Abstract
In this study, atomic force microscopy-based dynamic oscillatory and force-relaxation indentation was employed to quantify the time-dependent nanomechanics of native (untreated) and proteoglycan (PG)-depleted cartilage disks, including indentation modulus Eind, force-relaxation time constant τ, magnitude of dynamic complex modulus |E∗|, phase angle δ between force and indentation depth, storage modulus E′, and loss modulus E″. At ∼2 nm dynamic deformation amplitude, |E∗| increased significantly with frequency from 0.22 ± 0.02 MPa (1 Hz) to 0.77 ± 0.10 MPa (316 Hz), accompanied by an increase in δ (energy dissipation). At this length scale, the energy dissipation mechanisms were deconvoluted: the dynamic frequency dependence was primarily governed by the fluid-flow-induced poroelasticity, whereas the long-time force relaxation reflected flow-independent viscoelasticity. After PG depletion, the change in the frequency response of |E∗| and δ was consistent with an increase in cartilage local hydraulic permeability. Although untreated disks showed only slight dynamic amplitude-dependent behavior, PG-depleted disks showed great amplitude-enhanced energy dissipation, possibly due to additional viscoelastic mechanisms. Hence, in addition to functioning as a primary determinant of cartilage compressive stiffness and hydraulic permeability, the presence of aggrecan minimized the amplitude dependence of |E∗| at nanometer-scale deformation.
Introduction
Cartilage exhibits time-dependent mechanical behavior that has been documented macroscopically (1–9) and microscopically (10,11) in a variety of loading configurations. These time-dependent properties are essential for proper tissue function, energy dissipation, frequency-dependent stiffening, and reswelling after joint unloading (12). The observed time dependence has been interpreted using continuum theoretical models that include both poroelasticity and viscoelasticity (13–16). The biomechanical behavior of cartilage is related to the tissue's type II collagen network and the negatively charged aggrecan aggregates within the hydrated extracellular matrix (ECM) (12). Viscoelastic relaxation of the solid matrix involves the translational diffusion and reptation of aggrecan aggregate, aggrecan monomers, and glycosaminoglycan (GAG) side chains of aggrecan (17), as well as reconfiguration of the collagen network and associated proteins. Poroelastic energy dissipation is governed by the fluid flow through the porous ECM.
In hydrated cartilage tissue, the collagen fibrils are typically 30–80 nm in diameter, and the gaps between them are on the order of 100 nm (12,18). Between the fibrils, brush-like aggrecan monomers bind to hyaluronan, stabilized by link protein, to form aggregates (19). The aggrecan core protein has a contour length of ∼400 nm, and ∼40 nm-long GAG chains are substituted onto the core protein ∼2–3 nm apart (20). The high density of GAG chains has been identified as the main determinant of the tissue's hydraulic permability and, thereby, as an important constituent regulating cartilage poroelastic behavior (21). Deformation that takes place at the length scale of these molecules can affect local fixed charge density (22), hydraulic permeability (23), streaming potential (24), and other biophysical mediators of chondrocyte-cell signaling and mechanotransduction (25). The nanomechanics of isolated ECM macromolecules such as type II collagen (26), GAGs (27,28) and aggrecan (29–33) have been studied in vitro. However, there is still a lack of understanding of the ECM mechanical behavior within native tissue subjected to local deformations on the same length scale as macromolecular dimensions.
Hence, the objective of this study was to quantify the time- and frequency-dependent mechanics of native cartilage at deformation length scales comparable to its ECM molecular dimensions and the fluid flow pore sizes, which provides direct molecular-level insights into how these ECM constituents contribute to the tissue-level cartilage behavior. In this study, atomic force microscopy (AFM)-based techniques were employed to carry out sinusoidal dynamic oscillatory indentation of bovine cartilage disks as a function of oscillation frequency f (∼1–316 Hz), using previously reported methods (34,35). The results were compared to data obtained on the same cartilage specimens using classical nanoindentation and force-relaxation loading configurations. The contact radius between the AFM probe tips and the cartilage tissue samples (∼1 μm) corresponds to the length scale over which fluid-flow-related poroelastic phenomena can occur (i.e., a homogenized microscale region of the ECM). However, the dynamic deformation amplitudes utilized were in the range of ∼2–50 nm, a length scale as small as the GAG-GAG interstitial spacing (12,20,36) that encompasses the range of estimated pore sizes (21) that govern tissue hydraulic permeability, k, and, correspondingly, the degree of poroelastic fluid flow. This length scale also corresponds to the dimensions of the ECM macromolecular components (12,18–20) that contribute to elastic and viscoelastic molecular interactions. The contributions of poro- and viscoelasticity to the observed cartilage time dependence were investigated by relating the results to the associated length- and timescales of these mechanisms. By comparing the dynamic nanomechanical properties of native (untreated) and proteoglycan (PG)-depleted cartilage disks, the observed time and frequency dependence were interpreted in the context of the macromolecular structure and properties of the ECM constituents (e.g., collagen and aggrecan).
Methods
Sample preparation
Cartilage disks (9 mm in diameter and ∼0.5 mm thick) were harvested (37) from the middle zone of femoropatellar groove articular cartilage of 1- to 2-week-old bovine calves and maintained in sterile phosphate-buffered saline (PBS, without Mg2+, Ca2+) with protease inhibitors for <24 h before testing. Additional groups of disks were digested with 1 mg/mL trypsin in 0.15 M NaCl and 0.05 M Na3PO4, pH ∼7.2 (Sigma-Aldrich, St. Louis, MO), followed by digestion with 0.1 U/mL chondroitinase-ABC in 0.15 M NaCl and 0.05 M Tris-HCl, pH ∼8.0 (Seikagaku, Tokyo, Japan), both for 24 h at 37°C. Together, these enzyme treatments resulted in loss of >95% of matrix aggrecan, the most abundant PG in cartilage (38), as assessed by the GAG content via the dimethylmethyleneblue dye binding assay (39). It has been reported that the trypsin digestion does not produce any significant structural change in the collagen fibrillar network, either microscopically (40) or macroscopically (41), or the intrinsic tensile strength of collagen fibrils (42).
AFM-based nanomechanics experiments
Cantilever probe tips
Square pyramidal probe tips were employed with an end radius of R ∼ 50 nm, half-open angle of the pyramidal face of α ∼ 35°, nickel-plated tip D, silicon nitride V-shaped cantilever, and nominal spring constant, k, ∼0.58 N/m (Veeco, Santa Barbara, CA), as well as spherical borosilicate colloidal probe tips (R ∼ 2.5 μm and k ∼ 0.58 N/m, Bioforce Nanosciences, Ames, IA) (Fig. 1 a). Both probe tips were coated with 2 nm Cr and 50 nm Au, and functionalized with a neutral hydroxyl-terminated self-assembled monolayer (OH-SAM) by immersion in 3 mM 11-mercaptoundecanol (HS(CH2)11OH) (Sigma-Aldrich) ethanol solution for 24 h.
Figure 1.
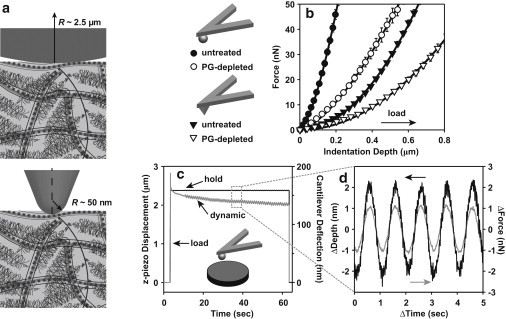
(a) Schematic of AFM-based nanoindentation of cartilage and its extracellular matrix components via spherical and pyramidal tips, adapted from Loparic et al. (56). (b) Typical force-indentation depth curves taken from a single location on untreated and PG-depleted cartilage disks using hydroxyl-functionalized spherical (R ∼ 2.5 μm) and pyramidal (R ∼ 50 nm, half-open angle of the pyramidal face α ∼ 35°) probe tips at 10 μm/s z-piezo displacement rate (mean ± SD of 10 repeats at each location to show the repeatability of the indentation at the same location). Data were analyzed using the effective contact point determination algorithm in Appendix A in the Supporting Material, and are shown with corresponding analytical model fits based on Eqs. 1 and 2 (solid lines). The number of data points shown here was reduced to increase clarity. (c) z-Piezo displacement and cantilever deflection data versus time during a typical force-relaxation experiment (∼2.3 μm z-piezo displacement applied at 10 μm/s, followed by 60-s hold at constant z-piezo displacement); a sinusoidal z-piezo displacement (1 Hz, ∼4 nm amplitude) was then initiated after ∼15 s of the holding period, superimposed onto the static displacement. (d) Sinusoidal steady-state dynamic force and indentation depth for five cycles. All the data from c and d correspond to those using an untreated cartilage disk with a hydroxyl-functionalized neutral spherical probe tip (R ∼ 2.5 μm) in PBS.
Classical nanoindentation
AFM-based classical nanoindentation were carried out in PBS using a maximum load of ∼70 nN in force mode, using a MultiMode AFM with a PicoForce piezo and Nanoscope IV controller (Veeco) over a range of constant z-piezo displacement rates (0.1–10 μm/s), where the closed-looped PicoFoce piezo in the z-direction (perpendicular to the sample plane) enabled the precise control and quantification of indentation force and depth. For each indentation curve, the cantilever deflection (in Volts) and z-piezo displacement (in μm) were converted and averaged to quantify the indentation force-depth data (see Appendix A in the Supporting Material). Three cartilage disk specimens were tested for each treatment condition (i.e., PG-depleted and untreated); separate groups of three disks each were used for testing with spherical and pyramidal probe tips. For each disk specimen, indentation was performed at two to three different locations on relatively flat regions (surface roughness <40 nm for 5 μm × 5 μm scans). At each location, indentation was repeated 10 times at 1 and 10 μm/s z-piezo displacement rate, and five times at 0.1 μm/s, with enough wait time between each repeat.
Force relaxation
Force-relaxation experiments were carried out at the same locations as the classical nanoindentation experiments via a number of programmed sequences as follows. 1), First, the sample was indented using the closed-loop force mode at a constant z-piezo displacement of ∼2 μm at 1 μm/s up to a maximum load of ∼85 nN, as described above. 2), The system was then changed to the open-loop script mode, in which the cantilever probe tip was programmed to move toward and into the surface at the same z-piezo displacement size and rate, which ensured that the maximum indentation force was kept constant at ∼85 nN for each test. 3), Immediately after indentation, the piezo was held at a constant position for 60 s before retracting from the specimen, during which period relaxation of indentation force took place. The script mode thus recorded the data for z-piezo displacement and cantilever deflection as a function of time during the indentation and hold period (steps 2 and 3) at a data acquisition rate of 1.02 kHz. At least three repeats were carried out at the same indentation location on each sample, with the time between each repeat sufficient to allow complete recovery.
Dynamic oscillatory loading
After each force-relaxation experiment, a dynamic oscillatory loading experiment was also carried out at the same specimen locations. A function generator (5100, Rockland Scientific, Victoria, British Columbia, Canada) was connected to the PicoForce piezo controller to enable sinusoidal displacement of the z-piezo at specified dynamic oscillation frequencies, f (f = 1–316 Hz), and z-piezo displacement amplitudes of ∼4, 25, and 125 nm. By subtracting the dynamic oscillation of cantilever deflection (in nm), the dynamic indentation depth amplitudes of cartilage were measured to be ∼2, 10, and 50 nm, respectively (see Appendix B in the Supporting Material). Prior to each specified frequency, f, and dynamic z-piezo displacement amplitude, a force-relaxation experiment was first carried out, as described above, and the dynamic oscillation was then superimposed onto the static indentation (Fig. 1 c) during the 60-s hold period at ∼15 s after the indentation (step 2 of the force-relaxation experiment). The same z-piezo displacement, cantilever deflection, and time data were acquired at rates of 1.02 kHz for f < 100 Hz and 11.74 kHz for f ≥ 100 Hz (Fig. 1 c). The offset static indentation depths were at 0.43 ± 0.04 μm and 1.01 ± 0.06 μm for the spherical and pyramidal tips on the untreated disks, respectively, and 0.77 ± 0.04 μm and 1.37 ± 0.12 μm (mean ± SE) for the spherical and pyramidal tips on the PG-depleted disks, respectively. Before the experiments on cartilage, control experiments were performed using a mica sample at the same z-piezo displacement frequencies and amplitudes for system calibration (see Appendix B in the Supporting Material).
Data analysis and modulus calculation
For AFM-based nanoindentation, the tip-sample contact point for each force-depth approach curve was determined via the Golden Section search method (43) (Appendix A in the Supporting Material). The effective indentation modulus, , was computed using analytical contact mechanics models via least-squares linear regression of the experimental loading F-D curves taken at the same sample position and z-piezo displacement rate. For the spherical colloidal probe tip with end radius R on the cartilage sample with thickness h >> R (here, h ∼ 0.5 mm, R ∼ 2.5 μm),
| (1) |
where F is the indentation force, D is the indentation depth (Appendix A in the Supporting Material), ν is the Poisson's ratio (ν = 0.1 for both the untreated and PG-depleted bovine cartilage disks (7,44)). For the sharp, pyramidal probe tip (here, R ∼ 50 nm << D) (45),
| (2) |
where α is the half-open angle of the pyramidal face (α = 35°, as measured with a scanning electron microscope (6060, JEOL, Peabody, MA). The analytical models (Eqs. 1 and 2) were used to account for the indentation geometry. It should be noted that is actually time- and rate-dependent (nonequilibrium), and hence, is utilized to assess trends in cartilage mechanical response to different experimental conditions (e.g., tip geometry and PG depletion) at a given indentation rate.
For each force-relaxation experiment, the loading data were first analyzed as described above to calculate the indentation depth, D. During the subsequent 60-s hold period, the z-piezo position (the sum of the cantilever deflection, d, and indentation depth, D) was held constant. The cantilever deflection decreased over time due to force relaxation, and the indentation depth increased subsequently to a small extent (∼5–7%). The time-dependent instantaneous indentation modulus, (t), was calculated via the analytical models (Eqs. 1 and 2) to account for the geometry and the change in both F and, to a much smaller extent, D during the hold period (Fig. 2 b). The equilibrium indentation modulus (after completion of relaxation), Eind,0, and the longest relaxation time constant, τ, were computed via a least squares nonlinear regression based on a three-element spring-dashpot model,
| (3) |
For the nanometer-scale dynamic oscillatory loading experiments, the amplitudes of sinusoidal force, , and indentation depth, , and the phase lag, δ, between them, were calculated at randomly selected intervals starting at ∼15 s after the initiation of dynamic z-piezo displacement. During this period, the dynamic oscillatory deformation was observed to be at steady state as , , and δ remained statistically constant over time. The calculation utilized five loading cycles at each frequency (e.g., Fig. 1, c and d; Appendix B in the Supporting Material). Within the dynamic oscillation region of Fig. 1 c, this calculation procedure was repeated 100 times, and the mean values of and amplitudes and phase angle δ were obtained. Thus, the total force, F, including static and dynamic components, can be described using the Hertz model with a Taylor series expansion in the indentation depth, D (46),
| (4) |
where |E∗| is the magnitude of the complex dynamic modulus. Higher-order terms in the expansion were neglected, since the dynamic amplitude, , is at least one to two orders of magnitude smaller than the offset indentation depth, D. Hence, |E∗| was calculated from the oscillatory component,
| (5) |
The same criteria were applied to the data obtained using the pyramidal tip:
| (6) |
We noted that the complex modulus E∗ can be represented in terms of its real part (the storage modulus, E′) and its imaginary part (the loss modulus, E″):
| (7) |
| (8) |
Figure 2.
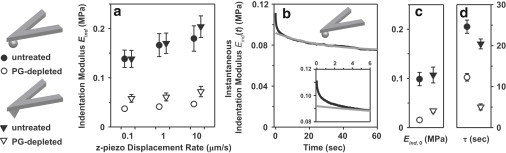
(a) Indentation modulus, Eind, of untreated and PG-depleted cartilage disks at z-piezo displacement rates of 0.1–10 μm/s, estimated using Eqs. 1 and 2. (b) Instantaneous indentation modulus, Eind(t), directly calculated, using the Hertz model (Eq. 1), from the relaxation data of a single force-relaxation test on an untreated cartilage disk (hydroxyl-functionalized neutral spherical tip, R ∼ 2.5 μm). The gray line is the best fit of a three-element model (Eq. 3) to the data. (c and d) Equilibrium indentation modulus, Eind,0 (c), and characteristic relaxation time constant, τ (d), of untreated and PG-depleted cartilage disks, estimated using Eqs. 1–3. All experiments in a, c, and d were performed using hydroxyl-functionalized spherical and pyramidal probe tips (n ≥ 6 different locations, mean ± SE).
Statistical analysis
To account for multiple measurements at the same indentation locations and to improve the robustness and power of the statistical test, we employed repeated-measures nonparametric statistical tests, which do not assume normal distribution or homoscedasticity of the data (47). Repeated measures analysis of variance (RM-ANOVA) on rank transforms (RT-1) of the entire set of original data and the Friedman test (nonparametric equivalent of one-way RM-ANOVA) were performed on the calculated dynamic modulus |E∗|, phase lag δ, storage modulus E′, loss modulus E″, and effective indentation modulus . The dynamic deformation frequency, f, amplitude, , and z-piezo displacement rate were taken as within-subject factors, whereas tip geometry and PG depletion were taken as between-subject factors. The Mann-Whitney U test (nonparametric equivalent of Student's t-test) was performed on the equilibrium modulus, Eind,0, and relaxation time constant, τ. In all the tests, a p-value of <0.05 was taken as statistically significant. For all statistical tests, data measured from different disks for the same experimental condition (e.g., enzymatic treatment, tip geometry) were pooled, as no significant difference (p > 0.05 for , ,0, τ, |E∗|, and δ) was found between the specimens using a two-way RM-ANOVA on RT-1 for |E∗|, δ, and (the specimen number was taken as the between-subject factor, frequency f as the within-subject factor), and Mann-Whitney U test for ,0 and τ.
Results
Classical indentation
In the applied indentation force range (∼0–70 nN), both the untreated and PG-depleted cartilage indentation behavior agreed well with the analytical model fits of Eqs. 1 and 2 (Fig. 1 b; R2 > 0.98 for all fits). Upon PG depletion, the cartilage samples exhibited increased compliance compared to the untreated samples with both spherical and pyramidal tips (Fig. 1 b). increased significantly with increasing z-piezo displacement rate (0.1–10 μm/s) for both untreated and PG-depleted disks, using both spherical and pyramidal tips (Fig. 2 a, Friedman test, p < 0.01 for all tests). significantly decreased by ∼70–80% after PG depletion (Fig. 2 a). A marginally significant tip geometry dependence was observed for the PG-depleted disks, whereas no significant tip geometry difference for the untreated disks (two-way RM-ANOVA on RT-1, z-piezo displacement rate as the only within-subject factor, p < 0.001 for untreated versus PG-depleted disks, p = 0.0493 for the tip geometry on PG-depleted disks).
Force relaxation
Fig. 2 b shows a typical individual force-relaxation experiment expressed in terms of the instantaneous indentation modulus, (t), versus time for the untreated cartilage disk, showing a stiffness relaxation from 0.11 MPa to 0.06 MPa during the 60-s hold period. The three-element model (Eq. 3) fit the observed force-relaxation data well for t > 5 s after the initiation of relaxation, but not for t ≤ 5 s (Fig. 2 b, overall R2 > 0.95 for all experimental data). ,0 for the untreated cartilage was 0.10 ± 0.01 MPa (mean ± SE, n ≥ 6 locations) using the spherical tip, and similar results were found using the pyramidal tip (Fig. 2 c, Mann-Whitney U test, p > 0.05). For the PG-treated cartilage, Eind,0 for the pyramidal tip was marginally greater than that for the spherical tip (Mann-Whitney U test, p = 0.0411) (Fig. 2 c). For both probe tips, PG depletion significantly decreased Eind,0 by 70–80% (Mann-Whitney U test, p = 0.0022 for both tips). In a similar way, tip geometry significantly affected τ for the PG-depleted disk, with ∼65% faster relaxation observed using the pyramidal tip, but not for the untreated disk (Fig. 2 d, Mann-Whitney U test, p < 0.01 for PG-depleted disks, p > 0.05 for untreated disks).
Dynamic oscillatory loading
For the dynamic oscillatory experiments, |E∗|, δ, E′, and E″ increased nonlinearly with frequency f in the range of 1–316 Hz for both untreated and PG-depleted cartilage disks, using both spherical and pyramidal tips (Fig. 3), and at all tested deformation amplitudes (data not shown, Friedman test, p < 0.001 for all cases). PG depletion significantly reduced |E∗|, E′, and E″, using both spherical and pyramidal tips (two-way RM-ANOVA on RT-1, f as the within-subject factor, p < 0.05 for all data sets). At dynamic deformation amplitude ∼ 2 nm, |E∗| was found to be linearly dependent on f1/2 for both untreated and PG-depleted disks using both probe tips (Fig. 4). |E∗|, E′ decreased significantly, and E″ increased significantly with increasing dynamic deformation amplitude, , for PG-depleted disks using both probe tips and for untreated disks using the pyramidal tip (two-way RM-ANOVA on RT-1, f and as within-subject factors, p < 0.01), as shown in Fig. 5, a, c, and d. For the untreated disk measured with the spherical tip, the change in |E∗|, E′, and E″ with amplitude, , was only marginally significant (p = 0.0442 for |E∗|, p = 0.0637 for E′, p = 0.0393 for E″).
Figure 3.
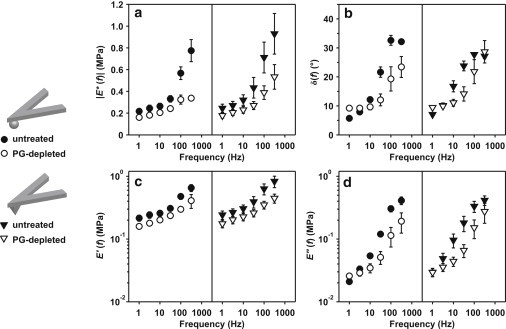
Dynamic mechanical properties |E∗(f)| (a), δ(f) (b), E′(f) (c), and E″(f) (d) versus frequency for untreated and PG-depleted cartilage disks in PBS, using hydroxyl-functionalized spherical (R ∼ 2.5 μm) and pyramidal (R ∼ 50 nm, α ∼ 35°) probe tips at ∼4 nm z-piezo displacement amplitude (∼2 nm cartilage dynamic indentation depth amplitude), estimated using Eqs. 5–8 for n ≥ 6 different locations (mean ± SE).
Figure 4.
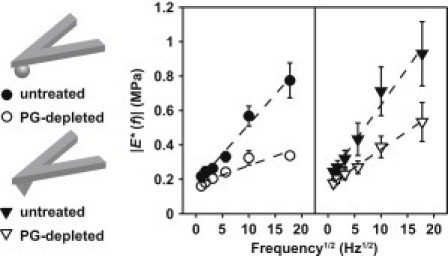
Poroelastic-like frequency dependence of |E∗(f)| (n ≥ 6, mean ± SE) on f1/2 for data obtained using both spherical and pyramidal probe tips, where dashed lines represent least square linear regression between the mean values of |E∗(f)| at each frequency and f1/2, R2 > 0.88.
Figure 5.
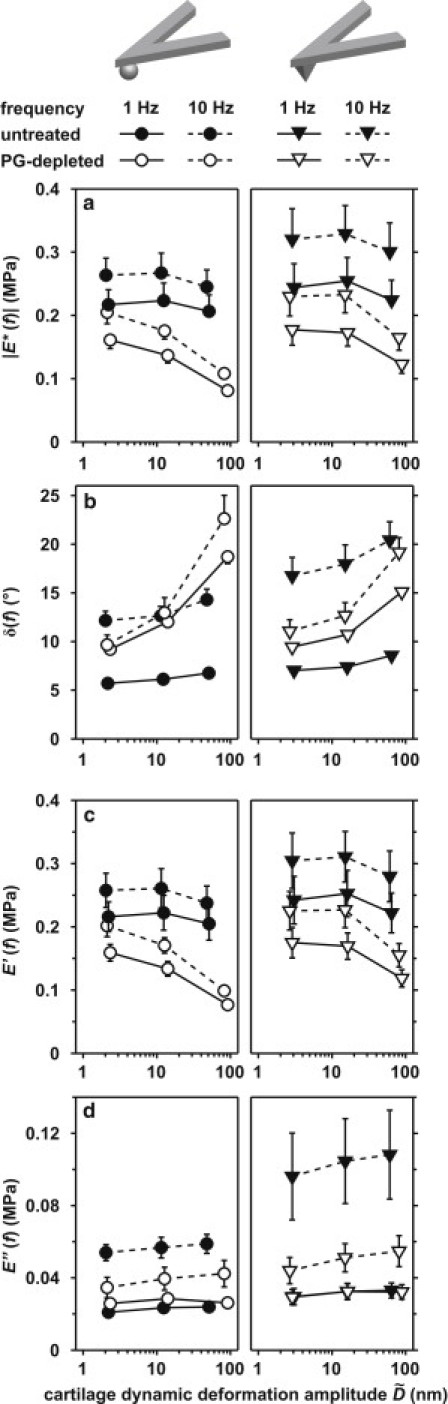
Dynamic mechanical properties |E∗(f)| (a), δ(f) (b), E′(f) (c), and E″(f) (d) as a function of cartilage dynamic deformation amplitude (∼2–50 nm) at 1- and 10-Hz deformation frequencies for untreated and PG-depleted cartilage disks in PBS, using hydroxyl-functionalized spherical (R ∼ 2.5 μm) and pyramidal (R ∼ 50 nm, α ∼ 35°) probe tips, estimated using Eqs. 5–8 for n ≥ 6 different locations (mean ± SE). The amplitude dependence was similar at 100-Hz frequency for both untreated and PG-depleted disks with both probe tips (not shown, for better clarity).
In addition, the relative percentage drop in |E∗|, E′, and E″ at higher with respect to the lowest (∼2 nm) was compared for untreated and PG-depleted disks at the tested frequencies (Fig. 5) using two-way RM-ANOVA on the RT-1 of the percentage drops, where f was taken as the within-subject factor, and PG depletion as the between-subject factor. For both spherical and pyramidal tips, PG-depleted disks showed a significantly larger percentage drop in |E∗| and E′ (but not E″) at higher (p < 0.001). When was increased from ∼2 nm to ∼50 nm, |E∗| of untreated disks decreased by only ∼3.4–4.6%, but by ∼25–48% for PG-depleted disks.
Discussion
Mechanistic origins of cartilage time-dependent nanomechanics
To assess the mechanistic origins of the observed time- and frequency-dependent dynamic nanomechanical properties, the characteristic poroelastic relaxation frequency, fp ∼ [(Hk)/Lp2], for the untreated cartilage was estimated to be ∼130 Hz (14). In this calculation, Lp, the characteristic length scale over which fluid flows, was approximated as equivalent to the linear dimension of the tip-sample contact zone: μm for the spherical tip and = 1.2 μm for the pyramidal tip. H is the equilibrium cartilage modulus, ∼0.1 MPa, calculated from the indentation and force-relaxation experiments (Fig. 2, a and c). The hydraulic permeability, k, of 1- to 2-week-old bovine femoropatellar groove cartilage was previously reported to be ∼3 × 10−15 m4 N−1·s−1 based on macroscopic (uniaxial confined compression) tests (15). To estimate fp for the experiments presented here, we first assumed that the same value of k is applicable at microscale dimensions of fluid flow, Lp, since k is dominated in general by GAG-associated pore sizes (21). The resulting fp is ∼130 Hz (for the spherical tip, ∼210 Hz for the pyramidal tip), which is the same order as the highest tested frequency values (100–316 Hz) at which the maximum in energy dissipation was observed (Fig. 3 b). |E∗| for the untreated cartilage disks was found to increase linearly with f1/2 (Fig. 4), consistent with the expected behavior for poroelastic deformation of cartilage in the frequency range f < fp (2,15,48), but not for viscoelastic deformation (5,49). In addition, the weak amplitude dependence of |E∗| and δ suggests that poroelasticity also dominates the observed energy dissipation mechanisms even at higher dynamic amplitudes (Fig. 5, a and b), as discussed in more detail below. Intrinsic viscoelastic behavior is expected to be associated with the local rearrangement of aggrecan/GAG and breakage/recombination of aggrecan adhesive interactions (32), and potential reconfiguration of collagen fibrils. Hence, because these possible deformation mechanisms take place at length scales much greater than the dynamic deformation amplitude, (17), they are expected to make a minimal contribution to the measured frequency dependence at such small deformation amplitudes. In addition, the known viscoelastic behavior of cartilage disks measured via torsional shear (in the absence of poroelastic behavior) exhibits a maximum of δ < 14° at larger macroscopic deformation scales (5), which is much smaller than the observed δ of Fig. 3 b at frequencies near fp. Furthermore, the frequency dependence of |E∗| and δ at higher deformation amplitudes, , remains consistent (Fig. 5), supporting the hypothesis that fluid flow tangential to the direction of dynamic oscillation dominates energy dissipation in this regime, rather than viscoelastic processes, which are most likely dominated by axially directed deformations. Hence, these results suggest that even at fluid-flow length scales much smaller than macroscopic load configurations, and deformation amplitudes on the scale of molecular dimensions, fluid-flow-related poroelasticity dominates energy dissipation in the f = 1–316 Hz frequency range. It is interesting that recent studies have also revealed that poroelasticity is the dominant mechanism governing the frequency-dependent behavior of newly synthesized cell-associated matrix surrounding individual chondrocytes, removed from 1- to 4-week alginate culture, and subjected to nanometer-scale dynamic oscillatory loading (35).
Since the force-relaxation behavior of untreated cartilage in Fig. 2, b and c, corresponds to t >> (characteristic poroelastic relaxation time, ms) and indentation depth ∼430 nm, it is hypothesized that the time dependence is dominated by the intrinsic viscoelastic behavior of the ECM. The fact that a single relaxation time constant could not fit the relaxation behavior of Fig. 2 b for intermediate time (1 < t < 5 s) suggests the presence of multiple viscoelastic relaxation mechanisms that may be associated with hierarchical structures of cartilage ECM at nano- and microscales (12,17). This viscoelastic behavior could be due to rearrangement of aggrecan/GAGs, adhesive interactions between aggrecan (32), reptation of aggrecan monomers and aggregates (17), and relaxation of interconnected collagen fibrils. At short times (t < 1 s), the force relaxation could be a combined effect of both poro- and viscoelastic mechanisms.
This time-dependent behavior of cartilage was also reflected in classical nanoindentation tests using the same probe tips. A single indentation test took place over ∼0.05 to 10 s, corresponding to z-piezo displacement rates of 10 to 0.1 μm/s, respectively. Hence, based on the above discussion, the observed rate dependence of Eind (Fig. 2 a) was likely due to the combined effects of poro- and viscoelasticity. The values of Eind are therefore effective responses of cartilage at given indentation rates, resulting from a combination of cartilage mechanical properties that affect its time dependence (e.g., Eind,0, τ, k). Because both these deformation mechanisms could occur during the indentation test, the contribution of each mechanism could not be individually quantified using only F-D indentation curves. Therefore, although classical nanoindentation results did show significant tissue time dependence (Fig. 2 a), the dynamic oscillatory indentation combined with force-relaxation tests are advantageous for direct probing of the time- and frequency-dependent behavior of cartilage.
Role of aggrecan in cartilage nanomechanics
Upon aggrecan depletion, ∼60–70% reduction in cartilage indentation modulus was observed in both microscale indentation (Fig. 2) and nanoscale dynamic (Figs. 3–5) deformation experiments. These results are consistent with previous tissue level studies showing that aggrecan accounts for >50% of cartilage macroscopic compressive modulus (12,50). Aggrecan is also the major determinant of cartilage tissue permeability, k (12). This is consistent with the increased fp (∼1 kHz) caused by depletion of aggrecan in the dynamic loading experiment (Fig. 3), in which the substantial increase in k outweighs the decrease in H as aggrecan is depleted. In addition, the presence of aggrecan increased the longest relaxation time τ in Fig. 2 d, likely associated with the constraints of aggrecan to the relaxation of the collagen network (τ ∼ 15 s for the PG-depleted disks versus 25 s for the untreated disks measured via the spherical tip).
Increasing the dynamic amplitude, , from ∼2 nm to ∼50 nm resulted in a significantly greater decrease in |E∗| for PG-depleted disks compared to untreated disks (Fig. 5). At the higher , which is comparable to collagen fibril diameters and interfibrillar distances (∼100 nm), collagen fibrils may undergo increased molecular mobility in the absence of constraints by aggrecan. In a similar way, it is hypothesized that strain softening at the tissue level, observed at large enough deformations, is due to the local release of tension and buckling of the collagen fibrils at deformations that overcome the osmotic swelling pressure of aggrecan (51–53). As buckling would mainly affect elastic deformations, the higher deformation amplitudes, , of Fig. 5 resulted in a significant drop in the elastic storage modulus, E′. At the same time, the increase in E″ and δ at higher could be related to the viscoelastic energy dissipation due to molecular friction between the buckling collagen fibrils in contact. On the other hand, in the presence of aggrecan, this amplitude-induced viscoelasticity effect was minimized (Fig. 5), as the GAG-GAG electrostatic repulsion can largely reduce the contacts between the ECM macromolecular components (32) and, hence, the viscoelastic energy dissipation associated with macromolecular frictions. As a result, poroelasticity remains the dominating energy dissipation mechanism for the untreated cartilage when increasing the dynamic deformation amplitudes, (Fig. 5).
Dynamic loading experiments using both spherical and pyramidal probe tips were performed using similar deformation amplitudes and contact areas, and no significant geometry dependence of |E∗| was observed for untreated or PG-depleted disk (Figs. 3–5). In contrast, at larger deformation amplitudes in the indentation force-relaxation experiments, τ and ,0 exhibited the geometry dependence for PG-depleted but not untreated disks, due to the different stress fields associated with probe tip geometry (Fig. 2, c and d). Similar to the effect on the dynamic amplitude dependence, in the presence of aggrecan and GAG-GAG electrostatic repulsion, cartilage exhibited more stable and uniform mechanical properties in response to different stress fields.
Comparison to tissue level cartilage dynamic properties
The frequency dependence of cartilage mechanical properties has been probed at the tissue level via dynamic compression in both confined (1,54,55) and unconfined (2,22) compression geometries. The frequency dependence of dynamic modulus and phase angle were well predicted by poroelastic theoretical models (2,15). In this study, fluid flow occurred within cartilage disks mounted between the fluid-impermeable substrate and the fluid-impermeable probe tip in an axisymmetric profile caused by vertical oscillation of the tip. Hence, we compared the observed frequency dependence with that reported for uniaxial unconfined compression of similar bovine calf cartilage disks between fluid-impermeable platens (2). In Fig. 6, the phase lag of the untreated disks measured using the spherical probe tip is plotted as a function of frequency normalized by fp ∼ Hk/Lp2, assuming Lp ∼ 1.5 μm, H ∼ 0.1 MPa, and k ∼ 3 × 10−15 m4 N−1 s−1, as discussed previously. Also shown in Fig. 6 is the previously reported phase lag of the dynamic modulus of one cartilage disk, 6.4 mm in diameter and 1 mm thick, tested in uniaxial unconfined compression (2). The macroscopic tests (2) focused on frequencies f > fp, since fp for the millimeter-sized disks in unconfined compression was ∼0.001 Hz (for the given disk geometry), and tests at lower frequencies were not possible. In this study, the frequency range was confined to the region f ≤ fp (∼100–300 Hz for the current geometry) due to AFM instrumentation constraints, which prevented measurements at frequencies >∼300 Hz. Overall, the normalized frequency dependence of the phase lag in Fig. 6 is qualitatively consistent with the poroelastic behavior that would be expected over the complete frequency range () in uniaxial unconfined compression as well as the nanoscale dynamic compression geometry in Fig. 1 a (2). In contrast to poroelasticity, the length scale dependence was much less remarkable for cartilage intrinsic viscoelasticity, as macroscale stress relaxation experiments via unconfined compression (3) resulted in viscoelastic relaxation time constants very similar to those reported by AFM-based nanoindentation (Fig. 2 d).
Figure 6.
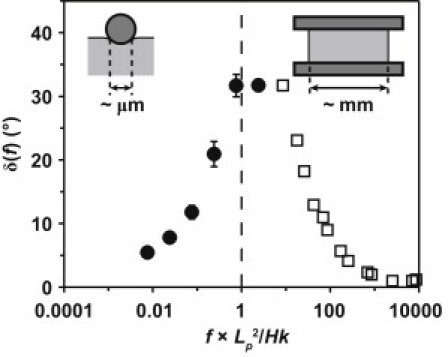
Phase angle δ(f) versus frequency normalized to the characteristic poroelastic relaxation frequency. Data on the left are from nanoscale dynamic oscillatory deformation experiments on normal, untreated bovine calf cartilage specimens, reported here using the spherical tip (R ∼ 2.5 μm) at ∼2 nm deformation amplitude superposed on a static ∼0.43-μm indentation depth (solid circles; n ≥ 6, mean ± SE). Data on the right are from macroscale unconfined compression experiments on one normal bovine calf cartilage disk, 6.4 mm in diameter and 1 mm thick, using an ∼10-μm deformation amplitude superimposed onto an ∼200-μm static compression (open squares) (adapted from Kim et al. (2)).
Conclusions
In this work, AFM-based indentation methods were used to quantify the time, frequency, and amplitude dependence of untreated and PG-depleted cartilage mechanical properties, including (classical indentation), Eind,0, τ (force relaxation), |E∗|, E′, E″, and δ (dynamic oscillatory loading). An important contribution that emerged from this study involves the comparison between untreated and PG-depleted cartilage disks at the small length scales that yielded insights into the contributions of the ECM molecular components to the measured mechanical properties. In particular, for the dynamic oscillatory experiments on the untreated cartilage disks, it was determined that fluid-flow-induced poroelasticity primarily governs frequency-dependent energy dissipation even at exceedingly small nanometer-scale deformation amplitudes, whereas the longer-timescale force relaxation mainly reflects viscoelasticity. Thus, using contact radii and deformation amplitudes that are several orders smaller than tissue-level testing configurations (2,8), the visco- and poroelastic contributions to cartilage time-dependent mechanical behavior could be deconvoluted. Furthermore, depletion of aggrecan caused a dramatic increase in energy dissipation at higher amplitudes (associated with increased δ and decreased E′ in Fig. 5), which is hypothesized to be due to the presence of additional viscoelastic mechanisms, i.e., collagen molecular interactions. Hence, the presence of aggrecan minimizes the amplitude dependence and provides a more uniform and stable mechanical response.
Acknowledgments
The authors thank the Institute for Soldier Nanotechnologies at MIT, funded through the U.S. Army Research Office, for use of instruments.
This work was supported by the National Science Foundation (grant CMMI-0758651), the National Institutes of Health (grant AR3326), and the National Security Science and Engineering Faculty Fellowship (grant N00244-09-1-0064).
Supporting Material
References
- 1.Lee R.C., Frank E.H., Roylance D.K. Oscillatory compressional behavior of articular cartilage and its associated electromechanical properties. J. Biomech. Eng. 1981;103:280–292. doi: 10.1115/1.3138294. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.-J., Bonassar L.J., Grodzinsky A.J. The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J. Biomech. 1995;28:1055–1066. doi: 10.1016/0021-9290(94)00159-2. [DOI] [PubMed] [Google Scholar]
- 3.June R.K., Ly S., Fyhrie D.P. Cartilage stress-relaxation proceeds slower at higher compressive strains. Arch. Biochem. Biophys. 2009;483:75–80. doi: 10.1016/j.abb.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Grodzinsky A.J., Roth V., Mow V.C. The significance of electromechanical and osmotic forces in the nonequilibrium swelling behavior of articular cartilage in tension. J. Biomech. Eng. 1981;103:221–231. doi: 10.1115/1.3138284. [DOI] [PubMed] [Google Scholar]
- 5.Hayes W.C., Bodine A.J. Flow-independent viscoelastic properties of articular cartilage matrix. J. Biomech. 1978;11:407–419. doi: 10.1016/0021-9290(78)90075-1. [DOI] [PubMed] [Google Scholar]
- 6.Jin M.S., Grodzinsky A.J. Effect of electrostatic interactions between glycosaminoglycans on the shear stiffness of cartilage: a molecular model and experiments. Macromolecules. 2001;34:8330–8339. [Google Scholar]
- 7.Buschmann M.D., Kim Y.-J., Grodzinsky A.J. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch. Biochem. Biophys. 1999;366:1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 8.Kempson G.E., Freeman M.A.R., Swanson S.A.V. The determination of a creep modulus for articular cartilage from indentation tests on the human femoral head. J. Biomech. 1971;4:239–250. doi: 10.1016/0021-9290(71)90030-3. [DOI] [PubMed] [Google Scholar]
- 9.Mow V.C., Gibbs M.C., Athanasiou K.A. Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J. Biomech. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S., Lin J., Pruitt L. A fiber reinforced poroelastic model of nanoindentation of porcine costal cartilage: a combined experimental and finite element approach. J. Mech. Behav. Biomed. Mater. 2009;2:326–337. doi: 10.1016/j.jmbbm.2008.09.003. discussion 337–338. [DOI] [PubMed] [Google Scholar]
- 11.Miller G.J., Morgan E.F. Use of microindentation to characterize the mechanical properties of articular cartilage: comparison of biphasic material properties across length scales. Osteoarthritis Cartilage. 2010;18:1051–1057. doi: 10.1016/j.joca.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maroudas A. Physicochemical properties of articular cartilage. In: Freeman M.A.R., editor. Adult Articular Cartilage. Pitman Medical; Tunbridge Wells, UK: 1979. pp. 215–290. [Google Scholar]
- 13.Biot M.A. Theory of propagation of elastic waves in a fluid-saturated porous solid. I. Low-frequency range. J. Acoust. Soc. Am. 1956;28:168–178. [Google Scholar]
- 14.Mow V.C., Kuei S.C., Armstrong C.G. Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J. Biomech. Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 15.Frank E.H., Grodzinsky A.J. Cartilage electromechanics—II. A continuum model of cartilage electrokinetics and correlation with experiments. J. Biomech. 1987;20:629–639. doi: 10.1016/0021-9290(87)90283-1. [DOI] [PubMed] [Google Scholar]
- 16.Mak A.F. The apparent viscoelastic behavior of articular cartilage—the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows. J. Biomech. Eng. 1986;108:123–130. doi: 10.1115/1.3138591. [DOI] [PubMed] [Google Scholar]
- 17.Papagiannopoulos A., Waigh T.A., Heinrich M. Solution structure and dynamics of cartilage aggrecan. Biomacromolecules. 2006;7:2162–2172. doi: 10.1021/bm060287d. [DOI] [PubMed] [Google Scholar]
- 18.Clark I.C. Articular cartilage: a review and scanning electron microscopy study. 1. The interterritorial fibrillar architecture. J. Bone Joint Surg. Br. 1971;53B:732–749. [PubMed] [Google Scholar]
- 19.Hardingham T.E., Muir H. The specific interaction of hyaluronic acid with cartilage proteoglycans. Biochim. Biophys. Acta. 1972;279:401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- 20.Ng L., Grodzinsky A.J., Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J. Struct. Biol. 2003;143:242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Maroudas A., Mizrahi J., Ziv I. Swelling pressure in cartilage. Adv. Microcirculat. 1987;13:203–212. [Google Scholar]
- 22.Buschmann M.D., Grodzinsky A.J. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J. Biomech. Eng. 1995;117:179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- 23.Reynaud B., Quinn T.M. Anisotropic hydraulic permeability in compressed articular cartilage. J. Biomech. 2006;39:131–137. doi: 10.1016/j.jbiomech.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg S.R., Grodzinsky A.J. Electrokinetic micromodel of extracellular matrix and other polyelectrolytes networks. PhysicoChem. Hydrodyn. 1988;10:517–539. [Google Scholar]
- 25.Guilak F., Ratcliffe A., Mow V.C. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J. Orthop. Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y.-L., Luo Z.-P., An K.N. Stretching type II collagen with optical tweezers. J. Biomech. 2004;37:1665–1669. doi: 10.1016/j.jbiomech.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Haverkamp R.G., Williams M.A., Scott J.E. Stretching single molecules of connective tissue glycans to characterize their shape-maintaining elasticity. Biomacromolecules. 2005;6:1816–1818. doi: 10.1021/bm0500392. [DOI] [PubMed] [Google Scholar]
- 28.Seog J., Dean D., Ortiz C. Nanomechanics of opposing glycosaminoglycan macromolecules. J. Biomech. 2005;38:1789–1797. doi: 10.1016/j.jbiomech.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Dean D., Han L., Ortiz C. Compressive nanomechanics of opposing aggrecan macromolecules. J. Biomech. 2006;39:2555–2565. doi: 10.1016/j.jbiomech.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Han L., Dean D., Grodzinsky A.J. Lateral nanomechanics of cartilage aggrecan macromolecules. Biophys. J. 2007;92:1384–1398. doi: 10.1529/biophysj.106.091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han L., Dean D., Grodzinsky A.J. Nanoscale shear deformation mechanisms of opposing cartilage aggrecan macromolecules. Biophys. J. 2007;93:L23–L25. doi: 10.1529/biophysj.107.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han L., Dean D., Ortiz C. Cartilage aggrecan can undergo self-adhesion. Biophys. J. 2008;95:4862–4870. doi: 10.1529/biophysj.107.128389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harder A., Walhorn V., Anselmetti D. Single-molecule force spectroscopy of cartilage aggrecan self-adhesion. Biophys. J. 2010;99:3498–3504. doi: 10.1016/j.bpj.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyrrell J.W.G., Attard P. Viscoelastic study using an atomic force microscope modified to operate as a nanorheometer. Langmuir. 2003;19:5254–5260. [Google Scholar]
- 35.Lee B., Han L., Grodzinsky A.J. Dynamic mechanical properties of the tissue-engineered matrix associated with individual chondrocytes. J. Biomech. 2010;43:469–476. doi: 10.1016/j.jbiomech.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai W.M., Hou J.S., Mow V.C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech. Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 37.Sui Y., Lee J.H., Grodzinsky A.J. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor α in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60:2985–2996. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 38.Hardingham T.E., Fosang A.J. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- 39.Farndale R.W., Buttle D.J., Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 40.Lewis J.L., Johnson S.L. Collagen architecture and failure processes in bovine patellar cartilage. J. Anat. 2001;199:483–492. doi: 10.1046/j.1469-7580.2001.19940483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonassar L.J., Frank E.H., Grodzinsky A.J. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum. 1995;38:173–183. doi: 10.1002/art.1780380205. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt M.B., Mow V.C., Eyre D.R. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J. Orthop. Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 43.Lin D.C., Dimitriadis E.K., Horkay F. Robust strategies for automated AFM force curve analysis—I. Non-adhesive indentation of soft, inhomogeneous materials. J. Biomech. Eng. 2007;129:430–440. doi: 10.1115/1.2720924. [DOI] [PubMed] [Google Scholar]
- 44.Kiviranta P., Rieppo J., Jurvelin J.S. Collagen network primarily controls Poisson's ratio of bovine articular cartilage in compression. J. Orthop. Res. 2006;24:690–699. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- 45.Bilodeau G.G. Regular pyramid punch problem. J. Appl. Mech. 1992;59:519–523. [Google Scholar]
- 46.Mahaffy R.E., Park S., Shih C.K. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys. J. 2004;86:1777–1793. doi: 10.1016/S0006-3495(04)74245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conover W.J., Iman R.L. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981;35:124–129. [Google Scholar]
- 48.Soltz M.A., Ateshian G.A. A conewise linear elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J. Biomech. Eng. 2000;122:576–586. doi: 10.1115/1.1324669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin M., Frank E.H., Grodzinsky A.J. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch. Biochem. Biophys. 2001;395:41–48. doi: 10.1006/abbi.2001.2543. [DOI] [PubMed] [Google Scholar]
- 50.Williamson A.K., Chen A.C., Sah R.L. Compressive properties and function-composition relationships of developing bovine articular cartilage. J. Orthop. Res. 2001;19:1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 51.Lanir Y. Biorheology and fluid flux in swelling tissues. II. Analysis of unconfined compressive response of transversely isotropic cartilage disc. Biorheology. 1987;24:189–205. doi: 10.3233/bir-1987-24211. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz M.H., Leo P.H., Lewis J.L. A microstructural model for the elastic response of articular cartilage. J. Biomech. 1994;27:865–873. doi: 10.1016/0021-9290(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 53.Bursać P., McGrath C.V., Stamenović D. A microstructural model of elastostatic properties of articular cartilage in confined compression. J. Biomech. Eng. 2000;122:347–353. doi: 10.1115/1.1286561. [DOI] [PubMed] [Google Scholar]
- 54.Frank E.H., Grodzinsky A.J. Cartilage electromechanics—I. Electrokinetic transduction and the effects of electrolyte pH and ionic strength. J. Biomech. 1987;20:615–627. doi: 10.1016/0021-9290(87)90282-x. [DOI] [PubMed] [Google Scholar]
- 55.Soltz M.A., Ateshian G.A. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 2000;28:150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- 56.Loparic M., Wirz D., Stolz M. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: validation with a gel-microfiber composite. Biophys. J. 2010;98:2731–2740. doi: 10.1016/j.bpj.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


