Abstract
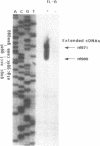
Transcription regulatory elements have been analyzed in upstream sequences of an Interleukin-6 (Il-6) primary response gene, MyD88. MyD88 2.3 kb mRNA is strongly and persistently induced in the course of myeloleukemic M1 cells differentiation with Il-6. MyD88 cDNA sequences were found in a region of 12 kb of mouse genomic DNA. Using Il-6 treated M1 cell RNAs, two transcription start sites have been localized, approximately 100 bp upstream from the 5' end of the cloned cDNA. We sequenced 1.4 kb of 5' genomic DNA including the first exon. In 5' of mRNA transcription start site, MyD88 nucleotidic sequence is 85% identical to 5' complementary sequences of the rat 3'-ketoacetyl CoA thiolase gene, over 1.2 kb. A DNA element conferring Il-6-inducible transcription to reporter genes, and localized 30 bp upstream of MyD88 first RNA start site, contains overlapping binding sites for cytokine activated transcription factors Stat and for the Interferon Regulatory Factor-1 and -2 (IRF-1 and IRF-2). In vitro binding assays showed that attachment of Stat factors to this element early in Il-6 treatment requires tyrosine kinase activation. IRF1, an activator of transcription, is also induced to bind to this sequence at later times. A model of persistent activation of MyD88 gene through these two types of factors is proposed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollahi A., Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991 Aug;2(8):401–407. [PubMed] [Google Scholar]
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Benech P., Vigneron M., Peretz D., Revel M., Chebath J. Interferon-responsive regulatory elements in the promoter of the human 2',5'-oligo(A) synthetase gene. Mol Cell Biol. 1987 Dec;7(12):4498–4504. doi: 10.1128/mcb.7.12.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Petsche D., Grunberger T., Freedman M. H. Interleukin 6 induces myeloid differentiation of a human biphenotypic leukemic cell line. Leuk Res. 1992 Aug;16(8):751–760. doi: 10.1016/0145-2126(92)90153-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Darnell J. E., Jr Two distinct alpha-interferon-dependent signal transduction pathways may contribute to activation of transcription of the guanylate-binding protein gene. Mol Cell Biol. 1991 Oct;11(10):5147–5153. doi: 10.1128/mcb.11.10.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Kimura Y., Miyamoto M., Barsoumian E. L., Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989 Jan 19;337(6204):270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- Gothelf Y., Raber J., Chen L., Schattner A., Chebath J., Revel M. Terminal differentiation of myeloleukemic M1 cells induced by IL-6: role of endogenous interferon. Lymphokine Cytokine Res. 1991 Oct;10(5):369–375. [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Kitagawa M., Tanaka N., Yamamoto H., Harada K., Ishihara M., Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993 Feb 12;259(5097):971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- Harroch S., Gothelf Y., Watanabe N., Revel M., Chebath J. Interleukin-6 activates and regulates transcription factors of the interferon regulatory factor family in M1 cells. J Biol Chem. 1993 Apr 25;268(12):9092–9097. [PubMed] [Google Scholar]
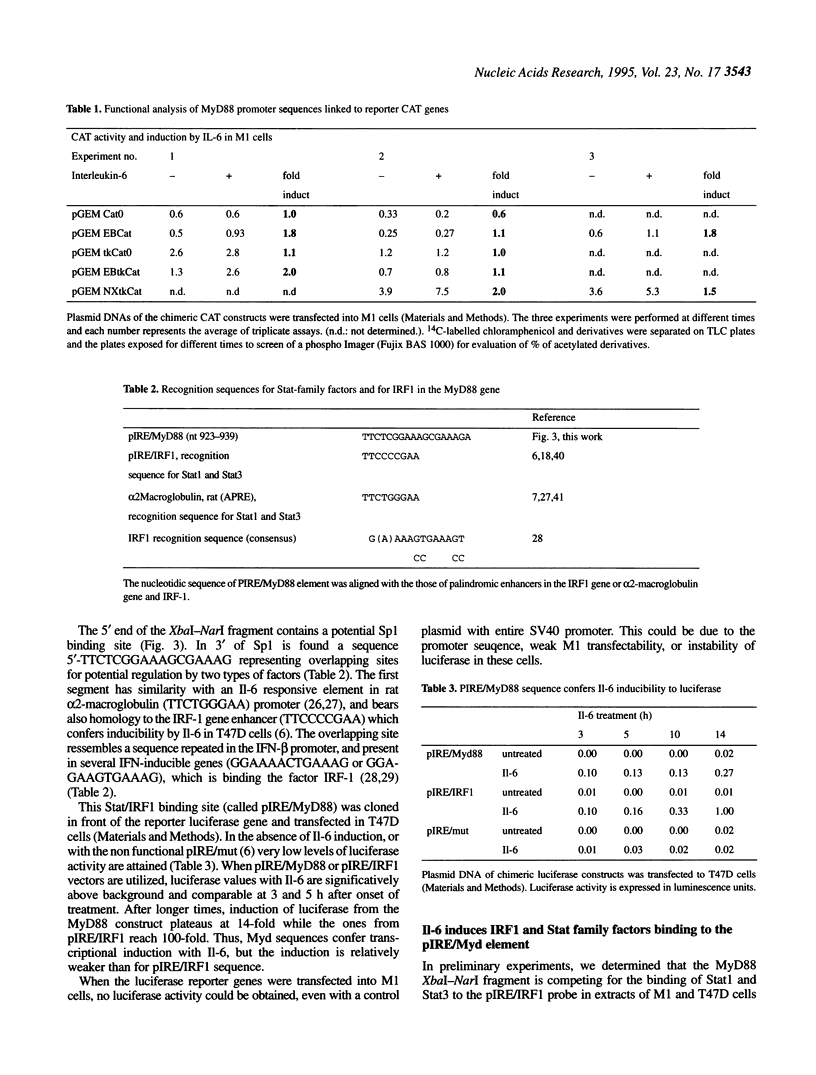
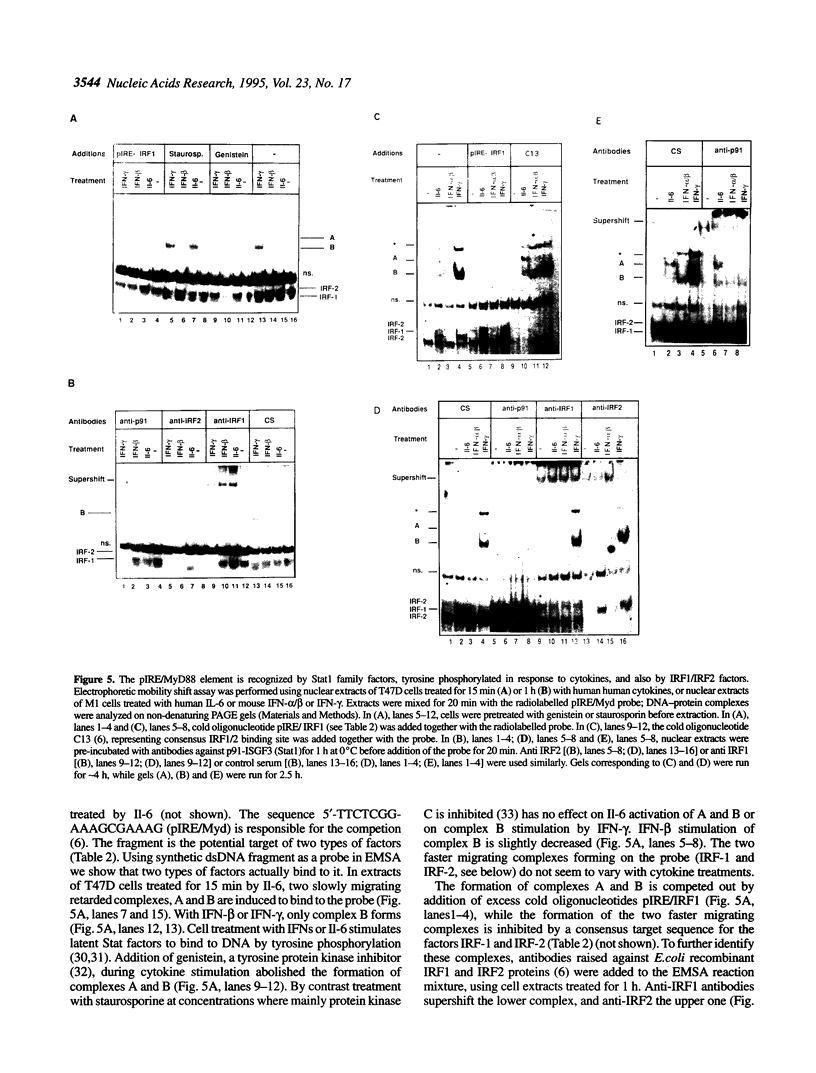
- Harroch S., Revel M., Chebath J. Induction by interleukin-6 of interferon regulatory factor 1 (IRF-1) gene expression through the palindromic interferon response element pIRE and cell type-dependent control of IRF-1 binding to DNA. EMBO J. 1994 Apr 15;13(8):1942–1949. doi: 10.1002/j.1460-2075.1994.tb06463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocke G. M., Barry D., Fey G. H. Synergistic action of interleukin-6 and glucocorticoids is mediated by the interleukin-6 response element of the rat alpha 2 macroglobulin gene. Mol Cell Biol. 1992 May;12(5):2282–2294. doi: 10.1128/mcb.12.5.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D. Macrophage differentiation marker MyD88 is a member of the Toll/IL-1 receptor family. Biochem Biophys Res Commun. 1994 Feb 28;199(1):144–146. doi: 10.1006/bbrc.1994.1206. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1969 Dec;74(3):223–234. doi: 10.1002/jcp.1040740303. [DOI] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Sugita T., Nishio Y., Hashimoto S., Pawlowski T., Suematsu S., Kishimoto T. Reciprocal expression of NF-IL6 and C/EBP in hepatocytes: possible involvement of NF-IL6 in acute phase protein gene expression. New Biol. 1991 Jan;3(1):63–70. [PubMed] [Google Scholar]
- Kanno Y., Kozak C. A., Schindler C., Driggers P. H., Ennist D. L., Gleason S. L., Darnell J. E., Jr, Ozato K. The genomic structure of the murine ICSBP gene reveals the presence of the gamma interferon-responsive element, to which an ISGF3 alpha subunit (or similar) molecule binds. Mol Cell Biol. 1993 Jul;13(7):3951–3963. doi: 10.1128/mcb.13.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Nakayama K., Penninger J., Kitagawa M., Harada H., Matsuyama T., Tanaka N., Kamijo R., Vilcek J., Mak T. W. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science. 1994 Jun 24;264(5167):1921–1924. doi: 10.1126/science.8009222. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Akira S., Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Taga T. IL-6 receptor and mechanism of signal transduction. Int J Immunopharmacol. 1992 Apr;14(3):431–438. doi: 10.1016/0192-0561(92)90173-i. [DOI] [PubMed] [Google Scholar]
- Kollmar R., Farnham P. J. Site-specific initiation of transcription by RNA polymerase II. Proc Soc Exp Biol Med. 1993 Jun;203(2):127–139. doi: 10.3181/00379727-203-43583. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K. A., Abdollahi A., Hoffman-Liebermann B., Liebermann D. A. Proto-oncogenes of the fos/jun family of transcription factors are positive regulators of myeloid differentiation. Mol Cell Biol. 1993 Feb;13(2):841–851. doi: 10.1128/mcb.13.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K. A., Abdollahi A., Thomas S. M., DeMarco M., Brugge J. S., Hoffman-Liebermann B., Liebermann D. A. Leukemia inhibitory factor and interleukin-6 trigger the same immediate early response, including tyrosine phosphorylation, upon induction of myeloid leukemia differentiation. Mol Cell Biol. 1991 Sep;11(9):4371–4379. doi: 10.1128/mcb.11.9.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Complexity of the immediate early response of myeloid cells to terminal differentiation and growth arrest includes ICAM-1, Jun-B and histone variants. Oncogene. 1990 Mar;5(3):387–396. [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990 Jul;5(7):1095–1097. [PubMed] [Google Scholar]
- Poli V., Mancini F. P., Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990 Nov 2;63(3):643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- Rio D. C. Splicing of pre-mRNA: mechanism, regulation and role in development. Curr Opin Genet Dev. 1993 Aug;3(4):574–584. doi: 10.1016/0959-437x(93)90093-5. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Sims S. H., Cha Y., Romine M. F., Gao P. Q., Gottlieb K., Deisseroth A. B. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol Cell Biol. 1993 Jan;13(1):690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T. Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991;201:340–347. doi: 10.1016/0076-6879(91)01030-6. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Kawakami T., Taniguchi T. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol Cell Biol. 1993 Aug;13(8):4531–4538. doi: 10.1128/mcb.13.8.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Taniguchi T. Cytokine gene regulation: regulatory cis-elements and DNA binding factors involved in the interferon system. Adv Immunol. 1992;52:263–281. doi: 10.1016/s0065-2776(08)60877-9. [DOI] [PubMed] [Google Scholar]
- Wegenka U. M., Buschmann J., Lütticken C., Heinrich P. C., Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993 Jan;13(1):276–288. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegenka U. M., Lütticken C., Buschmann J., Yuan J., Lottspeich F., Müller-Esterl W., Schindler C., Roeb E., Heinrich P. C., Horn F. The interleukin-6-activated acute-phase response factor is antigenically and functionally related to members of the signal transducer and activator of transcription (STAT) family. Mol Cell Biol. 1994 May;14(5):3186–3196. doi: 10.1128/mcb.14.5.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M., Merlie J. P., Sanes J. R. Interspecific comparisons reveal conserved features of the Drosophila Toll protein. Gene. 1994 Feb 25;139(2):223–228. doi: 10.1016/0378-1119(94)90760-9. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]