Abstract
Human γ-crystallins are long-lived unusually stable proteins of the eye lens exhibiting duplicated, double Greek key domains. The lens also contains high concentrations of the small heat shock chaperone α-crystallin, which suppresses aggregation of model substrates in vitro. Mature-onset cataract is believed to represent an aggregated state of partially-unfolded and covalently damaged crystallins. Nonetheless, the lack of cell or tissue culture for anucleate lens fibers and the insoluble state of cataract proteins has made it difficult to identify the conformation of the human γ-crystallin substrate species recognized by human α-crystallin. The three major human lens monomeric γ-crystallins, γD, γC, and γS, all refold in vitro in the absence of chaperones, on dilution from denaturant into buffer. However, off-pathway aggregation of the partially folded intermediates competes with productive refolding. Incubation with human αB-Crystallin chaperone during refolding suppressed the aggregation pathways of the three human γ-crystallin proteins. The chaperone did not dissociate or refold the aggregated chains under these conditions. The αB-crystallin oligomers formed long-lived, stable complexes with its γD-crystallin substrates. Using α-crystallin chaperone variants lacking tryptophans, we obtained fluorescence spectra of the chaperone/substrate complex. Binding of substrate γ-crystallins with two or three of the four buried tryptophans replaced by phenylalanines, showed that the bound substrate remained in a partially folded state with neither domain native-like. These in vitro results provide support for protein unfolding/protein aggregation models for cataract, with α-crystallin suppressing aggregation of damaged or unfolded proteins through early adulthood, but becoming saturated with advancing age.
Keywords: α-crystallin, cataracts, small heat shock protein, chaperone, aggregation
INTRODUCTION
The vertebrate lens has a unique architecture necessary for its transparency and refractive properties.1 Its nuclear region is composed of elongated, organelle-free, anucleate, fiber-like cells that contain high concentrations of the crystallin proteins and lack the capability of new protein synthesis.2 The ubiquitous crystallins, α-, β-, and γ-crystallin, account for approximately 90% of the total protein in lens fiber cells.
α-crystallin is a member of the small heat shock protein (sHsp) family of ATP-independent chaperones.3; 4 In adult human lenses, α-crystallin is a polydisperse oligomer (20-40 subunits) of two subunits αA- and αB-crystallin (αA- and αB-Crys) present in a 3αA:1αB ratio.2 In young human lenses, approximately 28% of the crystallin content is α-crystallin hetero-oligomers.5 α-crystallin has a dual role as the main protein chaperone system in mature fiber cells and as a structural protein.6-10
The β- and γ-crystallins are structural proteins whose high concentrations and native state interactions are important in maintaining the transparency and refractive index gradient of the lens. They are members of the same family of proteins, the βγ-crystallin. They are organized into two domains, with each domain composed of two intercalated Greek-key motifs.8; 11
γC-, γS- and γD-crystallin (γC-, γS- and γD-Crys) are the most abundant γ-crystallins (~21 kDa) and comprise 7, 9 and 11%, respectively, of the total protein content in young human eye lenses.5 Human γC- and γD-Crys are expressed in utero and localized to the nuclear region of the lens, while γS-Crys is expressed solely in the cortical region. Compared to other mesophilic proteins, the γ-crystallins are extremely stable with melting temperatures above 70°C. The high thermodynamic stability of their native states along with kinetic barriers to unfolding allow them to remain stably folded for long periods of time, a necessary prerequisite for maintaining lens transparency.12 Their buried tryptophan fluorescence is quenched in the native state relative to the unfolded state in the absence of metal ligands or cofactors.13-15 This quenching mechanism is believed to protect Trp residues from photo-damage (e.g. photolysis) caused by constant exposure to environmental UV radiation during our lifetime, thus contributing to the stability of the native protein.16
Disturbances to the proteins of fiber cells lead to cataract, a high molecular weight state of the lens crystallins that scatters visible light. Cataract is the leading cause of blindness in the world.17 Age-related or mature-onset cataract is the prevalent form of this disease, while congenital hereditary cataract occurs in early childhood and is relatively rare.18
Characterization of cataracts removed by surgery revealed that they are insoluble aggregated states, requiring denaturation for solubilization.19 β- and γ-crystallins isolated from water insoluble fractions of cataractous lenses contain a high percentage of glutamine and asparagine deamidation, methionine and tryptophan oxidation, and truncations.20; 21 Destabilization of β- and γ-crystallins resulting from the accumulation of these covalent damages could cause these proteins to partially unfold and populate aggregation-prone species. In vitro studies of deamidation-mimicking mutants have shown that these modifications can greatly affect the stability of these proteins.22; 23
Such pathways, in which partially folded species form high molecular weight aggregates, either within cells or outside them, underlie the pathology of many protein deposition diseases including Parkinson’s disease, Light chain amyloidosis, Alzheimer’s disease, and many other protein deposition diseases.24-26 For the well characterized chaperonins, for example GroEL-ES or TriC, such aggregation-prone species are their substrates.27
Horwitz6 showed that bovine α-crystallin had chaperone activity since it inhibited the thermally-induced aggregation of different enzymes and bovine β- and γ-crystallins. α-crystallin has also been shown to suppress the aggregation of different non-physiological substrates such as insulin, citrate synthase, α-lactalbumin and alcohol dehydrogenase in vitro.6; 28-31 αA- and αB-Crys subunits (~ 20 kDa) can also form polydisperse homo-oligomers with comparable structural and chaperone properties as the hetero-oligomeric α-crystallin.32 α-crystallin does not require ATP hydrolysis for chaperone function, although ATP binding has been shown to induce structural changes and enhance chaperone function.33
Sathish et al. investigated the interaction between human αA- and αB-crystallin and destabilized multimeric βB2-crystallin mutants. They showed that αA- and αB-crystallin recognized and bound mutants with altered equilibrium unfolding patterns observed using the fluorescence emission of a bimane probe attached to βB2-crystallin proteins.34 These mutants had similar thermodynamic stability as WT, but—unlike wild type—aggregated at high protein concentrations under native conditions. The authors proposed that changes in the stability of the substrate have to be accompanied by the population of a folding intermediate for α-crystallin to recognize the βB2-crystallin substrate.34 In addition, Evans et al.35 have shown that calf α-crystallin will bind to partially folded species of human and calf βB2-crystallin that structurally resemble an unfolding intermediate of human βB2-crystallin. α-crystallin is able to discriminate between species that are productive intermediates versus those intermediates involved in off-pathway aggregation of model substrate.29; 31; 35-37 This mode of chaperone function seems to be a conserved mechanism for other members of the sHsp family.38
Since primary lens fiber cells are terminally differentiated and enucleated, it has not been possible to culture them. In the absence of appropriate cell or tissue culture of the lens, it has been difficult to identify the actual crystallin conformers that are recognized and bound by α-crystallin in the lens. The characterization of the composition of cataractous material removed by surgery has required denaturing conditions such that the identity of the chaperone substrates and their conformation are lost. In the protein aggregation model for cataract, human α-crystallin would be expected to recognize partially unfolded molecules of the human lens γ-crystallins. Horwitz6, Carver et al.31, and Putilina et al.39 have previously shown that human αB-Crys is able to suppress the aggregation of a mixture of γ-crystallins from lens’ cells lysates. Most of these studies utilized heat denaturation to initiate competitive aggregation and the substrates usually consisted of all γ-crystallins from lenses purified via size exclusion chromatography (SEC). We report here experiments to identify the conformation of species of monomeric human γC-, γD- and γS-Crys that are recognized by the human αB-Crys chaperone.
Unfolding and refolding studies in vitro of human γD-Crys monitored the fluorescence emission of four tryptophan residues (two Trps per domain), each buried in a quadrant of the protein.40 These residues are very efficiently quenched in the folded state of the protein so that their fluorescence emission quantum yield increases upon unfolding.13; 14 The efficient quenching of Trp68 and Trp156 depends on an unusual conformation of the Trp ring with respect to its backbone amide, as well as the presence of two tightly bound H2O molecules with oppositely oriented dipoles. The other two tryptophans, Trp 42 and Trp 130, are quenched by Förster transfer to their counterpart Trps.14-16 As a result, the Trp fluorescence from the γ-crystallins is a very sensitive reporter of the protein’s conformation.
In equilibrium and kinetic refolding experiments, a stable, partially folded intermediate was identified with the N-terminal domain unfolded and C-terminal domain folded. When the protein was refolded from high to low concentrations of denaturant, off-pathway aggregation was observed that competed with productive refolding of human γD-Crys.41 Recombinant human γC-Crys behaved similarly under matching assay conditions (Dr. Yongting Wang, personal communication). Human γS-Crys, on the other hand, had a more cooperative unfolding/refolding transition during equilibrium experiments and aggregation was not detected during refolding.12 In this study, we have taken advantage of the in vitro aggregation reactions that compete with productive folding to examine individually the interactions of human α-crystallin with aggregating, partially unfolded, purified human γ-crystallins that are likely candidates for physiological chaperone substrates within the aging lens.
RESULTS
Purification and characterization of recombinant wild-type, human αB-Crys
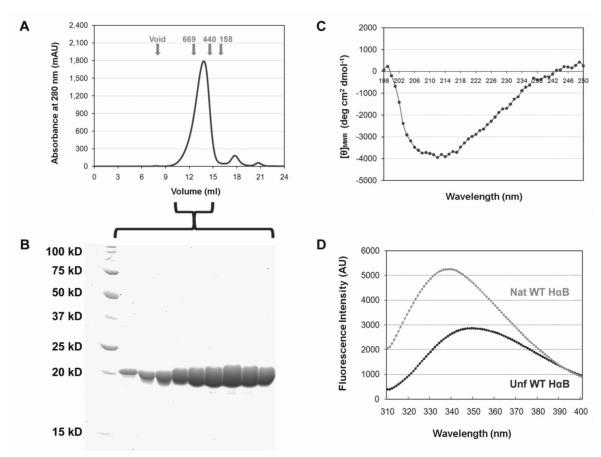
Human recombinant αA- and αB-Crys form large (400-600 kDa), polydisperse homo-oligomers when expressed in E. coli cells.32; 42 Sun et al.32 have shown that these homo-oligomers have similar structural characteristics as endogenous bovine α-crystallin, with small differences in surface hydrophobicity between recombinant homo-oligomers. Further, both human αA- and αB-Crys oligomers showed chaperone-like activity with αB-Crys oligomers suppressing aggregation at lower substrate to chaperone weight ratios. The multimeric state, heterogeneous character and absence of catalytic activity make it difficult to compare preparations of α-crystallin from different sources. Figure 1 describes the characteristics of the αB-Crys oligomers from our expression and purification protocols.
Fig. 1.
Purification and structural characterization of recombinant Human αB-Crys. Human WT αB-Crys was expressed and purified from E. coli cells (see Experimental Procedures). A) Representative size-exclusion chromatogram showing the final, polishing step of the purification procedure. WT αB-Crys eluted as a single peak (from 11 to 14.5 ml) with apparent molecular weight between 500-600 kDa. B) Fractions corresponding to the αB-Crys elution peak were run in a 14% SDS-PAGE gel. C) Far-UV CD spectra (198-250 nm) of native αB-Crys at pH 7.0 and 37°C. D) Fluorescence emission spectra of native ( ) and unfolded (●) αB-Crys at 37°C. Protein concentration for far-UV CD and fluorescence emission measurements was 100 μg/ml.
) and unfolded (●) αB-Crys at 37°C. Protein concentration for far-UV CD and fluorescence emission measurements was 100 μg/ml.
Recombinant, WT human αB-Crys was expressed at low temperature (18°C) and purified via anion exchange and SEC. Fractions collected during the final, polishing size-exclusion chromatography step were electrophoresed through a 14% SDS-PAGE gel to confirm purity of protein samples (Figure 1A and B). The secondary, tertiary and quaternary structures of purified WT αB-Crys were characterized using far-UV CD spectroscopy, fluorescence emission spectroscopy and SEC methods. Far-UV CD spectrum (Fig. 1C) of native αB-Crys showed a minimum at 214 nm consistent with previously published spectra.32 Human αB-Crys has two tryptophans at amino acid positions 9 and 60 in its N-terminal region. Comparable to previously published results,32 the fluorescence emission spectra showed maxima at wavelengths 339 and 349 nm for native and unfolded protein respectively (Fig. 1D). Quaternary structure characteristics were examined using a Superose 6 SEC column. Figure 1A shows a representative size-exclusion chromatogram. From elution profiles, recombinant WT αB-Crys oligomers had apparent molecular weights between 500-600 kDa (25-30 subunits per oligomer). This size distribution is consistent with previously published results 32.
WT human γC-, γD- and γS-Crys aggregate upon refolding out of high concentrations of denaturant into buffer with low concentrations of denaturant
Previous work by Kosinski-Collins and King41 showed that wild-type human γD-Crys can refold to its native state at 37°C after unfolding in high concentrations of GdnHCl in equilibrium refolding experiments that tracked tryptophan fluorescence changes between the folded and unfolded states. Upon dilution out of denaturant, refolding was already significant within the mixing dead-time of the experiment. The C-terminus reached its native-like state first, with a t1/2 of 35 sec, while the folding of the N-terminal, with a t1/2 of 130 sec, took significantly longer.22 Thus, there is a partially folded intermediate that is significantly populated during the first few minutes of refolding even at 37°C.22 However, when diluting to buffer or low denaturant concentrations (less than 1 M GdnHCl) early intermediates of γD-Crys aggregated under the experimental conditions (10 μg/ml, pH 7.0). Early, partially folded intermediates of WT human γC-Crys displayed analogous aggregation behavior in similar experiments (Dr. Yongting Wang, personal communication). On the other hand, partially folded intermediates of wild-type human γS-Crys did not aggregate under similar experimental refolding conditions during equilibrium refolding experiments.12 Given the structural and sequence similarity between these three γ-crystallins (~ 50% identity between human γS-Crys and γD-Crys),12 it seemed likely that partially folded species of γS-Crys would aggregate during refolding if the protein concentration was increased.43
Recombinant, WT human γD- and γS-Crys containing an N-terminal His6 tag were expressed in E. coli and purified via Ni-NTA affinity chromatography. Wild-type human γC-Crys, without any affinity tags, was also expressed in E. coli, but purified via SEC. Kosinski-Collins et al.13; 41 have shown no difference in protein stability and aggregation behavior between γD-Crys with or without a His6 tag.
Wild-type γD-, γC-, and γS-Crys were unfolded in 5.0 M GdnHCl at pH 7.0 and 37°C for 24 hours. Refolding at 37°C was initiated by a ten-fold dilution with Refolding Buffer (without GdnHCl) and thorough mixing of the sample. Aggregation was followed by monitoring solution turbidity measured as optical density at 350 nm (O.D.350 nm) without continuous stirring of the sample. The final protein concentration was 10 times higher than that used in the aforementioned equilibrium unfolding/refolding experiments. Figure 2 shows the O.D.350 nm, which is a direct measure of static light scattering due to formation of macromolecular aggregates, for each γ-Crys protein sample (1 γ:0 αB traces). The first recorded O.D. 350 nm values (at t=0) were higher than the buffer baseline due to the rapid formation of light-scattering species within the mixing dead-time of the experiment (~ 5 sec). Aggregates formed for all three γ-crystallins were confirmed to be amorphous by transmission electron microscopy (Fig. S1) and were visible to the naked eye minutes after initiation of refolding and aggregation.
Fig. 2.
Suppression of the aggregation of partially folded WT γD-, γS- and γC-crystallins by αB-Crys chaperone. A) γD-Crys, B) γS-Crys, and C) γC-Crys aggregate during refolding out of high concentrations of GdnHCl at a protein concentration of 100 μg/ml and a final GdnHCl of 0.5 M. The suppression reactions were done at 1 γ:1 αB and 1 γ:5 αB ratios.
WT γD-Crys had the highest increase in solution turbidity (~ 1.15 AU) followed by γC-Crys (~ 0.7 AU) and γS-Crys (~0.35 AU) (Fig. 2 and S2). At 37°C, O.D.350 nm traces for all three γ-crystallins displayed two phases. The traces increased sharply in the first 40 seconds and reached a plateau 200 seconds after initiation of refolding and aggregation (Fig. 2 and S2). The two-phase aggregation process observed in these traces for all partially folded γ-Crys was probably due to an initial, fast growth phase of the small-globular aggregates into fibril-like, amorphous structures. Eventually, the light scatter traces reached a plateau due to the formation of large aggregates within the first 30 minutes of the reaction. These aggregate species have been observed by Kosinski-Collins and King41 using atomic force microscopy.
αB-Crys chaperone suppresses the aggregation of partially folded WT γD-, γC- and γS-Crys
To test whether partially folded crystallins were substrates of α-crystallin, wild-type γD-, γC- or γS-Crys protein was allowed to refold and aggregate in the presence of WT αB-Crys chaperone at different mass-based ratios of γ-Crys to αB-Crys (Fig. 2). All γ-Crys to αB-Crys ratios described hereafter were mass-based ratios. Refolding buffer with αB-Crys chaperone was added to unfolded γ-Crys solutions, mixed thoroughly, and aggregation was monitored by measuring changes in O.D.350 nm in a UV-Vis Spectrophotometer without subsequent, continuous stirring. Unless otherwise stated, this mixing regime was followed for all of the short-term suppression experiments mentioned hereafter.
αB-Crys chaperone suppressed the aggregation of partially folded intermediates of all three γ-crystallins in assays measuring solution turbidity. Increasing the concentration of αB-Crys chaperone, from 1 γ:1 αB to 1 γ:5 αB, led to further reduction in light scattering (Fig. 2). WT γD- and γC-Crys aggregation was suppressed to similar levels (Fig 2A and C), while γS-Crys aggregation was not suppressed as well under identical experimental conditions (Fig. 2B). Further, we observed that only the partially folded γ-Crys substrate was incorporated into the aggregate while the αB-Crys chaperone was present as either free oligomers or bound to γ-Crys in high molecular weight complexes (data not shown).We also performed the same aggregation suppression assays in the presence of αB-Crys chaperone with different concentrations of γD-Crys ranging from 50-150 μg/ml. We did not observe significant differences in the suppression of aggregation levels at the same ratios within this range (data not shown).
Samples from these suppression reactions were further analyzed via SEC—performed at 4°C in SEC Buffer—2 h after samples were prepared. At this temperature, we did not observe any further aggregation after the samples had been filtered with a 0.2 μm filter prior to SEC. Chromatograms from the 1 γD:5 αB and 1 γC:5 αB samples showed the presence of a high molecular weight complex that eluted in the void volume (V0, ~7.6 ml) of the Superose 6 GL 10/300 SEC column, along with the signature peaks for αB-Crys chaperone and γD- or γC-Crys monomers (Fig. 3A and B, gray traces). The high molecular weight complexes constitute the γ—αB complexes. This early-eluting peak was only observed in samples from suppression reactions. Chromatograms from samples of native αB-Crys chaperone and native γD-Crys incubated together under final refolding conditions (0.5 M GdnHCl and Refolding Buffer), for 2 h at 37°C, lacked this early-eluting peak (Fig 3A and B, black traces). Chromatograms from 1 γ:0 αB aggregation reactions, native αB-Crys chaperone only, and native γ-Crys only also lacked this peak (data not shown). Interestingly, when 1 γS:5 αB samples were applied to the Superose 6 column, no early-eluting peak was observed (Fig. 3C). This result along with turbidity data shown in Fig. 2 indicates that αB-Crys did not suppress the aggregation of partially folded γS-Crys species as efficiently as the aggregation from partially folded γD- and γC-Crys. These results also show that the N-terminal His6 tag of γD- and γS-Crys does not seem to affect the chaperoning interactions between αB-Crys and that the changes in suppression efficiency observed for different, partially folded γ-Crys were due to particular characteristics of these proteins. This differential interaction with γS-Crys could be due to a shorter lifetime of the aggregation-prone species populated during γS-Crys refolding.12 Another possibility is that amino acid sequence differences in γS-Crys make it a poorer substrate for the αB-Crys chaperone.
Fig. 3.
Size-exclusion chromatograms for αB-Crys and γD-, γC-, or γS-Crys suppression reactions. A) 1 γD:5 αB suppression reaction ( ) and 1 γD:5 αB mixture of native proteins (—) chromatograms are shown. B) 1 γC:5 αB suppression reaction (
) and 1 γD:5 αB mixture of native proteins (—) chromatograms are shown. B) 1 γC:5 αB suppression reaction ( ) and 1 γC:5 αB mixture of native proteins (—) chromatograms are shown. C) 1 γS:5 αB suppression reaction (
) and 1 γC:5 αB mixture of native proteins (—) chromatograms are shown. C) 1 γS:5 αB suppression reaction ( ) and 1 γS:5 αB mixture of native proteins (—) chromatograms are shown. The suppression reactions shown in Fig. 2 were applied to a Superose 6 size-exclusion column (for the native mixture samples’ preparation see Materials and Methods) Arrows correspond to elution volume of standards. Numbers represent standards’ molecular weights in kDa.
) and 1 γS:5 αB mixture of native proteins (—) chromatograms are shown. The suppression reactions shown in Fig. 2 were applied to a Superose 6 size-exclusion column (for the native mixture samples’ preparation see Materials and Methods) Arrows correspond to elution volume of standards. Numbers represent standards’ molecular weights in kDa.
Destabilization of the C-terminal domain is required for the formation of the partially folded, aggregation-prone intermediate of γD-Crys
Since the crystal structure of γD-Crys has been solved to 1.25 Å resolution and its folding pathway has been previously described in detail, we chose γD-Crys to further study interactions between αB-Crys chaperone and this physiological substrate.13; 40; 41; 44; 45 Long-lived intermediates have been implicated as species responsible for the aggregation of several proteins because they might give rise to aggregation-prone species during refolding.43; 46 A stable intermediate is populated during WT γD-Crys unfolding/refolding equilibrium experiments and is manifested as a slight inflection in the equilibrium traces at 2.3 M GdnHCl.13; 41 This species is well-populated during the unfolding/refolding equilibrium experiment of a variety of crystallins carrying single amino acid substitutions.22; 44; 45 This species has its N-terminal domain unfolded and the C-terminal domain folded, hence the hydrophobic patches in the domains’ interfaces are exposed to solvent.13 Consequently, these hydrophobic patches could be candidate sites for intermolecular aggregate interactions.
Previous studies have shown that the two transitions observed during equilibrium unfolding/refolding experiments correspond to the successive unfolding of each domain, with the first transition (~ 2.2 M GdnHCl) corresponding to the unfolding/refolding of the N-terminal domain and the second transition (~ 2.8 M GdnHCl) to the unfolding/refolding of the C-terminal domain 13; 45. Therefore, it is possible to partially unfold γD-Crys at different concentrations of GdnHCl encompassing both transitions and to populate stable, partially-unfolded species.
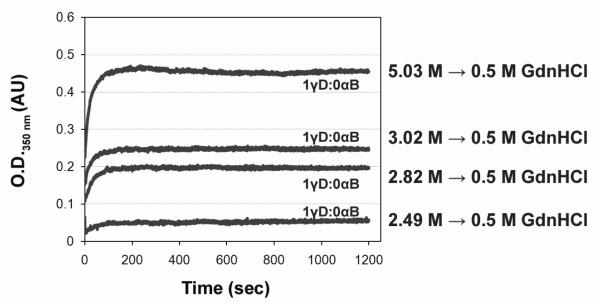
To explore the structural requirements for aggregation, two step reactions were performed. Wild-type γD-Crys was unfolded at different concentrations of GdnHCl in order to investigate whether the long-lived folding intermediate was the aggregation-prone species (2.49, 2.82, 3.09 and 5.03 M GdnHCl). γD-Crys unfolded at these concentrations does not self-associate and aggregate prior to dilution of the denaturant.44 After equilibrium conditions were reached, unfolded γD-Crys was diluted to lower concentrations of GdnHCl (0.5 M GdnHCl, final protein concentration was 50 μg/ml) (Fig. 4). The O.D.350 nm amplitudes were 0.2 and 0.25 AU for 2.82 M and 3.02 M GdnHCl unfolded samples respectively, while the final O.D.350 nm amplitude was 0.45 AU for 5.03 M GdnHCl unfolded samples (Fig. 4). This lower level in final O.D.350 nm was due to a higher proportion of protein refolding productively when diluted out of these intermediate GdnHCl concentrations (data not shown). These results indicate that the C-terminal domain of γD-Crys must be destabilized and partially unfolded for the protein to aggregate in vitro. Further, these result suggests that the stable folding intermediate, with its C-terminal domain folded and N-terminal domain unfolded, observed in unfolding/refolding equilibrium experiments, is likely not the aggregation-prone intermediate.
Fig. 4.
Aggregation of partially unfolded γD-Crys during refolding. 1 γD:0 αB reactions for γD unfolded at GdnHCl concentrations encompassing the intermediate to unfolded protein transition. γD was unfolded at 2.49, 2.82, 3.02, or 5.03 M GdnHCl for 24 h at 37°C to ensure equilibrium conditions. Protein was refolded by diluting the GdnHCl concentration to 0.5 M at 37°C. The final concentration protein concentration was 50 μg/ml for all samples.
Monitoring the conformation of the bound substrate
γD-Crys has four buried Trp residues, two in the N-terminal domain (Trp42 and Trp68, Fig. 6A) and two in the C-terminal domain (Trp130 and Trp156, Fig. 6A).40 Rapid electron transfer from the excited Trp indole ring to its amide backbone results in the efficient quenching of the weakly fluorescing, strongly quenched Trp68 and Trp156.14 The intra-domain mechanism of quenching of Trp fluorescence in the native state of γD-Crys is due to partial resonance energy transfer (FRET) from the strongly fluorescing tryptophans (Trp42 and Trp130) to their weakly fluorescing counterparts (Trp68 and Trp156). We took advantage of the structural information provided by the fluorescence emission of buried tryptophan residues in γD-Crys to further elucidate the structural nature of the substrate that is recognized by αB-Crys chaperone during aggregation.
Fig. 6.
Tryptophan fluorescence spectra from double-Trp γD-Crys mutants in complex with αB-Crys chaperone. A) Crystal structure of γD-Crys (PDB: 1HK0) showing Trp pairs conserved within the N-terminal domain (W130F/W156F γD-Crys) or the C-terminal domain (W42F/W68F γD-Crys).
B) Relative fluorescence emission spectra of W130F/W156F γD-Crys unfolded at different concentrations of GdnHCl. W130F/W156F γD-Crys (20 μg/ml) was incubated in Refolding buffer with 0, 1.6, 2.0, 2.6 and 5.1 M GdnHCl for 24 h at 37°C. Fluorescence emission spectra were recorded using a λEX set to 300 nm. All traces were normalized to the fluorescence intensity of the max. λEM for W130F/W156F γD-Crys unfolded at 5.1 M GdnHCl.
C) Relative fluorescence emission spectra of W42F/W68F γD-Crys unfolded at different concentrations of GdnHCl. W42F/W68F γD-Crys (20 μg/ml) was incubated in Refolding buffer with 0, 2.3, 2.6, 3.1 and 5.2 M GdnHCl for 24 h at 37°C. Fluorescence emission spectra were recorded using an λEX set to 300 nm. All traces were normalized to the fluorescence intensity of the max. λEM for W42F/W68F γD-Crys unfolded at 5.2 M GdnHCl.
D) SEC chromatograms for 1 γD:5 noTrp αB suppression reactions using W130F/W156F ( ) or W42F/W68F (
) or W42F/W68F ( ) γD-Crys as substrates. The shaded area on the trace indicates the fraction used for fluorescence measurements.
) γD-Crys as substrates. The shaded area on the trace indicates the fraction used for fluorescence measurements.
E) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex from 1 W130F/W156F γD: 5 noTrp αB suppression reaction ( ). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) W130F/W156F γD-Crys (50 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys.
). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) W130F/W156F γD-Crys (50 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys.
F) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex isolated from 1 W42F/W68F γD: 5 noTrp αB suppression reaction ( ). The λEX was set to 300 nm for all samples and the protein concentration for the native (○) and fully unfolded (●) W42F/W68F γD-Crys protein samples was 50 μg/ml. Traces were normalized as described above.
). The λEX was set to 300 nm for all samples and the protein concentration for the native (○) and fully unfolded (●) W42F/W68F γD-Crys protein samples was 50 μg/ml. Traces were normalized as described above.
Wild-type αB-Crys contains two tryptophan residues at positions 9 and 60. Mutating both residues to phenylalanine resulted in the elimination of fluorescence emission compared to wild-type protein when the excitation wavelength (λEX) was set to 300 nm to selectively excite tryptophan residues (Fig. 5A). Liang and colleagues47 have shown that individually mutating these tryptophan residues to phenylalanine does not result in secondary structural changes to αB-Crys. Structural changes in quaternary structure between wild-type and mutant proteins were not detected during SEC (Fig. 5C). Suppression assays using γD-Crys as the substrate showed that the chaperone activity was conserved in the W9F/W60F αB-Crys mutant (noTrp αB) (Fig. 5B).
Fig. 5.
Tryptophan fluorescence of γD-Crys in complex with αB-Crys chaperone. Tryptophan residues at positions 9 and 60 in αB-Crys were substituted to phenylalanines. A) Fluorescence emission spectra for native WT αB-Crys (●) and W9F/W60F αB-Crys ( ) at 25°C. λEX was set to 300 nm.
) at 25°C. λEX was set to 300 nm.
B) W9F/W60F αB-Crys suppressed the aggregation of γD-Crys under the same refolding and aggregation described previously (see Materials and Methods). Final γD-Crys concentration was 100 μg/ml and final GdnHCl concentration was 0.5 M at 37°C.
C) A high molecular weight complex could be isolated from the 1γD:5 noTrp αB suppression reactions during SEC. The volume highlighted by the gray bar corresponds to the fraction collected for fluorescence measurements.
D) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex ( ). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) WT γD-Crys (20 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys.
). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) WT γD-Crys (20 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys.
1 γD:5 noTrp αB suppression reactions were collected and loaded onto a Superose 6 SEC column (Fig. 5C). The fluorescence emission spectrum was recorded for the fraction corresponding to the high molecular weight complex (Fig. 5C, gray bar) at a λEX of 300 nm. Since αB-Crys contains no tryptophan residues, only the tryptophan residues from γD-Crys present in this complex would contribute to the fluorescence spectrum. The spectrum of the γD—αB-Crys complex (max. emission wavelength ~ 335 nm) did not coincide with either the spectra of the native or unfolded WT γD-Crys suggesting that the substrate might be in a partially-folded state (Fig. 5D).
To further investigate the conformation of γD-Crys in these complexes, we studied double- and triple-Trp γD-Crys mutants with the Trps replaced by phenylalanines.14 Double-Trp mutants had either the N-terminal (W130F/W156F γD-Crys, Fig. 6A) or C-terminal domain (W42F/W68F γD-Crys, Fig. 6A) buried tryptophan pair conserved in order to preserve the tryptophan fluorescence quenching mechanism within each domain. These mutants provided a more detailed picture of the folding status of each domain of γD-Crys when bound to αB-Crys during chaperoning interactions. Both W130F/W156F and W42F/W68F γD-Crys recapitulated the behavior of WT γD-Crys: their tryptophan fluorescence was quenched in the native state and, upon unfolding in high concentrations of denaturant, showed an increase in fluorescence intensity with a concurrent red-shift in their maximum emission wavelength (λEM) to ~350nm.14
Figures 6B and C show the relative fluorescence emission spectra for W130F/W156F and W42F/W68F γD-Crys after unfolding these proteins in increasing concentrations of GdnHCl. The red-shift in maximum λEM coincided with previously published transition GdnHCl concentrations that correspond to the unfolding of the N-terminal and the C-terminal domains of the wild-type protein.13; 41; 45 W130F/W156F γD-Crys (N-terminal Trp pair) retained the native-like fluorescence spectrum at 1.6 M GdnHCl when the λEX was 300 nm. Increasing the denaturant concentration to 2.0 M GdnHCl resulted in a red-shift in the max. λEM to 350 nm (Fig. 6B). The emission spectrum for W42F/W68F γD-Crys (C-terminal Trp pair), on the other hand, remained native-like at 2.3 M GdnHCl (λEX was 300 nm). At higher concentrations of GdnHCl, the spectra were red-shifted until reaching the maximum λEM for the fully unfolded protein, approximately 350 nm (3.1 M and 5.2 M GdnHCl traces, Fig. 6C).
Given the structural information provided by these γD-Crys mutants, we utilized W42F/W68F and W130F/W156F γD-Crys as substrates in suppression of aggregation reactions with Trp-less αB-Crys chaperone. Both double-Trp mutants aggregated to similar levels as the wild-type protein and Trp-less αB-Crys chaperone showed similar suppression efficiency as with the wild-type γD-Crys (data not shown). The substrate-chaperone complexes were isolated from 1 γD:5 noTrp αB suppression reactions via SEC using a Superose 6 SEC column (Fig. 6D). Fluorescence emission spectra (λEX = 300 nm) were recorded for the fraction corresponding to the substrate-chaperone peak for both double-Trp mutants (Fig. 6E and F). The fluorescence emission spectra for both double-Trp mutants in complex with Trp-less αB-Crys were similar (max. λEM ~ 333 nm). However, these spectra did not coincide with the spectra for their respective native or unfolded states (Fig. 6E and F). These results point to similar environments for both the N-terminal domain and C-terminal domain of γD-Crys in complex with αB-Crys. The tryptophan residues of γD-Crys in the complex did not seem to be exposed to solvent nor retained the conformation necessary for the quenching phenomenon observed in the native state. The tryptophans were either in buried but in non-native conformations, or they were directly interacting with the chaperone subunits.
Given the similarity in fluorescence spectra between wild-type and the double-Trp mutants of γD-Crys, it is likely that it is due to interaction of regions surrounding the Trp residues with αB-Crys in the chaperone-substrate complexes. In order to monitor binding interactions at specific regions of γD-Crys more closely, we took advantage of triple-Trp mutants that conserved the highly-fluorescent, buried Trp residues in either the N-terminal (W42only γD-Crys) or the C-terminal domain (W130only γD-Crys), while the remaining three tryptophans were changed to phenylalanines. Chen et al.14 previously characterized the buried tryptophan fluorescence behavior of these proteins and found that the quenching phenomenon observed in the native state of wild type and double-Trp mutants described above was absent in these triple-Trp γD-Crys mutants.
Since these tryptophan residues (Trp42 and Trp130) emit a robust fluorescence emission signal, we used these mutants in suppression aggregation reactions with Trp-less αB-Crys chaperone to reveal general features of the environment around these residues in the γD—αB complex. Both W42only and W130only γD-Crys aggregated after dilution out of denaturant and Trp-less αB-Crys suppressed their aggregation efficiently (at 1 γ:5 αB ratios). Figure 7A shows the emission spectrum of W42only γD-Crys in the γD—αB complex excited at 300 nm; the max. λEM was approximately 334 nm and the spectrum did not coincide with the spectra for the native and unfolded W42only γD-Crys controls. The fluorescence emission spectrum for W130only γD-Crys in the complex had a max. λEM at approximately 333 nm and also did not coincide with either the native or fully-unfolded tryptophan fluorescence spectra of controls (λEX=300 nm; Fig. 7B). These results indicate that the residues in close proximity to Trp42 and Trp130 are not solvent exposed in the γD—αB complex and might be involved in binding interactions with αB-Crys chaperone.
Fig. 7.
Fluorescence emission spectra of triple-Trp γD-Crys mutants in complex with αB-Crys. A) Relative fluorescence emission spectra for W42only γD-Crys in complex with noTrp αB-Crys ( ). B) Fluorescence emission spectra for W130only γD-Crys in complex with noTrp αB-Crys (
). B) Fluorescence emission spectra for W130only γD-Crys in complex with noTrp αB-Crys ( ). Spectra for their respective native (○) and unfolded (●) controls is also shown (protein concentration for W42only γD-Crys was 50 μg/ml and for W130only γD-Crys was 25 μg/ml). The λEX was 300 nm and the traces were normalized as described previously in Figure 6. Complexes were isolated from 1γD:5αB suppression reactions in which the final concentration of γD was 50 μg/ml.
). Spectra for their respective native (○) and unfolded (●) controls is also shown (protein concentration for W42only γD-Crys was 50 μg/ml and for W130only γD-Crys was 25 μg/ml). The λEX was 300 nm and the traces were normalized as described previously in Figure 6. Complexes were isolated from 1γD:5αB suppression reactions in which the final concentration of γD was 50 μg/ml.
αB-Crys chaperone forms stable complexes with partially folded γD-Crys
α-crystallin is the major chaperone system in differentiated lens fiber cells. Since there is no protein turnover, aggregation of covalently damaged crystallins is prevented through interactions with α-crystallin.2 Therefore, the complex between α-crystallin and its β/γ-crystallin substrates must be long-lived in order for lens transparency to be maintained. We investigated the lifetime of this complex in vitro by initiating the aggregation of γD-Crys in the presence of αB-Crys at a 1 γD:5 αB ratio. The suppression reaction was allowed to proceed at 37°C with moderate shaking and aliquots were removed at different times and applied to a Superose 6 SEC column. During this incubation period, we did not confirm whether any chemical modifications were introduced into γD-Crys or αB-Crys chaperone during the 4-day period.
Figure 8 shows the set of chromatograms collected within a time period of 30 min to 4 days after aggregation was initiated. The high molecular weight peak eluted at the V0, ~7.6 ml (Fig. 8, Inset). This peak was still present 4 days after the suppression reaction was initiated. Further, the absorbance at 280 nm of this peak at 7.6 ml increased after 30 min, decreased and remained constant thereafter for later time points. The WT αB-Crys chaperone peak (eluted at 13.5 ml) remained constant throughout the duration of the experiment. These results showed that the γD—αB complexes were stable for long periods of time supporting α-crystallin’s role as a chaperone in the lens.
Fig. 8.
SEC chromatograms of αB—γD complexes at different time points after suppression reaction. SEC chromatograms of 0.5 ml aliquots removed from a 1 γD:5 αB suppression reaction incubated at 37°C with continuous agitation. Time points at which aliquots were collected are shown on the chromatogram. The peak that eluted at 7.6 ml is the designated high molecular weight complex and eluted in the void of the column (V0= 8 ml). The peak that eluted at 13.5 ml corresponded to wild-type αB-Crys. Inset shows the high molecular weight peak that eluted at 7.6 ml in detail.
αB-Crys chaperone interacts with early and late aggregate species in the aggregation pathway of partially folded γD-Crys
Despite its chaperone function, α-crystallin is often found in cataractous inclusions in significant proportions.19; 20; 48; 49 Further, αA-Crys knock-out mice developed cataract at an early age in which αB-Crys was localized to the insoluble fraction of lens fiber cell cytoplasm.50 Therefore, we were interested in investigating the lifetime of the substrate species that is recognized by αB-Crys chaperone.
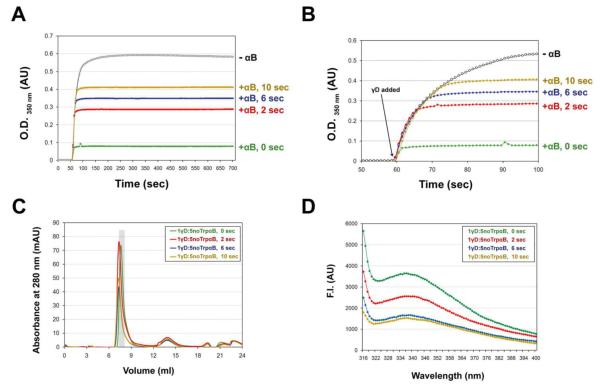
To do so, we added Trp-less αB-Crys chaperone at very early time points (2, 6, and 10 sec) after dilution of γD-Crys out of denaturant. Wild-type γD-Crys was added to Refolding Buffer that was constantly stirred with a spin-bar; the solution was stirred only for the first 30 sec after fully unfolded γD-Crys was added. The final O.D.350 nm levels did not change significantly using this method. Trp-less αB-Crys was added 0, 2, 6 or 10 sec after refolding and aggregation of γD-Crys were initiated. Solution turbidity increased the later Trp-less αB-Crys was added after refolding and aggregation were initiated (Fig. 9A and B). Controls were performed by adding H2O after 6 and 10 sec and showed no significant change in the kinetics or the level of aggregation of γD-Crys (data not shown).
Fig. 9.
Addition of αB-Crys at very early time points after initiation of refolding and aggregation of partially folded WT γD-Crys. A) O.D. 350nm data for no addition of αB-Crys (1 γD:0 αB ratio) and addition 0, 2, 6, and 10 sec after WT γD-Crys was added to Refolding buffer (1 γD:5 αB ratio). Buffer was constantly stirred with a magnetic spin bar and γD-Crys was added 60 sec after light scattering data collection was initiated. Samples were allowed to mix for the first 30 sec after γD-Crys addition. B) Light scattering data presented in (A) showing traces from 50-100 sec after recordings were initiated. C) SEC traces for suppression reactions shown in (A). Samples were loaded onto a Superose 6 SEC column. The fraction corresponding to the γD—αB complex was collected for fluorescence emission measurements (gray bar).
D) Raw fluorescence emission spectra for fractions highlighted in (C) and containing the γD—αB complex formed during addition of αB-Crys 0, 2, 6, and 10 sec after addition and initiation of γD-Crys refolding and aggregation. λEX was set to 300 nm.
Using Trp-less αB-Crys chaperone allowed us to record the fluorescence emission spectrum of the WT γD-Crys species bound to αB-Crys chaperone in the complexes isolated via SEC (Fig. 9C). All spectra had the same max. λEM (~336 nm), however, the fluorescence intensity decreased in samples from later addition time points indicating a decrease in the concentration of γD—αB complexes (Fig. 9D). These results indicate that the species recognized and bound to αB-Crys chaperone is a transient intermediate populated at very early times of refolding and aggregation.
Adding αB-Crys chaperone any time after 15 sec resulted in no suppression of γD-Crys aggregation (Fig. S3). Interestingly, when αB-Crys was added 5 minutes after the aggregation reaction was started there was an increase in the scatter of the aggregate compared to controls (Fig. S3). Similar results were also observed when αB-Crys was added 30 minutes after initiation of aggregation of partially folded γD-Crys . Given the structures of γD-Crys aggregates observed at these time points by Kosinski-Collins and King,41 it seems that αB-Crys chaperone is being incorporated into or binds to these preformed large fibril-like aggregates of γD-Crys, but does not disaggregate them. Similar behavior has been reported for IbpA and IbpB, sHsps in E. coli, which are known to be present in inclusion bodies formed during overexpression of heterologous protein.51; 52
DISCUSSION
The native states of the γ-crystallins are very soluble, very stable and among the longest lived proteins in the human body.2 They show little tendency to aggregate or associate even at high concentrations. Except for special cases such as sickle cell anemia, most well characterized protein aggregation reactions represent the polymerization of partially folded species.46; 53; 54 The examples of the well-characterized protein aggregation diseases, including Parkinson’s disease, prion protein encephalopathies, and Huntington’s disease, suggest that partially folded or unfolded intermediates are also the key species in the crystallin aggregation reactions that lead to cataract.24; 25 Extensive characterization of α-crystallins with model substrates support models in which partially folded species are the substrates.29; 31 However, the absence of a high resolution structure for the full-length protein chaperone, and the difficulty in characterizing the substrate conformations recognized within normal or cataractous lens, has limited our ability to define the conformation of the physiological substrates of α-crystallins.
Competitive off-pathway aggregation of γD-Crys during refolding conditions
Productive kinetic refolding experiments showed that two partially folded intermediates were populated during WT γD-Crys refolding.22 Significant refolding, as measured by changes in tryptophan fluorescence, occurred within the mixing dead time that initiated these kinetic refolding experiments. It is unlikely that a true random coil, as found in the fully denatured state, is significantly populated after the first seconds of the refolding reaction. The first two transitions, with t1/2 values of 8 and 35 sec, corresponded to the folding of C-terminal domain, while the third transition, with a t1/2 value of 130 sec, was significantly longer with and corresponded to the folding of the N-terminal domain.22 Flaugh et al.44; 45 showed that the residues on the interface of the C-terminal domain nucleated the folding of the N-terminal domain. Previously published work on deamidated γD-Crys mutants of the interface glutamine Page 16 of 43 residues22 and molecular dynamics simulations of γD-Crys unfolding56 suggest that the folding nucleus of the protein is located in the Greek key motif in the C-terminal domain closest to the domain interface of the protein (Motif IV).
Aggregation experiments with partially unfolded γD-Crys showed that the C-terminal domain must be partially unfolded for the protein to aggregate after dilution into low concentrations of denaturant (Fig. 4). Further, when γD-Crys was fully unfolded, αB-Crys recognized and bound to partially folded, aggregation-prone species populated during the first 10 sec after refolding and aggregation were initiated (Fig. 9A and B). The C-terminal domain also productively refolds during this time period.12; 13; 45 Experiments with the isolated single domain constructs of the N-terminal or C-terminal domain of γD-Crys showed that the single C-terminal domain of γD-Crys aggregated during dilution out of high concentrations of denaturant, while the single N-terminal domain of γD-Crys did not aggregate under similar conditions (unpublished results). Therefore, it is likely that the aggregation-prone region is located in the C-terminal domain and was recognized by the αB-Crys chaperone during these aggregation reactions.
Conformation of the partially folded γ-crystallin bound to αB-Crys chaperone oligomers
The long-lived nature of the γD—αB complex allowed us to characterize the conformation of the substrate bound to the chaperone (Fig. 8). The isolation using SEC of γD—αB-Crys complexes from 1 γD:5 noTrp αB suppression reactions indicated that γ-crystallin was present in the high molecular weight oligomeric complex. Since all tryptophans in αB-Crys were changed to phenylalanines and the excitation wavelength was set to preferentially excite tryptophan residues, the fluorescence emission spectra of these complexes provided structural information for bound WT γD-Crys (Fig. 5D). We compared this spectrum to the spectra from native and unfolded WT γD-Crys. The fluorescence emission spectrum for each conformation is distinct (i.e. the max. λEM is unique to each state). The fluorescence emission spectrum of γD-Crys from these complexes did not resemble the spectra for either native or fully unfolded γD-Crys (Fig. 5D).
Suppression reactions with double-Trp mutants of γD-Crys as substrates and Trp-less αB-Crys as the chaperone provided information on the folding status of each domain of γD-Crys in the γD—αB complex (Fig. 6). These double-Trp γD-Crys mutants only had the tryptophan pairs within the N-terminal (W130F/W156F γD-Crys) or C-terminal domain (W42F/W68F γD-Crys), so that the intra-domain native state quenching mechanism was conserved. If γD-Crys had either domain folded in the γD—noTrp αB complex, then fluorescence emission spectra of the substrate would be similar to the spectra of the native protein. Our results showed that both double-Trp mutants in γD—noTrp αB complexes had similar maximum λEM, and that their spectra did not resemble either their respective native or unfolded states (Fig. 6E and F). Further, these spectra were similar to the spectrum of the wild-type protein in complex with Trp-less αB-Crys chaperone (Fig. 5D). These results indicate that the intra-domain tryptophan pairs of γD-Crys were in similar environments in the γD—αB complexes.
The maximum λEM for tryptophan fluorescence is sensitive to local environment and changes in response to various degrees of solvent exposure.55 Chen et al.14 studied the fluorescence behavior of the buried tryptophans in the double- and triple-Trp mutants described previously (vide supra). For the native states of the triple-Trp γD-Crys mutants, they found that the highly fluorescing Trps (Trp42 and Trp130) had a max. λEM at ~322 nm, while the maximum λEM was 332 nm for the quenched Trps (Trp68 and Trp156). They compared these values to the max. λEM from fluorescence emission spectra of 3-methylindole (3-MI), a tryptophan indole ring analog, in different solvent mixtures. The maximum λEM for Trp42 and Trp130 native spectra closely matched the spectrum for 3-MI in a less polar solvent mixture (cyclohexane-dioxane, 83:17), while the fluorescence spectra and max. λEM for 3-MI in a more polar solvent mixture (methanol-dioxane, 25:75 and 10:90) were similar to native spectra from Trp68 and Trp156. Within the context of the quenching mechanism in the native state, these results bolstered the model of intra-domain partial resonance energy transfer from the highly-fluorescent Trps (donors) to the quenched Trps (acceptors) proposed by Chen and colleagues.14
This comprehensive investigation of the buried Trp fluorescence of γD-Crys allowed us to refine our model on the conformation of the aggregation-prone γD-Crys species that is bound to αB-Crys during the chaperoning process. We performed aggregation suppression assays with W42only and W130only γD-Crys triple-Trp mutants as substrates (Fig. 7). These mutants retained the highly fluorescing tryptophan from each domain thus allowing us to assess the environment surrounding these regions in both domains in the γD—noTrp αB complex. The fluorescence emission spectra of W42only or W130only γD-Crys in the substrate-chaperone complex did not coincide with their respective native and unfolded spectra. Therefore, Trp42 and Trp130 were neither fully exposed to solvent nor did they remain buried in the hydrophobic core of the native state (Fig. 7).
These results point to a possible interaction between the Trp residues, or the immediate regions surrounding them, and αB-Crys chaperone in the complex. One possible model to explain these results is that αB-Crys could initially recognize the exposed, buried aggregation-prone region in Motif 4 (which includes Trp130 and Trp156) in the C-terminal domain, bind to it, and keep it from interacting with other aggregation-prone regions. Binding to the homologous hydrophobic region in the N-terminal domain could occur after the initial recognition step. An exposed state of the Trp rings, which are intended to be buried in the native state, offers an attractive model for how the chaperone discriminates between partially folded intermediates and the fully folded state.
Another possible model is that the distinct fluorescence emission spectra of double- and triple-Trp γD-Crys mutants in complex with Trp-less αB-Crys could be due to their tryptophan residues being exposed to polar environments between γD-Crys chains in small, soluble aggregates formed in the first 10 sec after dilution out of denaturant. αB-Crys chaperone would bind these small γD-Crys oligomers prevent further growth into fibril-like aggregates. If this were the case, however, we would see similar or increased efficiency of suppression within these first 10 sec, rather than a progressive decrease in suppression of aggregation as judged by the increase in light scattering at 2, 6, or 10 sec (Fig. 9A and B).
As mentioned above, αB-Crys suppression activity was confined to early species along the aggregation pathway of γD-Crys, which may still be monomeric (Fig. 9). Adding αB-Crys at later times showed that the chaperone no longer suppressed aggregation, but may have been interacting with insoluble aggregates as judged by increases in solution turbidity (Fig. S3). Kosinski-Collins and King41 have shown that during this time period small fibrillar aggregates form. Small Hsps IbpA and B from E. coli are integrated into inclusion bodies when the amount of substrate exceeds the amount of sHsp in the cell.51; 52
Human γC-, γD-, and γS-Crys share a common aggregation-prone species that is recognized by αB-Crys chaperone
We have shown that the partially folded forms of all three human γ-crystallins, γC, γD and γS, will aggregate during refolding when diluted out of denaturant into buffer (Fig. 2 and Fig. S2). Their aggregation propensity points to a common species populated during refolding that is aggregation-prone. This species is populated very early during dilution out of high concentrations of denaturant (Fig. 9).13; 45
The lens is a protected environment lacking blood supply and with low metabolic activity, it is not clear what agents in that environment destabilize proteins, but the leading candidates are oxidative and photo-oxidative damage.21 Many of the crystallins found in insoluble inclusions from mature-onset cataractous lenses contain a host of covalent damages with deamidation of glutamine and asparagines, and oxidation of methionine and tryptophan residues being the most abundant.56 Lampi and coworkers have shown that deamidation-mimicking changes at interface residues of β-crystallins lower protein stability and stabilize intermediates during equilibrium unfolding/refolding experiments.23; 57 In the case of human γD-Crys, Flaugh et al.22 showed that the introduction of negative charges at the interface in gln→glu deamidated mutants led to the preferential destabilization of the N-terminal domain as shown by the population of the folding intermediate at lower concentrations of denaturant in equilibrium unfolding/refolding experiments. γ-crystallins, with their four buried tryptophans, also absorb UV-radiation and photo-oxidized tryptophans have been reported in cataractous lenses.14-16; 21 These damages would reduce the kinetic barrier to unfolding, facilitating further unfolding of the protein and exposure of the aggregation-prone region in the C-terminal domain. Once this region is exposed, the high concentrations of γ-crystallins in the lens would favor second-order aggregation reactions over productive refolding.
Features of αB-Crys chaperone involved in substrate recognition and aggregation suppression in vitro
Small heat shock proteins are divided at the level of their primary structure into three distinct regions: the conserved α-crystallin and the variable N-terminal domain and C-terminal extension.4 All three regions contribute to and are important for chaperone function in this family of heat shock proteins.28 In the case of αB-crystallin, Sharma et al.58 have shown that residues 57-69 in the N-terminal domain and residues 93-107 in the α-crystallin domain of bovine αB-crystallin are involved in chaperoning interactions with alcohol dehydrogenase. Further, Ghosh et al.59 using protein pin arrays identified seven regions in αB-Crys that were involved in chaperone-substrate interactions. Two regions were in the N-terminal domain, 4 regions in the α-crystallin domain and one region in the C-terminal extension. Two regions in the α-crystallin domain of αB-Crys interacted and prevented the thermal aggregation of βH-crystallin.59
Recently, Bagnéris et al.60 have solved the crystal structure of the α-crystallin domain of human αB-Crys to 2.9 Å resolution. They found that the homo-dimers formed an extended β-sheet with a shared groove. Based on known crystal structures of non-metazoan sHsps, they postulated that regions of the N-terminal of αB-Crys could bind to this groove in the full-length oligomers. They hypothesize that, in some vertebrate sHsps, disassembly of the oligomers into dimers could expose the groove and other pockets in the dimer where substrates, like the γ-crystallins, could bind during chaperoning interactions in vivo.
Many structural characteristics have been implicated in contributing to the chaperone function of α-crystallin. Hydrophobic regions in the N-terminal region of small heat shock proteins, for one, seem to play an important role.61 The α-crystallin domain and the C-terminal extension of sHsps are also important for chaperone function and interactions with the substrate.38 Subunit-exchange and oligomeric state of α-crystallin are also important factors in chaperone activity.28
Implications for cataract disease
The high molecular weight γD—αB-Crys complexes formed during the early stage of the suppression reactions were stable after four days at 37°C (Fig. 8). Given the incubation conditions, these proteins could have accumulated some non-enzymatic modifications (e.g. deamidation). However, as the SEC absorbance traces in Fig. 8 show, αB-Crys remained an oligomers during the four-day incubation period, which is an indication that the αB-Crys complexes remained stable despite the long incubation conditions. Nonetheless, these results show that αB-Crys forms stable complexes with aggregation-prone, partially folded species of γD-Crys in vitro. Similarly, water-soluble, high molecular weight species from normal aged lenses also consist of α-crystallin and γ-crystallin and it has been suggested that these complexes form when α-crystallin binds destabilized γ-crystallin proteins.19; 62
The finding that α-crystallin oligomers recognize and bind early, partially folded intermediates of the three human γ-crystallins is consistent with models of cataract formation from the aggregation of partially unfolded crystallins (Fig. 10). How these are generated in the protected environment of the lens remains to be determined, but multiple pathways of oxidative and photo-oxidative damage are all candidates for the etiology.16; 23; 57; 63 Since there is little protein degradation in lens fiber cells, and formation of high molecule aggregates needs to be avoided, it is not surprising that α-crystallin oligomers form long-lived complexes with their substrates. If this is true it may explain the age distribution of cataract, very rare below age of 50, then sharply increasing subsequently, reaching 50% of the population at age 80.64 In fact, α-crystallins binding many molecules of partially folded and/or covalently damaged β- and γ-crystallins may themselves end up aggregating, thus contributing to the growth of the cataract in the aged lens. Analysis of the water-soluble fraction from normal and cataractous age-matched lenses shows that the percentage of water-soluble high molecular weight species, consisting mostly of α-crystallin along with β- and γ-crystallins, increases not only with age but also in cataractous lenses. It is believed that these complexes are the precursors to water-insoluble crystallin aggregates responsible for cataract.65-67
Fig. 10.
A molecular model for age-related cataract formation in the lens. Environmental stresses could lead to covalent damage, such as deamidation or photo-oxidation, to the crystallins. This damage could destabilize γ-crystallins, which are otherwise very stable, populating aggregation-prone species (I*). α-crystallin would sequester such species in younger individuals (green arrow). But as we age, the finite levels of free α-crystallin in the mature lens fibers will be diminished leading to aggregation (orange arrows).
MATERIAL AND METHODS
Expression and Purification of human αB-Crys and γ-crystallins
Human γD-Crys cloning into pQE.1 was previously described in Kosinski-Collins et al.13 Human γS-Crys construct was previously described in Mills et al.12 Double- (W130F/W156F and W42F/W68F γD-Crys) and triple-Trp (W42only- and W130only γD-Crys) mutants were previously described in Chen et al.14 These recombinant proteins were expressed by transforming pQE.1 plasmids into E. coli M15 [pREP4] cells (Qiagen). Both constructs contained an N-terminal His6 tag. Proteins were purified following procedures described in Kosinski-Collins et al.13 using Ni-NTA (Qiagen) media for affinity chromatography. After purification, proteins were dialyzed into 10 mM ammonium acetate (pH 7.0) and stored at 4°C.
Human αB-Crys pAED4 plasmid was a generous gift from Dr. Jack Liang at Harvard Medical School. The wild-type αB-Crys containing plasmid was transformed into BL21 Gold (DE3) E. coli cells (Stratagene). Protein expression was induced by addition of IPTG and allowed to proceed over night at 18°C. Cells were harvested and stored at −80°C until future use. The αB-Crys purification protocol was modified from a previous protocol published by Horwitz and colleagues.68 Modifications included performing all steps for cell lysis at 4°C. Two rounds of ion-exchange chromatography were performed using HiPrep 16/10 Q Sepharose FF column (GE Lifesciences) followed by size-exclusion chromatography (SEC) in Superose 6 10/300 GL (GE Lifesciences). Protein was stored in 50 mM sodium phosphate and 150 mM sodium chloride buffer, pH 7.0 (SEC Buffer). αB-Crys aliquots were dialyzed into 10 mM ammonium acetate and concentrated using 10 kDa MWCO Amicon Ultra-4 concentrators (Millipore) prior to use in aggregation suppression assays. All protein batches were tested for chaperone efficiency in aggregation suppression assays at 1:1 and 1:5 γ-αB ratios, with WT γD-Crys as the substrate. Double tryptophan substitutions of residues 9 and 60 to phenylalanines were constructed using mutant primers (IDT-DNA) to amplify the αB-Crys gene in the pAED4 vector during site-directed mutagenesis. Amplified plasmid DNA was sequenced to confirm the substitutions (DNA Sequencing Facility, Massachusetts General Hospital). W9F/W60F αB-Crys was purified using the same procedures described above for the WT αB-Crys protein.
Wild-type human γC-Crys pET-15b plasmid was transformed into BL21 (DE3) E. coli cells (Stratagene). The plasmid and purification protocol were also kindly provided by Dr. Jack Liang 69. Briefly, protein expression was induced by addition of IPTG. Cells were lysed, centrifuged at high speeds, and the supernatant was subjected to two rounds of ammonium sulfate fractionation. The supernatant was centrifuged at high speeds and the pellet was resuspended in Lysis Buffer (50 mM Tris, 1 mM EDTA, 100 mM sodium chloride, pH 7.0). The resuspended pellet was run on a HiPrep 26/60 Sephacryl S-100 size-exclusion column connected to an AKTA FPLC (GE Lifesciences). Protein was concentrated to approximately 2 mg/ml and stored at 4°C until further use.
Protein concentrations were calculated using UV absorbance at 280 nm of unfolded proteins and the following extinction coefficients: 42,860 (γD-Crys, γS-Crys and γC-Crys), 31,860 (W42F/W60F and W130F/W156F γD-Crys), 26,735 (W42only and W130only γD-Crys) and 13,980 (αB-Crys) M−1cm−1.70; 71 Purity of all proteins mentioned herein was determined to be greater than 90% by SDS-PAGE.
Circular Dichroism Spectroscopy
WT αB-Crys was first incubated at a concentration of 100 μg/ml in 10 mM sodium phosphate buffer (pH 7.0) for 1 hour at 37°C. Far-UV CD spectra were then collected from 198 to 250 nm in a 1 mm cuvette in an AVIV model 202 CD spectrometer (Lakewood, NJ). The temperature was kept at 37°C using an internal Peltier thermoelectric temperature controller. Mean residual ellipticity was calculated after buffer signal had been subtracted from spectra.
Fluorescence Emission Spectroscopy
Native WT αB-Crys was incubated at a concentration of 100 μg/ml in Refolding Buffer (100 mM sodium phosphate, 1 mM EDTA, 5 mM DTT, pH 7.0) for 4 h at 37°C prior to collecting spectra. For the unfolded samples, αB-Crys was unfolded in Unfolding Buffer (100 mM sodium phosphate, 1 mM EDTA, 5 mM DTT, 5.0 M GdnHCl, pH 7.0) for 24 h at 37°C. The λEX was 295 nm (PMT voltage was 700 V) and the emission scans were collected from wavelengths 310-400 nm. The excitation and emission slit width were set to 10 nm and the temperature was kept at 37°C using a circulating water bath. For experiments comparing native WT and noTrp αB-Crys, the protein concentration was 20 μg/ml, the λEX was 300 nm, the PMT voltage was 950V, and the slit widths were 10 nm (at 25°C).
For experiments comparing the fluorescence emission spectra of WT γD-Crys—noTrp αB-Crys SEC fractions, the λEX was 300 nm and the emission scans were collected from wavelengths 316-400 nm (PMT voltage was 950 V). The excitation and emission slits were set to 10 nm and the temperature was kept at 25°C. These were the same conditions for experiments measuring the emission spectra of the W42only γD—noTrp αB and W130only γD—noTrp αB complex-containing fractions and their respective native and unfolded γD-Crys controls. However, the high molecular weight peak fractions were pooled (1 ml total), concentrated to 250 μl, and their fluorescence emission spectra were recorded with the λEX set to 300 nm. Fluorimeter settings were the same for experiments with W42F/W68F and W130F/W156F γD-Crys except the PMT voltage was set to 700 V. WT, double- and triple-Trp γD-Crys controls were fully unfolded in Unfolding Buffer for 24 h at 37°C and pre-equilibrated to 25°C prior to recordings. The native γD-Crys controls were equilibrated to 25°C for 1 h in Refolding Buffer prior to recording spectra. Protein concentration for WT and double Trp γD-Crys controls was 50 μg/ml. The concentration for W42only γD-Crys controls was 50 μg/ml, and for W130only γD-Crys controls was 25 μg/ml.
For experiments showing the emission spectra for double-Trp γD-Crys mutants at different concentrations of GdnHCl, the protein was unfolded in Unfolding Buffer with the matching concentration of GdnHCl for 24 h at 37°C. All fluorimeter settings were the same as described above except the PMT voltage was set to 700 V. Protein concentration for both double-Trp mutants was 20 μg/ml. Fluorescence spectra for respective buffers were collected and subtracted from the sample spectra for all experiments described herein. Spectra were collected using a Hitachi F-4500 fluorescence spectrophotometer equipped with a circulating water bath. All experiments described in this section were repeated as three separate trials.
Aggregation and Aggregation Suppression Assays
For aggregation experiments where γD-Crys was unfolded at intermediate GdnHCl concentrations, γD-Crys was unfolded in Unfolding Buffer at the target concentration (2.49, 2.82, 3.02, and 5.03 M GdnHCl) and incubated at 37°C for 24 hours. Aggregation was initiated by diluting the GdnHCl concentration to 0.5 M with Refolding Buffer and thorough mixing of the samples. The final protein concentrations were the same for all samples (50 μg/ml). Light scatter was recorded as O.D.350 nm. All aggregation reactions were carried out at 37°C without continuous stirring.
For all aggregation suppression assays, unfolded γ-Crys was diluted ten-fold to initiate refolding and aggregation; αB-Crys chaperone was already added to the Refolding Buffer and equilibrated to 37°C. Samples were mixed thoroughly upon addition of Refolding Buffer. The final γ-Crys protein concentration was 100 μg/ml for assays with WT γD-, γC-, γS- and double-Trp γD-Crys protein substrates. The γ-Crys protein concentration was 50 μg/ml for assays with triple-Trp γD-Cry as substrates. Final GdnHCl concentration for all assays described above was 0.5 M GdnHCl. Different mass-based ratios of γ-Crys to αB-Crys were used to assay the chaperone activity of the α-crystallin oligomers. Turbidity of the solutions was measured at 37°C without continuous stirring using a Cary 50 UV-Vis spectrophotometer.
For the early time point addition experiments, WT γD-Crys was unfolded in Unfolding Buffer for 24 h at 37°C. Unfolded protein was added to constantly stirred Refolding Buffer (± noTrp αB-Crys) at 37°C (dead time ~ 1sec); unfolded protein was added 60 sec after O.D.350 nm measurements were started. Samples were stirred only for the first 30 sec after addition of γD-Crys. For the 0 sec addition, αB-Crys chaperone had already been added to the Refolding Buffer, which had been equilibrated to 37°C for 1 h. For subsequent time point additions, γD-Crys was added followed by addition of noTrp αB-Crys after 2, 6, or 10 sec. NoTrp αB-Crys had been equilibrated for 1 h at 37°C in 10 mM ammonium acetate buffer at pH 7.0. Data collection was not paused during addition of either γD- or αB-Crys. Suppression reactions were performed at a 1 γD:5 αB ratio and a γD-Crys protein concentration of 50 μg/ml. All experiments described in this section were repeated as three separate trials.
Size-exclusion chromatography
Aggregation and aggregation suppression samples (0.5 ml) were collected after UV spectrophotometer measurements, incubated at 37°C for 2 h. Samples for double-Trp and triple-Trp γD-Crys suppression experiments and early time point addition suppression experiments were incubated for 24 h at 37°C. One-hundred μl of 6X SEC Buffer (300 mM sodium phosphate, 900 mM sodium chloride, pH 7.0) were added and samples (500 μl) were filtered using Ultrafree-MC Centrifugal filter units with a 0.22 μm membrane (Millipore) and then equilibrated to 4°C. Samples were injected into a 0.5 ml Teflon loop (GE Lifesciences) and loaded onto a Superose 6 GL 10/300 (CV=24 ml, V0= 8 ml, GE Lifesciences) attached to an AKTA FPLC (GE Lifesciences). Chromatography runs were performed at a flow rate of 0.5 ml/min in SEC Buffer (50 mM sodium phosphate, 150 mM sodium chloride, pH 7.0, degassed) at 4°C. For the native mixture samples, native γ-Crys at 100 μg/ml and native αB-Crys at 500 μg/ml were mixed together and incubated at 37°C for 2 h in Refolding Buffer with 0.5 M GdnHCl.
For experiments assaying the lifetime of the γD—αB-Crys complexes, γD-Crys was unfolded in Unfolding Buffer for 24 h at 37°C. Aggregation was initiated by ten-fold dilution of the unfolded protein solution with Refolding Buffer plus αB-Crys (ratio was 1 γD:5 αB) at 37°C; the final protein concentration for γD-Crys was 100 μg/ml and the final GdnHCl concentration was 0.5 M. The final volume for this reaction was 5.0 ml and the sample was left at 37°C in a Nutator shaker (BD Diagnostics) for 4 days. Aliquots (0.5 ml) were removed, 100 μl of 6X SEC Buffer were added, samples were filtered using Ultrafree-MC Centrifugal units with a 0.22 μm filter and equilibrated at 4°C. Filtered samples were loaded onto a Superose 6 GL SEC column equilibrated in SEC Buffer and run at a flow rate of 0.5 ml/min at 4°C. All experiments described in this section were repeated as three separate trials.
Supplementary Material
ACKNOWLEDGEMENTS
We greatly appreciate the gift of the pAED4-WT αB-Crys plasmid provided by Dr. Jack Liang (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA). We would also like to thank Dr. Bing-Fen Liu for her initial help purifying recombinant αB-Crys (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA). We thank Dr. Yongting Wang and Daniel Goulet for providing γC-Crys protein. We thank Dr. Shannon Thol, Dr. Ishara Mills-Henry and Dr. Jiejin Chen for helpful discussions and critical reading of the manuscript. The Biophysical Instrumentation Facility for the Study of Complex Macromolecular Systems (NSF-0070319 and NIH GM68762) is gratefully acknowledged. This work was supported by a NEI grant (EY015834), a grant to the Center for Protein Folding Machinery (EY016525) and a NIH grant (GM017980) awarded to J. K.
Abbreviations used
- AU
Arbitrary Units
- sHsp
small heat shock protein
- Crys
crystallin
- γD-Crys
γD-crystallin
- DTT
dithiothreitol
- WT
wild-type
- Trp
tryptophan
- SEC
size-exclusion chromatography
- GdnHCl
guanidinium hydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ponce A, Sorensen C, Takemoto L. Role of short-range protein interactions in lens opacifications. Mol Vis. 2006;12:879–84. [PubMed] [Google Scholar]
- 2.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–85. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982;79:2360–4. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103–26. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- 5.Robinson NE, Lampi KJ, Speir JP, Kruppa G, Easterling M, Robinson AB. Quantitative measurement of young human eye lens crystallins by direct injection Fourier transform ion cyclotron resonance mass spectrometry. Mol Vis. 2006;12:704–11. [PubMed] [Google Scholar]
- 6.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–53. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz J. Proctor Lecture. The function of alpha-crystallin. Invest Ophthalmol Vis Sci. 1993;34:10–22. [PubMed] [Google Scholar]
- 8.Tardieu A. Eye lens proteins and transparency: from light transmission theory to solution X-ray structural analysis. Annu Rev Biophys Biophys Chem. 1988;17:47–70. doi: 10.1146/annurev.bb.17.060188.000403. [DOI] [PubMed] [Google Scholar]
- 9.Rao PV, Huang QL, Horwitz J, Zigler JS., Jr. Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim Biophys Acta. 1995;1245:439–47. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 10.Reddy GB, Reddy PY, Vijayalakshmi A, Kumar MS, Suryanarayana P, Sesikeran B. Effect of long-term dietary manipulation on the aggregation of rat lens crystallins: role of alpha-crystallin chaperone function. Mol Vis. 2002;8:298–305. [PubMed] [Google Scholar]
- 11.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–7. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 12.Mills IA, Flaugh SL, Kosinski-Collins MS, King JA. Folding and stability of the isolated Greek key domains of the long-lived human lens proteins gammaD-crystallin and gammaS-crystallin. Protein Sci. 2007;16:2427–44. doi: 10.1110/ps.072970207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosinski-Collins MS, Flaugh SL, King J. Probing folding and fluorescence quenching in human gammaD crystallin Greek key domains using triple tryptophan mutant proteins. Protein Sci. 2004;13:2223–35. doi: 10.1110/ps.04627004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Flaugh SL, Callis PR, King J. Mechanism of the highly efficient quenching of tryptophan fluorescence in human gammaD-crystallin. Biochemistry. 2006;45:11552–63. doi: 10.1021/bi060988v. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Toptygin D, Brand L, King J. Mechanism of the efficient tryptophan fluorescence quenching in human gammaD-crystallin studied by time-resolved fluorescence. Biochemistry. 2008;47:10705–21. doi: 10.1021/bi800499k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Callis PR, King J. Mechanism of the very efficient quenching of tryptophan fluorescence in human gamma D- and gamma S-crystallins: the gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage. Biochemistry. 2009;48:3708–16. doi: 10.1021/bi802177g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brian G, Taylor H. Cataract blindness--challenges for the 21st century. Bull World Health Organ. 2001;79:249–56. [PMC free article] [PubMed] [Google Scholar]
- 18.Graw J. Congenital hereditary cataracts. Int J Dev Biol. 2004;48:1031–44. doi: 10.1387/ijdb.041854jg. [DOI] [PubMed] [Google Scholar]
- 19.Harrington V, McCall S, Huynh S, Srivastava K, Srivastava OP. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 2004;10:476–89. [PubMed] [Google Scholar]
- 20.Harrington V, Srivastava OP, Kirk M. Proteomic analysis of water insoluble proteins from normal and cataractous human lenses. Mol Vis. 2007;13:1680–94. [PubMed] [Google Scholar]
- 21.Hains PG, Truscott RJ. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res. 2007;6:3935–43. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- 22.Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. J Biol Chem. 2006;281:30782–93. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- 23.Lampi KJ, Amyx KK, Ahmann P, Steel EA. Deamidation in human lens betaB2-crystallin destabilizes the dimer. Biochemistry. 2006;45:3146–53. doi: 10.1021/bi052051k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta. 2004;1698:131–53. doi: 10.1016/j.bbapap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JW. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol. 1998;8:101–6. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 26.Baden EM, Sikkink LA, Ramirez-Alvarado M. Light chain amyloidosis - current findings and future prospects. Curr Protein Pept Sci. 2009;10:500–8. doi: 10.2174/138920309789351949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barral JM, Broadley SA, Schaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Semin Cell Dev Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–41. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 29.Lindner RA, Kapur A, Carver JA. The interaction of the molecular chaperone, alpha-crystallin, with molten globule states of bovine alpha-lactalbumin. J Biol Chem. 1997;272:27722–9. doi: 10.1074/jbc.272.44.27722. [DOI] [PubMed] [Google Scholar]
- 30.Leroux MR, Melki R, Gordon B, Batelier G, Candido EP. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J Biol Chem. 1997;272:24646–56. doi: 10.1074/jbc.272.39.24646. [DOI] [PubMed] [Google Scholar]
- 31.Carver JA, Guerreiro N, Nicholls KA, Truscott RJ. On the interaction of alpha-crystallin with unfolded proteins. Biochim Biophys Acta. 1995;1252:251–60. doi: 10.1016/0167-4838(95)00146-l. [DOI] [PubMed] [Google Scholar]
- 32.Sun TX, Das BK, Liang JJ. Conformational and functional differences between recombinant human lens alphaA- and alphaB-crystallin. J Biol Chem. 1997;272:6220–5. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 33.Biswas A, Das KP. Role of ATP on the interaction of alpha-crystallin with its substrates and its implications for the molecular chaperone function. J Biol Chem. 2004;279:42648–57. doi: 10.1074/jbc.M404444200. [DOI] [PubMed] [Google Scholar]
- 34.Sathish HA, Koteiche HA, McHaourab HS. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: the role of a folding intermediate. J Biol Chem. 2004;279:16425–32. doi: 10.1074/jbc.M313402200. [DOI] [PubMed] [Google Scholar]
- 35.Evans P, Slingsby C, Wallace BA. Association of partially folded lens betaB2-crystallins with the alpha-crystallin molecular chaperone. Biochem J. 2008;409:691–9. doi: 10.1042/BJ20070993. [DOI] [PubMed] [Google Scholar]
- 36.Treweek TM, Lindner RA, Mariani M, Carver JA. The small heat-shock chaperone protein, alpha-crystallin, does not recognize stable molten globule states of cytosolic proteins. Biochim Biophys Acta. 2000;1481:175–88. doi: 10.1016/s0167-4838(00)00109-6. [DOI] [PubMed] [Google Scholar]
- 37.Carver JA, Lindner RA, Lyon C, Canet D, Hernandez H, Dobson CM, Redfield C. The interaction of the molecular chaperone alpha-crystallin with unfolding alpha-lactalbumin: a structural and kinetic spectroscopic study. J Mol Biol. 2002;318:815–27. doi: 10.1016/S0022-2836(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 38.Nakamoto H, Vigh L. The small heat shock proteins and their clients. Cell Mol Life Sci. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putilina T, Skouri-Panet F, Prat K, Lubsen NH, Tardieu A. Subunit exchange demonstrates a differential chaperone activity of calf alpha-crystallin toward beta LOW- and individual gamma-crystallins. J Biol Chem. 2003;278:13747–56. doi: 10.1074/jbc.M208157200. [DOI] [PubMed] [Google Scholar]
- 40.Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. J Mol Biol. 2003;328:1137–47. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- 41.Kosinski-Collins MS, King J. In vitro unfolding, refolding, and polymerization of human gammaD crystallin, a protein involved in cataract formation. Protein Sci. 2003;12:480–90. doi: 10.1110/ps.0225503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphaA-crystallin. J Biol Chem. 1996;271:31973–80. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- 43.Speed MA, Wang DI, King J. Specific aggregation of partially folded polypeptide chains: the molecular basis of inclusion body composition. Nat Biotechnol. 1996;14:1283–7. doi: 10.1038/nbt1096-1283. [DOI] [PubMed] [Google Scholar]
- 44.Flaugh SL, Kosinski-Collins MS, King J. Interdomain side-chain interactions in human gammaD crystallin influencing folding and stability. Protein Sci. 2005;14:2030–43. doi: 10.1110/ps.051460505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flaugh SL, Kosinski-Collins MS, King J. Contributions of hydrophobic domain interface interactions to the folding and stability of human gammaD-crystallin. Protein Sci. 2005;14:569–81. doi: 10.1110/ps.041111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wetzel R. Mutations and off-pathway aggregation of proteins. Trends Biotechnol. 1994;12:193–8. doi: 10.1016/0167-7799(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 47.Liang JJ, Sun TX, Akhtar NJ. Spectral contribution of the individual tryptophan of alphaB-crystallin: a study by site-directed mutagenesis. Protein Sci. 1999;8:2761–4. doi: 10.1110/ps.8.12.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 49.Heys KR, Friedrich MG, Truscott RJ. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–15. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 50.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr., Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–9. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen SP, Polazzi JO, Gierse JK, Easton AM. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J Bacteriol. 1992;174:6938–47. doi: 10.1128/jb.174.21.6938-6947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiao W, Li P, Zhang J, Zhang H, Chang Z. Small heat-shock proteins function in the insoluble protein complex. Biochem Biophys Res Commun. 2005;335:227–31. doi: 10.1016/j.bbrc.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 53.Mitraki A. a. K. J. A. Protein Folding Intermediates and Inclusion Body Formation. Bio/Technology. 1989;7:690–697. [Google Scholar]
- 54.Ecroyd H, Carver JA. Unraveling the mysteries of protein folding and misfolding. IUBMB Life. 2008;60:769–74. doi: 10.1002/iub.117. [DOI] [PubMed] [Google Scholar]
- 55.Vivian JT, Callis PR. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys J. 2001;80:2093–109. doi: 10.1016/S0006-3495(01)76183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–66. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takata T, Oxford JT, Brandon TR, Lampi KJ. Deamidation alters the structure and decreases the stability of human lens betaA3-crystallin. Biochemistry. 2007;46:8861–71. doi: 10.1021/bi700487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma KK, Kaur H, Kester K. Functional elements in molecular chaperone alpha-crystallin: identification of binding sites in alpha B-crystallin. Biochem Biophys Res Commun. 1997;239:217–22. doi: 10.1006/bbrc.1997.7460. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh JG, Clark JI. Insights into the domains required for dimerization and assembly of human alphaB crystallin. Protein Sci. 2005;14:684–95. doi: 10.1110/ps.041152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal Structures of alpha-Crystallin Domain Dimers of alphaB-Crystallin and Hsp20. J Mol Biol. 2009 doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 61.Eifert C, Burgio MR, Bennett PM, Salerno JC, Koretz JF. N-terminal control of small heat shock protein oligomerization: changes in aggregate size and chaperone-like function. Biochim Biophys Acta. 2005;1748:146–56. doi: 10.1016/j.bbapap.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Santhoshkumar P, Udupa P, Murugesan R, Sharma KK. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–85. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 64.Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O’Colmain B, Wu SY, Taylor HR. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 65.Roy D, Spector A. High molecular weight protein from human lenses. Exp Eye Res. 1976;22:273–9. doi: 10.1016/0014-4835(76)90055-5. [DOI] [PubMed] [Google Scholar]
- 66.Srivastava OP, Srivastava K, Silney C. Levels of crystallin fragments and identification of their origin in water soluble high molecular weight (HMW) proteins of human lenses. Curr Eye Res. 1996;15:511–20. doi: 10.3109/02713689609000762. [DOI] [PubMed] [Google Scholar]
- 67.Clark R, Zigman S, Lerman S. Studies on the structural proteins of the human lens. Exp Eye Res. 1969;8:172–82. doi: 10.1016/s0014-4835(69)80029-1. [DOI] [PubMed] [Google Scholar]
- 68.Horwitz J, Huang QL, Ding L, Bova MP. Lens alpha-crystallin: chaperone-like properties. Methods Enzymol. 1998;290:365–83. doi: 10.1016/s0076-6879(98)90032-5. [DOI] [PubMed] [Google Scholar]
- 69.Graw J, Loster J, Soewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, de Angelis M. Hrabe. Characterization of a new, dominant V124E mutation in the mouse alphaA-crystallin-encoding gene. Invest Ophthalmol Vis Sci. 2001;42:2909–15. [PubMed] [Google Scholar]
- 70.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–23. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–52. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.