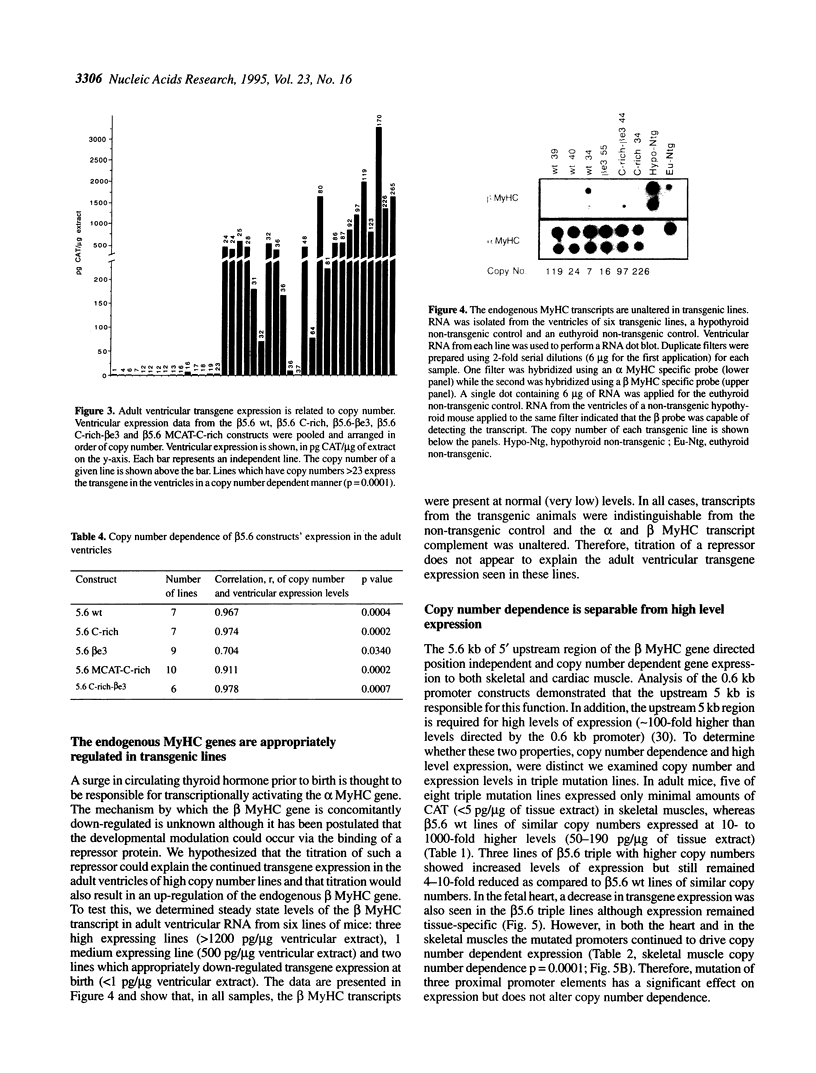
Transgenic mice generated with constructs containing 5.6 kb of the beta myosin heavy chain (MyHC) gene's 5' flanking region linked to the cat reporter gene express the transgene at high levels. In all 47 lines analyzed, tissue-specific accumulation of chloramphenicol acetyltransferase was found at levels proportional to the number of integrated transgene copies. Deletion constructs containing only 0.6 kb of 5' upstream region showed position effects in transgenic mice and did not demonstrate copy number dependence although transgene expression remained muscle-specific. The 5.6 kb 5' upstream region conferred appropriate developmental control of the transgene to the cardiac compartment and directs copy number dependent and position independent expression. Lines generated with a construct in which three proximal cis-acting elements were mutated showed reduced levels of transgene expression, but all maintained their position independence and copy number dependence, suggesting the presence of distinct regulatory mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adom J., Carr K. D., Gouilleux F., Marsaud V., Richard-Foy H. Chromatin structure of hormono-dependent promoters. J Steroid Biochem Mol Biol. 1991;40(1-3):325–332. doi: 10.1016/0960-0760(91)90198-e. [DOI] [PubMed] [Google Scholar]
- Allen N. D., Cran D. G., Barton S. C., Hettle S., Reik W., Surani M. A. Transgenes as probes for active chromosomal domains in mouse development. Nature. 1988 Jun 30;333(6176):852–855. doi: 10.1038/333852a0. [DOI] [PubMed] [Google Scholar]
- Aronow B. J., Silbiger R. N., Dusing M. R., Stock J. L., Yager K. L., Potter S. S., Hutton J. J., Wiginton D. A. Functional analysis of the human adenosine deaminase gene thymic regulatory region and its ability to generate position-independent transgene expression. Mol Cell Biol. 1992 Sep;12(9):4170–4185. doi: 10.1128/mcb.12.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Grimber G., Briand P., Nicolas J. F. Patterns of expression of position-dependent integrated transgenes in mouse embryo. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6331–6335. doi: 10.1073/pnas.87.16.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carson S., Wiles M. V. Far upstream regions of class II MHC Ea are necessary for position-independent, copy-dependent expression of Ea transgene. Nucleic Acids Res. 1993 May 11;21(9):2065–2072. doi: 10.1093/nar/21.9.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. W., Vasavada H. A., Ganguly S., Weissman S. M. Identification of cis sequences controlling efficient position-independent tissue-specific expression of human major histocompatibility complex class I genes in transgenic mice. Mol Cell Biol. 1991 Jul;11(7):3564–3572. doi: 10.1128/mcb.11.7.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R. A., Zak R. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem. 1984 Oct 25;259(20):12628–12632. [PubMed] [Google Scholar]
- Crossley M., Orkin S. H. Regulation of the beta-globin locus. Curr Opin Genet Dev. 1993 Apr;3(2):232–237. doi: 10.1016/0959-437x(93)90028-n. [DOI] [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Fedor M. J. Chromatin structure and gene expression. Curr Opin Cell Biol. 1992 Jun;4(3):436–443. doi: 10.1016/0955-0674(92)90009-2. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Edwards J. G., Bahl J. J., Liew C. C., Sole M., Morkin E. Characterization of a strong positive cis-acting element of the human beta-myosin heavy chain gene in fetal rat heart cells. J Biol Chem. 1992 May 15;267(14):9917–9924. [PubMed] [Google Scholar]
- Flink I. L., Morkin E. Interaction of thyroid hormone receptors with strong and weak cis-acting elements in the human alpha-myosin heavy chain gene promoter. J Biol Chem. 1990 Jul 5;265(19):11233–11237. [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Gulick J., Subramaniam A., Neumann J., Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991 May 15;266(14):9180–9185. [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Bahl J. J., Morkin E. Thyroid hormone regulates expression of a transfected alpha-myosin heavy-chain fusion gene in fetal heart cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3122–3126. doi: 10.1073/pnas.84.10.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Knotts S., Rindt H., Neumann J., Robbins J. In vivo regulation of the mouse beta myosin heavy chain gene. J Biol Chem. 1994 Dec 9;269(49):31275–31282. [PubMed] [Google Scholar]
- Li Q., Stamatoyannopoulos J. A. Position independence and proper developmental control of gamma-globin gene expression require both a 5' locus control region and a downstream sequence element. Mol Cell Biol. 1994 Sep;14(9):6087–6096. doi: 10.1128/mcb.14.9.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Knight J. D., Jackson S. P., Tjian R., Botchan M. R. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell. 1991 May 3;65(3):493–505. doi: 10.1016/0092-8674(91)90467-d. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Lyons G. E., Schiaffino S., Sassoon D., Barton P., Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990 Dec;111(6 Pt 1):2427–2436. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I. A., Courey A. J., Wall J. S., Jackson S. P., Hough P. V. DNA looping and Sp1 multimer links: a mechanism for transcriptional synergism and enhancement. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5670–5674. doi: 10.1073/pnas.88.13.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano C. A., Allen L. F., Rockman H. A., Dolber P. C., McMinn T. R., Chien K. R., Johnson T. D., Bond R. A., Lefkowitz R. J. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994 Apr 22;264(5158):582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- Milano C. A., Dolber P. C., Rockman H. A., Bond R. A., Venable M. E., Allen L. F., Lefkowitz R. J. Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkin E. Regulation of myosin heavy chain genes in the heart. Circulation. 1993 May;87(5):1451–1460. doi: 10.1161/01.cir.87.5.1451. [DOI] [PubMed] [Google Scholar]
- Neznanov N., Thorey I. S., Ceceña G., Oshima R. G. Transcriptional insulation of the human keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Apr;13(4):2214–2223. doi: 10.1128/mcb.13.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. A., Grupp I. L., Subramaniam A., Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res. 1991 Jun;68(6):1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud G., Roux J., Pictet R., Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991 Nov 29;67(5):977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- Rindt H., Gulick J., Knotts S., Neumann J., Robbins J. In vivo analysis of the murine beta-myosin heavy chain gene promoter. J Biol Chem. 1993 Mar 5;268(7):5332–5338. [PubMed] [Google Scholar]
- Rindt H., Knotts S., Robbins J. Segregation of cardiac and skeletal muscle-specific regulatory elements of the beta-myosin heavy chain gene. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1540–1544. doi: 10.1073/pnas.92.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Gulick J., Sanchez A., Howles P., Doetschman T. Mouse embryonic stem cells express the cardiac myosin heavy chain genes during development in vitro. J Biol Chem. 1990 Jul 15;265(20):11905–11909. [PubMed] [Google Scholar]
- Robbins J., Palermo J., Rindt H. In vivo definition of a cardiac specific promoter and its potential utility in remodeling the heart. Ann N Y Acad Sci. 1995 Mar 27;752:492–505. doi: 10.1111/j.1749-6632.1995.tb17458.x. [DOI] [PubMed] [Google Scholar]
- Samuel J. L., Rappaport L., Syrovy I., Wisnewsky C., Marotte F., Whalen R. G., Schwartz K. Differential effect of thyroxine on atrial and ventricular isomyosins in rats. Am J Physiol. 1986 Mar;250(3 Pt 2):H333–H341. doi: 10.1152/ajpheart.1986.250.3.H333. [DOI] [PubMed] [Google Scholar]
- Schedl A., Montoliu L., Kelsey G., Schütz G. A yeast artificial chromosome covering the tyrosinase gene confers copy number-dependent expression in transgenic mice. Nature. 1993 Mar 18;362(6417):258–261. doi: 10.1038/362258a0. [DOI] [PubMed] [Google Scholar]
- Sharpe J. A., Wells D. J., Whitelaw E., Vyas P., Higgs D. R., Wood W. G. Analysis of the human alpha-globin gene cluster in transgenic mice. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11262–11266. doi: 10.1073/pnas.90.23.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Prior G., Umeda P. K., Zak R. cis-acting elements responsible for muscle-specific expression of the myosin heavy chain beta gene. Nucleic Acids Res. 1992 Apr 11;20(7):1793–1799. doi: 10.1093/nar/20.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Josephson B., Zhang J. W., Li Q. Developmental regulation of human gamma-globin genes in transgenic mice. Mol Cell Biol. 1993 Dec;13(12):7636–7644. doi: 10.1128/mcb.13.12.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Su W., Jackson S., Tjian R., Echols H. DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 1991 May;5(5):820–826. doi: 10.1101/gad.5.5.820. [DOI] [PubMed] [Google Scholar]
- Subramaniam A., Gulick J., Neumann J., Knotts S., Robbins J. Transgenic analysis of the thyroid-responsive elements in the alpha-cardiac myosin heavy chain gene promoter. J Biol Chem. 1993 Feb 25;268(6):4331–4336. [PubMed] [Google Scholar]
- Thompson W. R., Nadal-Ginard B., Mahdavi V. A MyoD1-independent muscle-specific enhancer controls the expression of the beta-myosin heavy chain gene in skeletal and cardiac muscle cells. J Biol Chem. 1991 Nov 25;266(33):22678–22688. [PubMed] [Google Scholar]
- Tsika R. W., Bahl J. J., Leinwand L. A., Morkin E. Thyroid hormone regulates expression of a transfected human alpha-myosin heavy-chain fusion gene in fetal rat heart cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):379–383. doi: 10.1073/pnas.87.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C. A., Nasr-Schirf D., Luna V. J. Identification of transgenic mice carrying the CAT gene with PCR amplification. Biotechniques. 1989 Nov-Dec;7(10):1065–1070. [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Dong J. M., Chiu J. F. Regulation of alpha-fetoprotein gene expression by antagonism between AP-1 and the glucocorticoid receptor at their overlapping binding site. J Biol Chem. 1991 May 5;266(13):8248–8254. [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]