Abstract
Aims/hypothesis
Nicotinamide phosphoribosyltransferase (NAMPT) is a multifunctional protein potentially involved in obesity and glucose metabolism. We systematically studied the association between circulating NAMPT, obesity, interventions and glucose metabolism and investigated potential underlying inflammatory mechanisms.
Methods
Fasting morning NAMPT serum levels were measured in cohorts of lean vs obese children, cohorts of intervention by lifestyle, exercise and bariatric surgery, and during an OGTT. In addition, mRNA expression, protein production and enzymatic activity of NAMPT were assessed from isolated leucocytes and subpopulations.
Results
Circulating NAMPT was significantly elevated in obese compared with lean children and declined after obesity interventions concomitantly with the decline in BMI, high-sensitivity C-reactive protein (hsCrP) and leucocyte counts. Circulating NAMPT significantly correlated with glucose metabolism and cardiovascular variables in univariate analyses, but only the association with glucose response during an OGTT was independent from BMI. We therefore assessed the NAMPT dynamic following an oral glucose load and found a significant decline of NAMPT levels to 77.0 ± 0.1% as a function of time, and insulin-to-glucose ratio during an OGTT in obese insulin-resistant adolescents. Circulating NAMPT was, however, most strongly associated with leucocyte counts (r = 0.46, p < 0.001). The leucocyte count itself determined significantly and independently from BMI insulin resistance in multiple regression analyses. We systematically evaluated NAMPT expression among several tissues and found that NAMPT was predominantly expressed in leucocytes. In subsequent analyses of leucocyte subpopulations, we identified higher NAMPT protein concentrations in lysates of granulocytes and monocytes compared with lymphocytes, whereas granulocytes secreted highest amounts of NAMPT protein into cell culture supernatant fractions. We confirmed nicotinamide mononucleotide enzymatic activity of NAMPT in all lysates and supernatant fractions. In monocytes, NAMPT release was significantly stimulated by lipopolysaccharide (LPS) exposure.
Conclusions
Leucocytes are a major source of enzymatically active NAMPT, which may serve as a biomarker or even mediator linking obesity, inflammation and insulin resistance.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-010-2042-z) contains supplementary material, which is available to authorised users.
Keywords: Adipocytokines, Children, Glucose metabolism, Insulin sensitivity, NAMPT, Obesity, PBEF, Visfatin
Introduction
Nicotinamide phosphoribosyltransferase (NAMPT), formerly known as visfatin or pre-B cell colony enhancing factor (PBEF), is a protein that has been implicated in various pathophysiological conditions, although some of these ascribed features are controversial and are still being discussed. Originally, NAMPT had been identified as PBEF [1], a cytokine capable of stimulating the maturation of B cell precursors. NAMPT attracted most attention, however, when it was re-discovered as an adipocytokine named ‘visfatin’ and claimed to be predominantly secreted from visceral adipose tissue, exerting insulin-like actions [2]. More recently, NAMPT has been identified as the crucial NAD biosynthetic enzyme [3] and thereby to play an important role in the regulation of cellular metabolism and insulin secretion [4, 5].
So far, clinical studies in adults have provided controversial findings concerning the role of NAMPT in obesity and glucose metabolism [6], with positive, negative or no associations found [7–10]. In addition, knowledge of the main origin of NAMPT is still elusive [11, 12]. Nevertheless, no matter which potential NAMPT function is considered (NAD biosynthetic enzyme, pro-inflammatory cytokine and/or adipocytokine), NAMPT may have acute as well as chronic associations with obesity and may influence major metabolic functions. In particular, the direct interactions with sirtuin-1 (SIRT1), a key mediator that coordinates metabolic responses to nutritional availability, are of interest [13, 14].
Paediatric cohorts allow a less biased exploration of the clinical context and potential underlying mechanisms linking NAMPT with obesity and glucose metabolism, as they are less confounded by co-morbidities and their treatment than adult cohorts. Studies in children have indeed revealed that circulating NAMPT was elevated in obesity but failed to detect a direct association with BMI [15, 16].
In this study, we not only provide clinical evidence of the association of NAMPT with obesity and related metabolic and cardiovascular sequelae, but also show that NAMPT is acutely downregulated after glucose provocation. In particular, we identify leucocytes as a major source of enzymatically active NAMPT and reveal that the association of circulating NAMPT with leucocyte count outranges that with obesity.
Methods
Cohort and patient characteristics
Leipzig Schoolchildren cohort
This is a representative cohort of the Leipzig paediatric population with detailed characterisation of anthropometric and biochemical variables of physical and pubertal maturation [17]. We have selected 134 children that have been previously evaluated for the dynamics of adipocytokines during pubertal development (see Electronic supplementary material [ESM] Table 1) [18].
Leipzig Atherobesity Childhood cohort
We recruited 86 obese white children and adolescents and 70 lean controls of similar age, sex and pubertal stage (ESM Table 2).
Fasting blood samples were obtained at 08:00 hours and were immediately centrifuged, aliquoted and stored at −80°C. Metabolic variables were analysed by a certified laboratory. Insulin and C-peptide were measured using the Liaison C-peptide and insulin luminescence immunoassay on the Liaison analyzer (DiaSorin, Saluggia, Italy). Analytical sensitivity of these test systems was 1.2 and 3 pmol/l, respectively. Mean intra- and interassay coefficients of variation were below 4.1% for insulin and 4.4% for C-peptide.
An OGTT was performed in all participants. HOMA-IR [19] and Matsuda insulin sensitivity index (ISI) [20] were calculated as measures of insulin sensitivity. Ambulatory blood pressure monitoring was performed for 24 h (SpaceLabs Medical Inc., Redmond, USA); a recording was considered successful if >80% of readings were valid. Endothelial function was evaluated by the reactive hyperaemia index (RHI), and intima media thickness (IMT) by high-resolution B-mode ultrasound as described previously [21].
Lifestyle intervention cohort
Thirty-six obese children (ESM Table 3) completed the 1-year lifestyle intervention ‘Obeldicks’ as described in detail elsewhere [22].
Bariatric surgery intervention cohort
Fourteen extremely obese patients (ten adults, four adolescents; ESM Table 3) were subjected to bariatric surgery by sleeve gastrectomy for clinical indications according to current guidelines of the Bariatric Scientific Collaborative Group and the American Pediatric Surgical Association [23, 24]. We obtained serum samples before and 6 months after surgery in all patients.
Exercise intervention cohort
Fifteen young healthy, lean adults (ESM Table 3) participated in a 6 month exercise programme consisting of sessions on 3 days of the week. Each training session included 20 min biking or running, 45 min circuit training and 20 min warming-up and cooling-down periods. At baseline and after 6 months of training, blood samples were obtained in the fasting state.
In all cohorts, height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg using a digital balance. In children, BMI data were standardised by age and sex applying German reference data [25], and given as the BMI standard deviation score (SDS). A cutoff of ≥1.23 and ≥1.88 SDS (90th and 97th centile) classified the children as being overweight or obese. Detailed inclusion criteria for all cohorts are given in supplementary methods (see ESM Methods).
Written informed consent was obtained from all parents and from children ≥12 years. All studies were approved by the local ethics committees of the Universities of Leipzig and Witten/Herdecke (reg. nos.: 782-1998 (schoolchildren), 029-2006 (Atherobesity cohort), 341-1 (adolescent bariatric surgery), 031-2006 (adult bariatric surgery), 274-2008 (exercise study), 64/2001 and 15/2006 (‘Obeldicks’).
Quantification of endothelial progenitor cells in peripheral blood
Endothelial progenitor cells (EPCs) were determined as CD34 and kinase insert domain receptor (KDR) double-positive cells applying flow cytometry [26] (Beckman Coulter Epics XL, Krefeld, Germany). Number of EPCs is given as EPCs per 500,000 counted cells.
Isolation, cultivation and stimulation of leucocytes and subpopulations
Cells were isolated applying density gradient centrifugation (Biocoll Separating Solution, Biochrom, Germany) of 20 ml peripheral blood from 12 children and adolescents (age, 11.7 ± 2.7 years; BMI SDS, 2.14 ± 1.12). An incubation step of 2 h at 37°C in RPMI medium containing 0.5% FCS, penicillin and streptomycin (culture medium [CM]) was used for separation of monocytes and lymphocytes. Cell fractions were cultivated in CM for 6 h at 37°C before harvesting of cells and supernatant fractions. Cell integrity was confirmed by Trypan Blue staining and lactase dehydrogenase assay (BIOCAT, Heidelberg, Germany).
For stimulation experiments, granulocytes and monocytes were incubated in CM supplemented with 1 μg/ml lipopolysaccharide (LPS; Sigma), 40 mmol/l d-glucose and 40 mmol/l l-glucose as osmotic control for 24 h at 37°C before harvesting of cells and supernatant fractions.
In addition, leucocytes were isolated from peripheral blood after oral glucose provocation at 0, 60 and 120 min, and cultivated in CM for 45 min before harvest of cells and supernatant fractions.
Analysis of NAMPT and SIRT1 mRNA expression
We obtained commercially available tissue RNAs (Clontech-Takara Bio Europe, Saint-Germain-en-Laye, France) and cDNAs (Biochain, Heidelberg, Germany). RNA from cell fractions was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany). Reverse transcription was performed using 200 U M-MLV reverse transcriptase per microgram total RNA with oligo (dT) primers. NAMPT and SIRT1 mRNA expression was determined using quantitative real-time PCR with TaqMan probe-based gene expression assay on the ABI 7500 Sequence Detection System (Applied Biosystems, Darmstadt, Germany). To normalise gene expression, housekeeping genes beta actin (ACTB), TBP and HPRT were quantified simultaneously in each sample.
Quantification of NAMPT and enzyme activity in serum samples, cell lysates and supernatant fractions
NAMPT concentrations were measured by ELISA following the manufacturer’s protocol (Adipogen, Seoul, South Korea). Assay quality variables including sensitivity and specificity have been validated previously [27]. NAMPT amounts in cell lysates and supernatant fractions were normalised to total protein amount (BCA Protein Assay, Thermo Fisher Scientific, Bonn, Germany).
Preparation of samples and determination of enzymatic activity were performed according to the method by Elliott et al. [28] with minor modifications [12, 28] (see ESM Methods).
Statistical analyses
Logarithmic transformation of non-normally distributed data was performed before analysis. For comparison of quantitative traits between two groups, two-tailed Student’s t test was applied. For more groups and time courses ANOVA was applied, and for categorical data χ 2 test was applied. Correlation analyses were performed using Pearson correlation analysis or partial correlation analysis with adjustment for BMI SDS. For multiple regression analyses, the stepwise forward model was employed. For all tests, the significance level was set at 0.05. Statistical analyses were performed using the software package Statistica 7.1 (StatSoft, Tulsa, OK, USA).
Results
Association of NAMPT with normal development in healthy lean children
We identified no differences in circulating NAMPT levels with progression through puberty in normal healthy lean boys or girls (ESM Fig. 1a). Also, there was no correlation between NAMPT levels and height SDS, circulating IGF-I and markers of pubertal development and adrenarche, including oestradiol, testosterone or dehydroepiandrosterone-sulfate (DHEA-S). There were no differences between boys and girls in the entire cohort (ESM Fig. 1b) or if analysis was restricted to adolescent children.
Association of NAMPT with obesity and obesity interventions
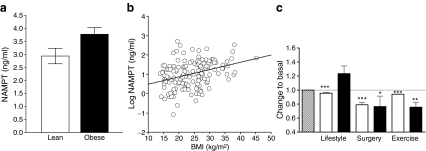
We applied the Leipzig Atherobesity Childhood cohort (ESM Table 2) to evaluate differences in NAMPT serum concentrations between lean and obese children. NAMPT levels were significantly higher in the obese children compared with lean controls (Fig. 1a). NAMPT levels correlated with BMI (Fig. 1b) and other variables of obesity and body fat, including BMI SDS as a normalised index of the degree of overweight, leptin as a biomarker of fat mass, and skinfold thickness as an index of subcutaneous fat (Table 1).
Fig. 1.
NAMPT association with obesity. a Obese children (n = 86) had significantly higher NAMPT levels (p = 0.031) compared with lean children (n = 70). Data are mean ± SEM. b NAMPT serum levels correlated with BMI (r = 0.35, p < 0.001). c Changes of NAMPT levels and BMI after 1-year lifestyle intervention in obese children and adolescents (n = 36), 6 months following bariatric surgery in severely obese adults and adolescents (n = 14), and 6 month exercise programme in normal weight adults (n = 15). Data for BMI (open bars) and NAMPT levels (black bars) are given relative to the basal situation (hatched bar), which was set to 1.0. Data are mean ± SEM. Statistical significance was assessed by paired t test. *p < 0.05, **p < 0.01, ***p < 0.001
Table 1.
Association of NAMPT levels with anthropometric, metabolic and cardiovascular variables in lean and obese children of the Leipzig Atherobesity Childhood cohort (n = 156)
| Variable | Univariate correlation | Partial correlation | ||
|---|---|---|---|---|
| r | p value | r a | p a value | |
| Anthropometric variables | ||||
| BMI SDS | 0.31 | <0.0001 | ||
| BMI (kg/m2) | 0.35 | <0.0001 | ||
| Serum leptin (ng/ml)b | 0.25 | 0.007 | ||
| WHR | 0.06 | 0.426 | ||
| Skinfold thickness (cm) | 0.31 | <0.0001 | ||
| Metabolic variables | ||||
| Fasting BG (mmol/l) | 0.26 | 0.001 | 0.07 | 0.401 |
| 120 min BG (mmol/l) | 0.20 | 0.012 | 0.13 | 0.129 |
| Mean BG (mmol/l) | 0.25 | 0.001 | 0.21 | 0.009 |
| AUC BG (mmol/l × min) | 0.28 | <0.0001 | 0.23 | 0.004 |
| FPIb (pmol/l) | 0.26 | 0.001 | 0.08 | 0.346 |
| Peak insulinb (pmol/l) | 0.24 | 0.003 | 0.10 | 0.237 |
| AUCbIns (pmol/l × min) | 0.27 | 0.001 | 0.13 | 0.124 |
| HOMA-IRb | 0.23 | 0.004 | 0.11 | 0.395 |
| Matsuda ISIb | −0.29 | <0.0001 | −0.09 | 0.112 |
| Belfiore ISI | −0.31 | <0.0001 | −0.19 | 0.021 |
| AUCIns/AUCBG (pmol/mmol) | 0.24 | 0.003 | 0.10 | 0.215 |
| Cardiovascular variables | ||||
| Systolic BP (mmHg) | 0.20 | 0.012 | 0.09 | 0.241 |
| Diastolic BP (mmHg) | 0.09 | 0.226 | 0.03 | 0.676 |
| Mean systolic BP in 24 h (mmHg) | 0.18 | 0.043 | 0.06 | 0.491 |
| Mean diastolic BP in 24 h (mmHg) | 0.10 | 0.247 | 0.06 | 0.515 |
| IMT (cm) | 0.11 | 0.174 | −0.04 | 0.582 |
| Endothelial function (RHI) | −0.23 | 0.004 | −0.10 | 0.232 |
| EPC count (per 50,0000 counts)b | −0.29 | <0.0001 | −0.25 | 0.002 |
| EPC migrationb | 0.01 | 0.871 | 0.04 | 0.669 |
| WBC count (×109/l)b | 0.49 | <0.0001 | 0.44 | <0.0001 |
| hsCrP (ng/ml)b | 0.34 | <0.0001 | 0.27 | 0.001 |
Pearson correlation analysis was performed for log-transformed NAMPT serum levels, n = 156
BP, blood pressure; FPI, fasting plasma insulin; Ins, insulin
aPearson partial correlation analysis adjusted for BMI SDS
bIndicates log-transformed variables
We further assessed NAMPT serum concentrations in the context of three distinct intervention studies: (1) a 12 month lifestyle intervention programme in children and adolescents; (2) bariatric surgery by sleeve gastrectomy in severely obese adolescents and adults; and (3) a 6 month exercise intervention in normal weight young healthy adults (ESM Table 3). The strategies differed in the extent of weight loss and improvement of HOMA-IR achieved (Fig. 1c, ESM Table 3).
After both the bariatric surgery and the exercise interventions we found significantly lower NAMPT serum concentrations, whereas we observed no significant change after lifestyle intervention (Fig. 1c). The decrease in NAMPT correlated significantly with the decrease in BMI in the surgery group only (ESM Fig. 2d).
In addition to BMI, the bariatric surgery group showed the strongest decrease in white blood cell count (WBC) and high-sensitivity C-reactive protein (hsCrP; ESM Fig. 2a–c) and there was a significant correlation between decrease in hsCrP and NAMPT in this group (ESM Fig. 2f). In multiple regression analyses, decrease in hsCrP (β = 0.65, p = 0.033) and decrease in BMI (β = 0.86, p = 0.029) were significant predictors for the NAMPT decrease, whereas decrease in WBC and basal WBC, hsCrP and BMI did not contribute (R 2 = 0.83, p = 0.035 for the model).
Association of NAMPT with glucose metabolism
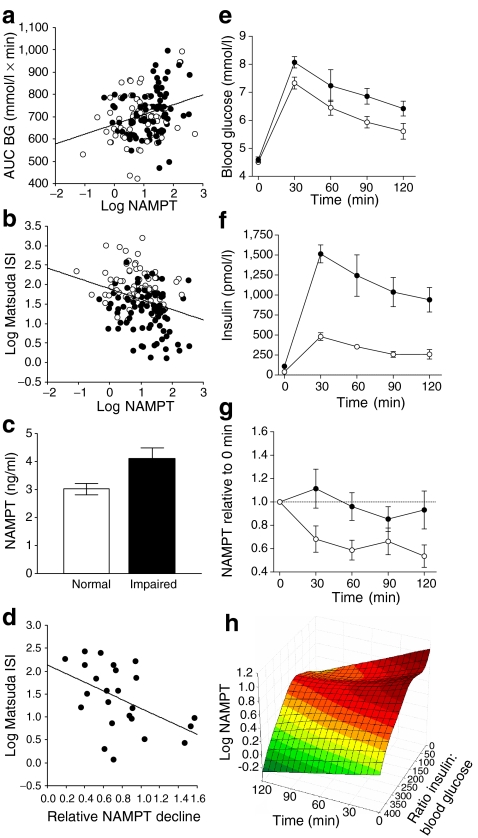
NAMPT significantly correlated with all variables of glucose and insulin metabolism (Table 1, Fig. 2a, b). Children with impaired insulin sensitivity had higher NAMPT levels compared with normal insulin-sensitive children (Fig. 2c). When we adjusted for BMI SDS in partial correlation analyses, only mean blood glucose (BG) and AUC BG as dynamic variables during OGTT remained significant, but most other significances were lost (Table 1). In multiple regression analyses, NAMPT was significantly associated with AUC BG (β = 0.24 ± 0.08, p = 0.004), whereas BMI SDS, age, sex and pubertal stage did not contribute significantly.
Fig. 2.
NAMPT association with glucose/insulin metabolism. Correlation of NAMPT serum levels with AUC BG during OGTT (a) (r = 0.25, p = 0.001) and Matsuda ISI (b) (r = −0.29, p < 0.001). White circles = lean children, black circles = obese children. c Children with impaired insulin sensitivity according to Matsuda ISI < 4 (n = 44) had higher NAMPT levels compared with normal insulin sensitivity (n = 105, p = 0.003). Data are given as mean ± SEM and were analysed by t test of log-transformed NAMPT. d Correlation of NAMPT decline (given as mean ratio of NAMPT between 60–120 min to basal NAMPT) and Matsuda ISI (r = 0.48, p = 0.017). Course of blood glucose (e), insulin (f) and NAMPT (g) serum levels during OGTT in insulin-sensitive (n = 11) and insulin-resistant (n = 13) obese children. For NAMPT, data are given as ratio of NAMPT levels at single time points compared with basal NAMPT at t = 0 (p Anova = 0.003). White circles, normal insulin; black circles, hyperinsulinemia. h Three-dimensional plot of NAMPT as a function of insulin-to-glucose ratio and time during OGTT
To investigate this finding in more detail, we analysed the dynamic of NAMPT serum concentrations during an OGTT in 24 obese adolescents (age 11.7 ± 4.1 years, BMI SDS 2.60 ± 0.53). Compared with basal levels, NAMPT levels declined to 77 ± 0.1% (p = 0.0029). This decline was stronger in the insulin-sensitive obese participants, as evidenced by a negative correlation of NAMPT with Matsuda ISI (Fig. 2d). We thus stratified groups into insulin-resistant and insulin-sensitive patients on the basis of peak insulin during OGTT >1,000 and <600 pmol/l, respectively (Fig. 2e, f). Accordingly, Matsuda ISI was significantly impaired in the hyperinsulinaemic group (8.02 ± 0.70 vs 2.59 ± 0.29, p < 0.0001). There was no difference in the degree of obesity between the two groups (2.72 ± 0.11 vs 2.48 ± 0.20 BMI SDS, p > 0.2). The decline of NAMPT, however, was significantly more pronounced in the insulin-sensitive group (Fig. 2g) and correlated with AUC-insulin-to-AUC-BG ratio (r = 0.43, p = 0.038). Likewise, individual NAMPT levels during OGTT correlated with the insulin-to-glucose ratio at each time point (r = −0.19, p = 0.034). Thus, NAMPT levels declined after an oral glucose load as a function of time and insulin-to-glucose ratio (Fig. 2h).
Association of NAMPT with cardiovascular parameters and leucocytes
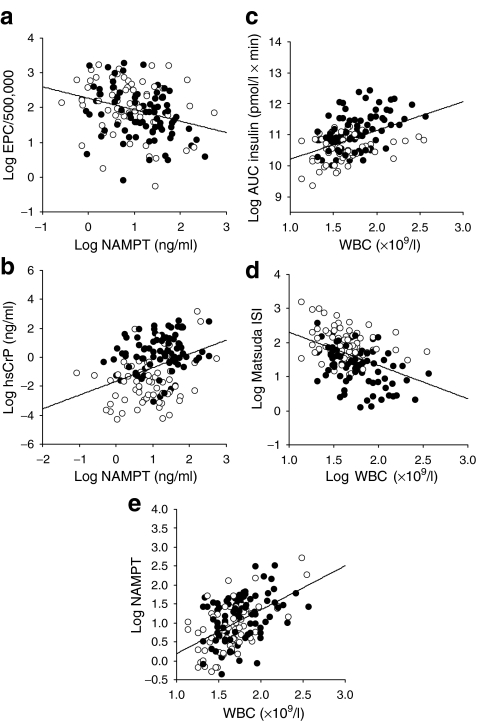
We assessed the correlation of NAMPT levels with cardiovascular variables (random and 24 h blood pressure), functional variables of early vascular impairment (IMT, endothelial function), and regenerative capacity (EPC count and migratory function) in our Atherobesity Childhood cohort. We identified significant correlations of NAMPT with systolic blood pressure and endothelial function that did, however, not withstand adjustment for BMI SDS in partial correlation analyses (Table 1). By contrast, the cellular variables EPC and WBC counts, and hsCrP, were significantly associated with NAMPT serum levels (Fig. 3a, b, Table 1). In multiple regression analyses, NAMPT was the strongest predictor for EPC as well as for WBC counts (Table 2).
Fig. 3.
NAMPT association with leucocytes in the Leipzig Atherobesity Childhood cohort. Correlation of NAMPT serum levels with EPC count (a) (r = −0.29, p < 0.001) and hsCrP (b) (r = 0.34, p < 0.001). Correlations of WBC count with metabolic variables AUC insulin (c) (r = 0.35, p < 0.001) and Matsuda ISI (d) (r = −0.33, p < 0.001). The strongest correlation was achieved between NAMPT and WBC count (e) (r = 0.46, p < 0.001). White circles, lean children; black circles, obese children. Correlation coefficients were determined by Pearson correlation analyses of log-transformed variables
Table 2.
Multiple regression analyses for independent associations of NAMPT serum levels
| Step | Variable | R 2 | β ± SEM | B ± SEM | p value |
|---|---|---|---|---|---|
| Dependent variable: log EPC counta | |||||
| 1 | log NAMPT | 0.08 | −0.22 ± 0.10 | −0.23 ± 0.10 | 0.023 |
| 2 | Leptin | 0.03 | −0.16 ± 0.10 | −0.007 ± 0.004 | 0.095 |
| 3 | Systolic BP SDS | 0.01 | −0.12 ± 0.09 | −0.07 ± 0.06 | 0.197 |
| Dependent variable: log WBC countb | |||||
| 1 | log NAMPT | 0.240 | 0.35 ± 0.08 | 0.15 ± 0.03 | <0.001 |
| 2 | log AUCIns | 0.105 | 0.40 ± 0.10 | 0.19 ± 0.05 | <0.001 |
| 3 | log EPC count | 0.022 | −0.16 ± 0.08 | −0.06 ± 0.03 | 0.045 |
| 4 | Leptin | 0.015 | −0.21 ± 0.10 | −0.003 ± 0.002 | 0.046 |
| 5 | log hsCrP | 0.014 | 0.13 ± 0.09 | 0.02 ± 0.01 | 0.124 |
| Dependent variable: log Matsuda ISIc | |||||
| 1 | BMI SDS | 0.360 | −0.54 ± 0.06 | −0.23 ± 0.03 | <0.001 |
| 2 | log WBC | 0.070 | −0.26 ± 0.06 | 0.56 ± 0.14 | <0.001 |
| 3 | Age | 0.032 | −0.17 ± 0.06 | −0.03 ± 0.01 | 0.006 |
| 4 | Sex | 0.008 | 0.09 ± 0.06 | 0.11 ± 0.07 | 0.140 |
| Step | Variable | R 2 | β ± SEM | B ± SEM | p value |
|---|---|---|---|---|---|
| Dependent variable: log EPC counta | |||||
| 1 | log NAMPT | 0.08 | −0.22 ± 0.10 | −0.23 ± 0.10 | 0.023 |
| 2 | Leptin | 0.03 | −0.16 ± 0.10 | −0.007 ± 0.004 | 0.095 |
| 3 | Systolic BP SDS | 0.01 | −0.12 ± 0.09 | −0.07 ± 0.06 | 0.197 |
| Dependent variable: log WBC countb | |||||
| 1 | log NAMPT | 0.240 | 0.35 ± 0.08 | 0.15 ± 0.03 | <0.001 |
| 2 | log AUCIns | 0.105 | 0.40 ± 0.10 | 0.19 ± 0.05 | <0.001 |
| 3 | log EPC count | 0.022 | −0.16 ± 0.08 | −0.06 ± 0.03 | 0.045 |
| 4 | Leptin | 0.015 | −0.21 ± 0.10 | −0.003 ± 0.002 | 0.046 |
| 5 | log hsCrP | 0.014 | 0.13 ± 0.09 | 0.02 ± 0.01 | 0.124 |
| Dependent variable: log Matsuda ISIc | |||||
| 1 | BMI SDS | 0.360 | −0.54 ± 0.06 | −0.23 ± 0.03 | <0.001 |
| 2 | log WBC | 0.070 | −0.26 ± 0.06 | 0.56 ± 0.14 | <0.001 |
| 3 | Age | 0.032 | −0.17 ± 0.06 | −0.03 ± 0.01 | 0.006 |
| 4 | Sex | 0.008 | 0.09 ± 0.06 | 0.11 ± 0.07 | 0.140 |
Ins, insulin; PH, pubic hair stage
a R 2 = 0.12, p = 0.0019 (Childhood Atherobesity cohort, n = 156). Independent variables: BMI SDS, leptin, systolic BP SDS, log AUCIns, log ISI, log hsCrP, log NAMPT, log EPC count
b R 2 = 0.40, p < 0.0001(Childhood Atherobesity cohort, n = 156). Independent variables: BMI SDS, leptin, systolic BP SDS, log AUCIns, log ISI, log hsCrP, log NAMPT, log EPC count
c R 2 = 0.47, p < 0.0001(Childhood Atherobesity cohort, n = 156). Independent variables: BMI SDS, log NAMPT, log WBC, age, sex, PH
Considering this predominant association of NAMPT with WBCs, we hypothesised that WBCs may influence the associations of NAMPT with metabolic variables and evaluated the correlation of WBCs themselves with metabolic and cardiovascular variables. We identified significant BMI-independent correlations with AUC insulin, Matsuda ISI (Fig. 3c, d), systolic blood pressure (r = 0.18, p = 0.024), but most strongly with NAMPT serum levels (Fig. 3e). Likewise, in multiple regression analyses, WBCs contributed significantly and independently of age, sex and BMI SDS to Matsuda ISI (Table 2).
Hence, NAMPT is most strongly associated with WBC count and WBCs themselves are significantly associated with variables of insulin resistance.
Expression pattern of NAMPT in tissues and leucocytes
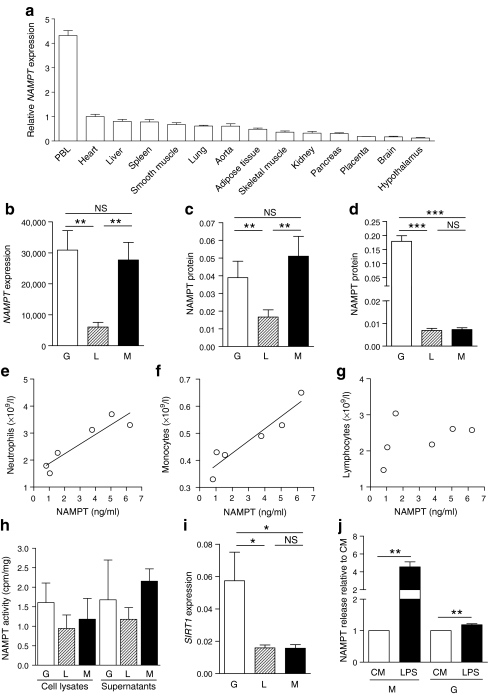
Considering the strong association of circulating NAMPT levels with leucocytes in the clinical study, we systematically evaluated the expression pattern of NAMPT among 14 metabolically, endocrine and immunologically active tissues. We found that NAMPT mRNA was predominantly expressed in peripheral blood leucocytes (Fig. 4a).
Fig. 4.
NAMPT expression pattern, production and secretion by leucocyte subpopulations. a The expression of NAMPT mRNA was significantly higher in PBL than in all other tissues, including adipose tissue and liver (p Anova < 0.0001). Significance was calculated by one-way ANOVA with Dunnett’s post test (compared with PBL). b The mRNA expression of NAMPT was more than fivefold higher in granulocytes and monocytes compared with lymphocytes. c Higher amounts of NAMPT protein were detected in cell lysates of granulocytes and monocytes compared with lymphocytes. d Granulocytes secreted more than 22-fold higher amounts of NAMPT protein into cell culture supernatant fractions (n = 12) when normalised to total protein. Serum concentrations of NAMPT were highly correlated to leucocyte count in particular to neutrophil granulocyte (e) (r = 0.92, p = 0.009) and monocyte (f) (r = 0.94, p = 0.005) count but not to lymphocyte count (g) (n = 6, p = 0.41). h NAMPT enzymatic activity was present in cell lysates and supernatant fractions of all leucocyte subpopulations (n = 5). i SIRT1 mRNA expression was significantly higher in granulocytes compared with lymphocytes and monocytes (n = 12). j The release of NAMPT was significantly increased from monocytes and granulocytes after stimulation with 1 μg/ml LPS for 24 h in n = 3 independent experiments. Data are mean ± SEM. Statistical significance was assessed by Student’s t test and Pearson correlation analysis: *p < 0.05, **p < 0.01, ***p < 0.0001. G, granulocytes; L, lymphocytes; M, monocytes; PBL, peripheral blood leucocytes
To determine which subpopulation(s) of leucocytes potentially contribute to NAMPT levels in peripheral blood, we assessed NAMPT mRNA and NAMPT protein expression in granulocytes, lymphocytes and monocytes isolated from peripheral blood of 12 children and adolescents. The mRNA expression was more than fivefold higher in granulocytes and monocytes compared with lymphocytes (Fig. 4b). Consistent with this, we detected a higher amount of NAMPT protein in cell lysates of granulocytes and monocytes compared with lymphocytes (Fig. 4c). Granulocytes secreted highest amounts of NAMPT protein into cell culture supernatant fractions (Fig. 4d). Consistently, serum concentrations of NAMPT were highly correlated to leucocyte count, particularly to neutrophil granulocyte and monocyte count but not to lymphocyte count (Fig. 4e–g). Hence, NAMPT is predominantly produced and secreted by leucocytes, in particular by granulocytes.
NAMPT function and interaction with SIRT1 and LPS in distinct leucocyte subpopulations
We evaluated the enzymatic activity of the NAMPT protein by quantification of nicotinamide mononucleotide (NMN) synthesis from nicotinamide and confirmed that NAMPT enzymatic activity was present in cell lysates and supernatant fractions of all leucocyte subpopulations (Fig. 4h).
To assess a downstream target of NAMPT activity, we examined SIRT1 mRNA expression in the leucocyte subpopulations. The expression of SIRT1 was significantly higher in granulocytes compared with lymphocytes and monocytes (Fig. 4i).
To finally evaluate whether NAMPT is stimulated by inflammatory agents, we analysed NAMPT release following stimulation with LPS. LPS significantly increased NAMPT release from monocytes and slightly enhanced NAMPT release from granulocytes (Fig. 4j).
Leucocyte counts and NAMPT release from leucocytes during OGTT
We assessed whether the decline of NAMPT during OGTT is attributable to reduced leucocyte counts or reduced NAMPT release from leucocytes in 11 additional patients. The leucocyte, lymphocyte and neutrophil counts did not change significantly after oral glucose intake; only monocytes were significantly decreased after 60 min (ESM Fig. 3a). Ex vivo, we found a slight decrease in NAMPT release after 120 min (ESM Fig. 3b). Finally, to assess whether glucose has a direct effect on NAMPT release, we incubated isolated leucocytes with glucose, but we did not find significant changes (ESM Fig. 3c).
Discussion
In this study, we showed that NAMPT is not only associated with obesity and glucose metabolism, but has the strongest relationship with leucocyte count and is in fact predominantly produced in leucocytes in its enzymatically active form. The circulating leucocyte count strongly correlated to insulin resistance, and the association of NAMPT with obesity and glucose metabolism may reflect an underlying relation with leucocytes.
Circulating NAMPT is affected by obesity and obesity intervention
NAMPT has drawn considerable interest in the fields of obesity, type 2 diabetes and inflammatory diseases. Some studies reported that circulating NAMPT is elevated in obesity and type 2 diabetes [8, 10], whereas others found no association with adiposity and metabolic variables [9]. In our study, circulating NAMPT was significantly higher in obese compared with lean children, and correlated with the extent of obesity. If NAMPT is related to obesity, one would expect a decrease after weight loss, as has been shown by some studies [8, 29, 30]. We show that NAMPT was reduced by significant weight loss after bariatric surgery but not by moderate weight loss due to lifestyle intervention. Exercise was also successful in reducing NAMPT levels as well as improving insulin sensitivity. Interestingly, bariatric surgery and exercise led to a concomitant reduction in WBC and hsCrP, and the decrease in NAMPT not only correlated with the decrease in BMI but also with the decrease in hsCrP. This suggests that in addition to a significant reduction in adipose tissue mass and/or insulin resistance, improvement in the inflammatory state may account for a reduction of NAMPT by interventions.
Circulating NAMPT declines after oral glucose provocation
NAMPT was associated with metabolic variables in our study. However, when analyses were adjusted for the degree of obesity, most associations were abolished, thus indicating that the correlation with insulin resistance was mainly secondary to obesity. Only dynamic variables of blood glucose during an OGTT remained significant after adjustment for BMI. Considering this finding, and that NAMPT has been shown to play a crucial role in beta cell function [5], we investigated the acute response of NAMPT to oral glucose provocation. As NAMPT has been reported to be increased by hyperglycaemia through i.v. glucose infusions and experimentally in adipocytes [31], one may expect an increase of NAMPT during an OGTT. Surprisingly, NAMPT levels declined following glucose challenge. Our results contrast with the findings of other studies, which reported a transient increase of NAMPT [32] or have failed to demonstrate any NAMPT response during an OGTT [33]. These contrasting findings may result from different patient characteristics, specifically the distinct degree of obesity, stratification for glucose vs for insulin resistance study, and in particular from the fact that all previous studies were performed in adults, whereas we investigated children. Children are at the beginning of the pathophysiologic development of insulin resistance and less biased by co-morbidities and their treatment; they may hence allow a better exploration of a potential association of NAMPT with glucose metabolism. Also different analytical methods need to be considered that have been shown to have a crucial impact on the quantification of NAMPT/visfatin [27].
In our study, the decline of NAMPT was a function of time and insulin-to-glucose ratio. Time was the strongest predictor for the change of NAMPT serum concentration after glucose provocation. This may indicate that the natural circadian variation may affect NAMPT. A rhythmic oscillation in mRNA and protein levels of NAMPT was recently discovered [34, 35]. However, considering that: (1) NAMPT is lower with higher insulin-per-glucose secretion in our study; (2) our in vitro findings that did not show a stimulation of NAMPT release by glucose; and (3) the proposed role for NAMPT in beta cell function [5], one may conclude that NAMPT augments glucose stimulated insulin secretion and is then negatively regulated by insulin. This feedback regulation seems to be disturbed in insulin-resistant participants, as the decline in NAMPT is predominant in insulin-resistant participants [10, 36]. In addition, other groups provided evidence that glucose directly downregulates NAMPT production in different cell types [37, 38]. Hence, the glucose stimulus and/or resulting insulin release and/or natural circadian variations in NAMPT levels may contribute to the decline of NAMPT in OGTT, although the precise mechanisms remain unclear at this point.
NAMPT is produced and secreted by leucocyte subpopulations
In our cross-sectional study, we found the strongest independent associations of circulating NAMPT with leucocyte counts. As there is no systematic evaluation of the origin of NAMPT so far, this prompted us to evaluate NAMPT expression among several metabolically, endocrine and immunologically active tissues. We found highest amounts of NAMPT mRNA in leucocytes compared with all other tissues, including adipose tissue and liver. Higher expression of NAMPT in non-fat cells and adipose tissue derived macrophages compared with fat cells has been reported before [11, 39], further supporting the hypothesis that adipose tissue is not the major source of NAMPT. We subsequently evaluated which leucocyte subpopulation(s) may account for the high NAMPT production and found that NAMPT mRNA expression was higher in granulocytes and monocytes compared with lymphocytes, and that granulocytes released significantly more NAMPT protein into cell culture supernatant fractions. Considering that neutrophil granulocytes constitute 50% to 60% of the total circulating WBCs, we hypothesise that granulocytes represent a major source of circulating NAMPT. To our knowledge, none of the previous clinical studies have adjusted their analyses for leucocyte counts, which may contribute to the conflicting results reported in the clinical studies so far.
NAMPT as a marker of low grade inflammation in obesity
Given that leucocytes are a major source of circulating NAMPT, one may hypothesise that the increase of NAMPT in obese participants [40, 41] may be related to low-grade inflammation seen in obesity. This is substantiated by the association of NAMPT with inflammatory markers observed here, and in other studies [42]. In addition, we demonstrated that LPS stimulated NAMPT production in monocytes and granulocytes, which is in line with a new study showing that NAMPT is induced in human macrophages [43]. Taken together, this suggests a role for NAMPT and immune cells in chronic low grade inflammation observed in obesity and type 2 diabetes. These findings also indicate that the association of NAMPT with obesity and type 2 diabetes may be partly secondary to an underlying association of NAMPT with leucocytes.
This may in consequence have an impact on downstream targets, such as the NAD consuming factor SIRT1, which in turn has been shown to exert antiapoptotic effects [3]. Supporting this hypothesis, SIRT1 mRNA expression was significantly higher in granulocytes in our study, which may suggest that increased NAMPT levels delay apoptosis of granulocytes in obesity via induction of SIRT1. Another recent study has shown that NAMPT-mediated NAD biosynthesis plays a crucial role in myeloid differentiation in humans [44]. These results may further explain the high correlation between circulating NAMPT and leucocyte counts, in that the high NAMPT levels may promote myeloid differentiation through SIRT1 and eventually increase the number of peripheral blood leucocyte counts. This would indicate that there is a mechanistic link between circulating NAMPT and leucocyte counts, and this may in consequence be relevant for the obesity-related increase in inflammatory status.
Leucocytes as major source of NAMPT: clinical implications
Our major new finding is that NAMPT is predominantly produced by leucocytes, independent from BMI. Interestingly, the decline of NAMPT after weight loss due to bariatric surgery, exercise [45], and glucose provocation [46] was paralleled by a decline in leucocyte counts. Furthermore we show that leucocytes per se, and independent from BMI, are associated with insulin sensitivity markers in obese children. Hence we speculate that this direct link between NAMPT and leucocytes may underlie the association between NAMPT and obesity and insulin resistance, and in particular the low-grade inflammatory state seen in these conditions. There is support for this notion from recent studies showing that NAMPT is related with systemic inflammation in diabetic patients [47], that NAMPT serum concentrations and mRNA expression levels in leucocytes are increased in obese patients and that plasma NAMPT levels are related to inflammation in fatty liver disease [48]. Finally, NAMPT was one of five markers that discriminated between patients with chronic inflammation and healthy controls [49].
In summary, we not only provide clinical evidence on the association of NAMPT with obesity and obesity-related metabolic and cardiovascular sequelae in children, but show that NAMPT is acutely downregulated after glucose provocation. In particular, we identified leucocytes as a major source of enzymatically active NAMPT and reveal that the association of circulating NAMPT with leucocyte count outranges the one with obesity. This finding may be of particular importance linking obesity, inflammation and insulin resistance, for which NAMPT may be a marker or even a mediator.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Cohort characteristics of the normal lean cohort (Leipzig Schoolchildren project) (PDF 94.5 kb)
Cohort characteristics of lean and obese children of the Leipzig Childhood Atherobesity cohort (PDF 80.0 kb)
Cohort characteristics of intervention cohorts (PDF 59.7 kb)
Association of NAMPT with development in healthy lean children. a Dynamics of NAMPT levels during pubertal development. Plots are median with interquartile range. b Comparison of NAMPT levels between boys and girls. Number of participants is indicated in parentheses. PH, pubic hair stage (PDF 4.44 kb)
Dynamics of NAMPT, WBC and hsCrP with intervention. The effect of intervention on BMI (a) WBC (b) (exercise: p = 0.052) and CrP (c) is given relative to basal levels before intervention (set at 1.0) as mean ± SEM. For bariatric surgery intervention, the correlation between decrease in BMI (d) (r = 0.70, p = 0.007), WBC (e) and hsCrP (f) (r = 0.72, p = 0.019) with the NAMPT decrease is shown. D, difference **p < 0.01 (PDF 11.4 kb)
Effect of glucose on leucocyte population and NAMPT release. a Dynamics of leucocyte and subpopulations counts after oral glucose provocation in n = 11 obese patients. Plots are mean ± SEM. Analysis was performed with ANOVA. White circles, lymphocytes (p = 0.192); black circles, monocytes (p = 0.023); white squares, neutrophils (p = 0.242); black squares, leucocytes (p = 0.123). b NAMPT release ex vivo from total leucocytes obtained from n = 4 during an OGTT. Leucocytes were isolated from peripheral blood at 0, 60 and 120 min after glucose provocation and were cultured for 45 min. NAMPT concentrations in the supernatant fractions were determined by ELISA. NAMPT was significantly reduced at 120 min (p = 0.001). c NAMPT release from monocytes and granulocytes in response to direct glucose provocation in vitro with 40 mmol/l d-glucose and 40 mmol/l l-glucose as osmotic control. *p < 0.05, **p < 0.01 (PDF 7.33 kb)
(PDF 68.3 kb)
Acknowledgements
We thank all children who participated in the studies. We very gratefully appreciate the help of the study nurses and physicians of our daycare clinic, the technical assistants (A. Berthold, R. Tauscher), who performed the clinical examinations, data collection and experimental studies. We are grateful to our collaborators in the Department of Surgery (A. Dietrich) and the Department of Pediatric Surgery, especially to H. Till and R. Böhm. This work was supported by grants from the German Research Council (DFG) KFO 152 ‘Atherobesity’ KO3512/1-1 (to AK and SE, also JK, MB, MS and WK); the Federal Ministry of Education and Research (BMBF), Germany, IFB Adiposity Diseases #01EO1001 (project ADI-K7-10); the German Diabetes Association (to DF and AK); the Else Kröner-Fresenius Foundation (to A. Körner); and the LARGE consortium funded by the German Federal Ministry of Education and Research (BMBF) within the scope of the competence network ‘Obesity’ (to A. Körner, T. Reinehr and W. Kiess).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- BG
Blood glucose
- CM
Culture medium
- EPC
Endothelial progenitor cells
- hsCrP
High-sensitivity C-reactive protein
- IMT
Intima–media thickness
- ISI
Insulin sensitivity index
- LPS
Lipopolysaccharide
- NAMPT
Nicotinamide phosphoribosyltransferase
- NMN
Nicotinamide mononucleotide
- PBEF
Pre-B cell colony enhancing factor
- RHI
Reactive hyperaemia index
- SDS
Standard deviation score (synonymous for z score)
- WBC
White blood cell
References
- 1.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 3.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 4.Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revollo JR, Körner A, Mills KF, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiess W, Petzold S, Töpfer M, et al. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab. 2008;22:135–153. doi: 10.1016/j.beem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Berndt J, Klöting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 8.Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–1581. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 9.Ingelsson E, Larson MG, Fox CS, et al. Clinical correlates of circulating visfatin levels in a community-based sample. Diabetes Care. 2007;30:1278–1280. doi: 10.2337/dc06-2353. [DOI] [PubMed] [Google Scholar]
- 10.Retnakaran R, Youn BS, Liu Y, et al. Correlation of circulating full-length visfatin (PBEF/NAMPT) with metabolic variables in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol Oxf. 2008;69:885–893. doi: 10.1111/j.1365-2265.2008.03264.x. [DOI] [PubMed] [Google Scholar]
- 11.Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 12.Garten A, Petzold S, Barnikol-Oettler A, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun. 2010;391:376–381. doi: 10.1016/j.bbrc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Imai S. SIRT1 and caloric restriction: an insight into possible trade-offs between robustness and frailty. Curr Opin Clin Nutr Metab Care. 2009;12:350–356. doi: 10.1097/MCO.0b013e32832c932d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai S. The NAD World: a new systemic regulatory network for metabolism and aging—Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haider DG, Holzer G, Schaller G, et al. The adipokine visfatin is markedly elevated in obese children. J Pediatr Gastroenterol Nutr. 2006;43:548–549. doi: 10.1097/01.mpg.0000235749.50820.b3. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Jiang B, Tang J, et al. Serum visfatin concentrations in obese adolescents and its correlation with age and high-density lipoprotein cholesterol. Diab Res Clin Pract. 2008;79:412–418. doi: 10.1016/j.diabres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Reich A, Müller G, Gelbrich G, Deutscher K, Godicke R, Kiess W. Obesity and blood pressure—results from the examination of 2365 schoolchildren in Germany. Int J Obes Relat Metab Disord. 2003;27:1459–1464. doi: 10.1038/sj.ijo.0802462. [DOI] [PubMed] [Google Scholar]
- 18.Böttner A, Kratzsch J, Muller G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Friebe D, Neef M, Erbs S et al (2011) Retinol binding protein 4 (RBP4) is primarily associated with adipose tissue mass in children. Int J Pediatr Obes. doi:10.3109/17477166.2010.491228 [DOI] [PubMed]
- 22.Reinehr T, de Sousa G, Toschke AM, Andler W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr. 2006;84:490–496. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- 23.Fried M, Hainer V, Basdevant A, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts. 2008;1:52–59. doi: 10.1159/000113937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 25.Kromeyer-Hauschild K, Wabitsch M, Geller FJ, et al. Perzentilen für den Body Mass Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben [Centiles for body mass index for children and adolescents derived from distinct independent German cohorts] Monatsschr Kinderheilkd. 2001;149:807–818. doi: 10.1007/s001120170107. [DOI] [Google Scholar]
- 26.Krankel N, Adams V, Linke A, et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25:698–703. doi: 10.1161/01.ATV.0000156401.04325.8f. [DOI] [PubMed] [Google Scholar]
- 27.Körner A, Garten A, Bluher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- 28.Elliott GC, Ajioka J, Okada CY. A rapid procedure for assaying nicotinamide phosphoribosyltransferase. Anal Biochem. 1980;107:199–205. doi: 10.1016/0003-2697(80)90512-6. [DOI] [PubMed] [Google Scholar]
- 29.Bo S, Ciccone G, Baldi I, et al. Plasma visfatin concentrations after a lifestyle intervention were directly associated with inflammatory markers. Nutr Metab Cardiovasc Dis. 2009;19:423–430. doi: 10.1016/j.numecd.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Brema I, Hatunic M, Finucane F, et al. Plasma visfatin is reduced after aerobic exercise in early onset type 2 diabetes mellitus. Diab Obes Metab. 2008;10:600–602. doi: 10.1111/j.1463-1326.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 31.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 32.Hofso D, Ueland T, Hager H, et al. Inflammatory mediators in morbidly obese subjects: associations with glucose abnormalities and changes after oral glucose. Eur J Endocrinol. 2009;161:451–458. doi: 10.1530/EJE-09-0421. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diab. 2006;114:544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 34.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 37.de Kreutzenberg SV, Ceolotto G, Papparella I, et al. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orimo M, Minamino T, Miyauchi H, et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–894. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 39.Fain JN, Tagele BM, Cheema P, Madan AK, Tichansky DS. Release of 12 adipokines by adipose tissue, nonfat cells, and fat cells from obese women. Obes Silver Spring. 2010;18:890–896. doi: 10.1038/oby.2009.335. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 41.Zaldivar F, McMurray RG, Nemet D, Galassetti P, Mills PJ, Cooper DM. Body fat and circulating leucocytes in children. Int J Obes Lond. 2006;30:906–911. doi: 10.1038/sj.ijo.0803227. [DOI] [PubMed] [Google Scholar]
- 42.Seo JA, Jang ES, Kim BG, et al. Plasma visfatin levels are positively associated with circulating interleukin-6 in apparently healthy Korean women. Diab Res Clin Pract. 2008;79:108–111. doi: 10.1016/j.diabres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Mayi TH, Duhem C, Copin C, et al. Visfatin is induced by peroxisome proliferator-activated receptor gamma in human macrophages. FEBS J. 2010;277:3308–3320. doi: 10.1111/j.1742-4658.2010.07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skokowa J, Lan D, Thakur BK, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- 45.Lee KJ, Shin YA, Lee KY, Jun TW, Song W. Aerobic exercise training-induced decrease in plasma visfatin and insulin resistance in obese female adolescents. Int J Sport Nutr Exerc Metab. 2010;20:275–281. doi: 10.1123/ijsnem.20.4.275. [DOI] [PubMed] [Google Scholar]
- 46.von Kanel R, Mills PJ, Dimsdale JE. Short-term hyperglycemia induces lymphopenia and lymphocyte subset redistribution. Life Sci. 2001;69:255–262. doi: 10.1016/S0024-3205(01)01127-4. [DOI] [PubMed] [Google Scholar]
- 47.Kang YS, Song HK, Lee MH, Ko GJ, Cha DR. Plasma concentration of visfatin is a new surrogate marker of systemic inflammation in type 2 diabetic patients. Diab Res Clin Pract. 2010;89:141–149. doi: 10.1016/j.diabres.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Catalan V, Gomez-Ambrosi J, Rodriguez A et al (2011) Association of increased Visfatin/PBEF/NAMPT circulating concentrations and gene expression levels in peripheral blood cells with lipid metabolism and fatty liver in human morbid obesity. Nutr Metab Cardiovasc Dis. doi:10.1016/j.numecd.2009.09.008 [DOI] [PubMed]
- 49.Mesko B, Poliska S, Szegedi A, et al. Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med Genomics. 2010;3:15. doi: 10.1186/1755-8794-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cohort characteristics of the normal lean cohort (Leipzig Schoolchildren project) (PDF 94.5 kb)
Cohort characteristics of lean and obese children of the Leipzig Childhood Atherobesity cohort (PDF 80.0 kb)
Cohort characteristics of intervention cohorts (PDF 59.7 kb)
Association of NAMPT with development in healthy lean children. a Dynamics of NAMPT levels during pubertal development. Plots are median with interquartile range. b Comparison of NAMPT levels between boys and girls. Number of participants is indicated in parentheses. PH, pubic hair stage (PDF 4.44 kb)
Dynamics of NAMPT, WBC and hsCrP with intervention. The effect of intervention on BMI (a) WBC (b) (exercise: p = 0.052) and CrP (c) is given relative to basal levels before intervention (set at 1.0) as mean ± SEM. For bariatric surgery intervention, the correlation between decrease in BMI (d) (r = 0.70, p = 0.007), WBC (e) and hsCrP (f) (r = 0.72, p = 0.019) with the NAMPT decrease is shown. D, difference **p < 0.01 (PDF 11.4 kb)
Effect of glucose on leucocyte population and NAMPT release. a Dynamics of leucocyte and subpopulations counts after oral glucose provocation in n = 11 obese patients. Plots are mean ± SEM. Analysis was performed with ANOVA. White circles, lymphocytes (p = 0.192); black circles, monocytes (p = 0.023); white squares, neutrophils (p = 0.242); black squares, leucocytes (p = 0.123). b NAMPT release ex vivo from total leucocytes obtained from n = 4 during an OGTT. Leucocytes were isolated from peripheral blood at 0, 60 and 120 min after glucose provocation and were cultured for 45 min. NAMPT concentrations in the supernatant fractions were determined by ELISA. NAMPT was significantly reduced at 120 min (p = 0.001). c NAMPT release from monocytes and granulocytes in response to direct glucose provocation in vitro with 40 mmol/l d-glucose and 40 mmol/l l-glucose as osmotic control. *p < 0.05, **p < 0.01 (PDF 7.33 kb)
(PDF 68.3 kb)