Abstract
Acute intermittent hypoxia (AIH) facilitates phrenic motor output by a mechanism that requires spinal serotonin (type 2) receptor activation, NADPH oxidase activity and formation of reactive oxygen species (ROS). Episodic spinal serotonin (5-HT) receptor activation alone, without changes in oxygenation, is sufficient to elicit NADPH oxidase-dependent phrenic motor facilitation (pMF). Here we investigated: 1) whether serotonin 2A and/or 2B (5-HT2a/b) receptors are expressed in identified phrenic motor neurons, and 2) which receptor subtype is capable of eliciting NADPH-oxidase-dependent pMF. In anesthetized, artificially ventilated adult rats, episodic C4 intrathecal injections (3 × 6µl injections, 5 min intervals) of a 5-HT2a (DOI) or 5-HT2b (BW723C86) receptor agonist elicited progressive and sustained increases in integrated phrenic nerve burst amplitude (i.e. pMF), an effect lasting at least 90 minutes post-injection for both receptor subtypes. 5-HT2a and 5-HT2b receptor agonist-induced pMF were both blocked by selective antagonists (ketanserin and SB206553, respectively), but not by antagonists to the other receptor subtype. Single injections of either agonist failed to elicit pMF, demonstrating a need for episodic receptor activation. Phrenic motor neurons retrogradely labeled with cholera toxin B fragment expressed both 5-HT2a and 5-HT2b receptors. Pre-treatment with NADPH oxidase inhibitors (apocynin and DPI) blocked 5-HT2b, but not 5-HT2a-induced pMF. Thus, multiple spinal type 2 serotonin receptors elicit pMF, but they act via distinct mechanisms that differ in their requirement for NADPH oxidase activity.
Keywords: Serotonin receptors, NADPH oxidase, respiratory neuroplasticity
Serotonin (5-HT) receptor activation is necessary for the induction, but not maintenance, of phrenic long-term facilitation (pLTF), a form of respiratory plasticity elicited by acute intermittent hypoxia (AIH) or carotid chemoafferent neuron activation (Millhorn et al. 1980a; Bach and Mitchell, 1996; Fuller et al. 2001a; McGuire et al. 2004). pLTF induction arises in part from intermittent activation of peripheral chemoreceptors, stimulating brainstem serotonergic neurons, which have been hypothesized to release 5-HT in the phrenic motor nucleus (Kinkead et al. 2001). Subsequent 5-HT2 receptor activation within the phrenic motor nucleus is necessary for pLTF (Kinkead et al. 1998; Fuller et al. 2001; Baker-Herman and Mitchell, 2002; MacFarlane et al. 2008). Additional requirements of AIH-induced pLTF include NADPH oxidase activity (MacFarlane et al. 2009) and reactive oxygen species (ROS) formation (MacFarlane and Mitchell, 2008). Episodic spinal 5-HT receptor activation in the absence of AIH is sufficient to induce NADPH oxidase-dependent phrenic motor facilitation (pMF), an effect similar in magnitude and time course to AIH-induced pLTF. Specific 5-HT receptor subtype(s) responsible for 5-HT induced pMF are not known (MacFarlane and Mitchell 2009). Since multiple 5-HT2 receptor sub-types are expressed in respiratory regions (Kubin and Volgin, 2008; Basura et al., 2001), we sought to determine which 5-HT2 receptor subtypes are capable of eliciting pMF, suggesting a possible role in AIH induced pLTF.
Two subtypes of metabotropic Gq-protein coupled 5-HT2 receptors stimulate NADPH oxidase activity (Peng et al. 2006). For example, sensory long-term facilitation of ex vivo carotid chemoafferent neurons can be elicited by episodic bath application of 5-HT (3×15 sec, 5 minute intervals), and this facilitation requires 5-HT2a receptor-dependent increases in NADPH oxidase activity (Peng et al. 2006). Exogenous 5-HT also increases NADPH oxidase activity and ROS formation in renal mesangial cells, an effect inhibited by pre-treatment with a 5-HT2a receptor antagonist (Grewal et al. 1999). On the other hand, angiotensin II elicits 5-HT2b receptor-dependent increases in NADPH oxidase activity in rat cardiac fibroblasts (Monassier et al. 2008). Further, 5-HT2b receptor activation increases NADPH oxidase-derived ROS formation, and increased phosphorylation of cytosolic subunits necessary for catalytic activation of the NADPH oxidase complex in differentiated 1C11 clonal cell lines (Schneider et al. 2006). Since 5-HT2a and 5-HT2b metabotropic receptors both couple to Gq proteins (Hannon and Hoyer, 2008) and both activate NADPH oxidase, thereby increasing ROS formation, they both appear to have the requisite characteristics to induce NADPH oxidase-dependent pMF.
Since multiple 5-HT2 type receptors stimulate NADPH oxidase activity without intermittent hypoxia, we investigated: 1) whether episodic spinal 5-HT2a or 5-HT2b receptor activation (without hypoxia) is sufficient to elicit phrenic motor facilitation in vivo (i.e. pMF), and 2) whether these forms of pMF require spinal NADPH oxidase activity. Whereas both receptor subtypes elicit pMF, only 5-HT2b receptors did so by an NADPH oxidase dependent mechanism.
1.0 Experimental Procedures
Experiments were performed on 3–4 month old male Sprague Dawley rats (Colony 218A, Harlan, Indianapolis, IN. USA). All experiments were approved by the Animal Care and Use Committee at the School of Veterinary Medicine, University of Wisconsin-Madison. All attempts were made to minimize the quantities of animals used in these studies.
1.1 Surgery
Surgical procedures were performed on isoflurane (~3.5%) anesthetized rats with 50% inspired O2 (balance N2) on a stainless steel heated surgical table. Immediately on induction of anesthesia, a rectal thermistor (Fisher Scientific, USA) was inserted, and body temperature was maintained constant (37–38°C) by adjusting the temperature of a water bath that perfused water to the surgical table. An O2 sensor (TED 60T, Teledyne Analytical Instruments, USA) monitored inspired O2 concentration which was accurately adjusted when necessary by manually switching the mixed ratio’s of N2 and O2 supplied from gas tanks. A tail vein catheter (24 gauge, Surflo, Elkton, MD, USA) was inserted to allow an infusion pump (Cole-Palmer, Vernon Hills, IL,USA) to deliver a slow (1.5–2ml/hr) infusion of a 1:1 lactated Ringers:hetastarch solution to assist in maintenance of blood pressure (6% Hetastarch; Hospira Inc., IL, USA) and base excess (Lactated Ringers, Baxter, IL, USA). A small amount (1:20) of sodium bicarbonate (8.4% Hospira Inc., IL, USA) was also added to the infusion solution. Rats received an initial 1ml intravenous injection of lactated ringers over a 5 minute period to minimize early changes in base excess.
Rats were tracheotomized and bilaterally vagotomised through a midline ventral incision made in the neck. A polyethylene catheter (PE50, I.D/O.D.-0.58mm/0.965mm; Intramedic MD, USA) connected to a pressure sensitive transducer (Gould Pressure Transducer, P23, USA) was inserted into the femoral artery for monitoring of mean arterial blood pressure (MAP). This also permitted sampling of arterial blood for analysis of partial pressure of O2 (Po2) and CO2 (Pco2), pH, and base-excess using a blood gas analyzer (ABL 500, Radiometer, Copenhagen) throughout the experimental protocol (see below). The left phrenic and hypoglossal (XII) nerves were carefully dissected using a dorsal approach, cut distally, de-sheathed covered with saline soaked cotton wool to prevent desiccation. The rat was then slowly converted to urethane anesthesia (1.8g/kg) and simultaneously weaned off isoflurane. During conversion to urethane anesthesia, an intrathecal surgery was also performed for sub-dural placement of a silicone catheter (O.D. 0.6mm; Access Technologies, IL, USA) as described previously (Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009). In brief, using a dorsal approach, the muscles overlying the cervical spinal cord were partially separated to expose the cervical vertebra (~C1-C3); the dorsal section of C2 was carefully removed to expose the underlying spinal cord. The silicone catheter was primed with the relevant compound (drug and/or vehicle) and inserted ~2mm through a small incision made in the dura so that the tip was positioned just rostral to C3 for delivery of the compound near the phrenic motor nucleus.
Following placement of the intrathecal catheter, the nerves were submerged in mineral oil and placed on bipolar silver recording electrodes. Once nerve activity was detected, the rat was paralyzed with pancuronium bromide (1mg/kg; Sicor Pharmaceuticals, CA, USA) and then allowed one hour for stabilization of electroneurograms and blood pressure. The raw nerve activity was amplified (gain, 10,000; A-M systems, Everett, WA), bandpass-filtered (100 Hz to 10 kHz), and integrated (CWE 821 filter; Paynter, Ardmore, PA; time constant, 50 msec). The signal was then digitized and recorded using the WINDAQ data acquisition system (DATAQ Instruments, Akron, OH) and later analyzed using custom-designed software on a platform of LabView.
Throughout surgery and experimental protocols, rats were ventilated with ~50% inspired O2, which establishes a baseline Pao2 ~250 mmHg. End-tidal CO2 was monitored throughout the experiment using a flow-through capnograph (Novametrix, Wallingford, CT). Rats were ventilated at a frequency of ~60–70 br/min and a ventilator volume of 2.5ml or less using a small animal ventilator (Rodent Ventilator, model 683; Harvard Apparatus, South Natick, MA, USA). This level of ventilation typically resulted in hypocapnia as detected by the end-tidal CO2 sensor and required a small amount of CO2 to be added to the inspired gas. After a stabilization period of 1hr, the apneic CO2 threshold was determined by progressively lowering the inspired CO2 until phrenic nerve activity ceased; the CO2 recruitment threshold was determined by then progressively raising the inspired CO2 until nerve activity resumed; the end tidal CO2 was then set 2–3 mmHg above the CO2 recruitment threshold (see Bach and Mitchell, 1996). PaCO2 throughout the experiment was maintained within 1mmHg of baseline by manipulating inspired CO2. Blood gases and blood pressure were monitored and corrected as necessary. Negative base excess values more severe than −3 mEq/L were corrected with intravenous sodium bicarbonate, and progressive reductions in blood pressure were offset with intravenous injection of lactated Ringer's solution. Phrenic and XII nerve activity was recorded continuously. Blood (0.3 ml in a heparinized syringe) was sampled once baseline nerve recordings were stabilized and then again at 30, 60 and 90 minutes post-episodic injections of either vehicle or 5-HT receptor agonists (see below). Measurements of phrenic and XII burst frequency and amplitude were evaluated in one minute bins immediately prior to each blood sample. At the end of the experiment, rats were dispatched by urethane overdose in accordance with ethical standards of the institution. Some rats were also perfused with 4% paraformaldeyde (PFA), the cervical spinal cord was removed, stored in PFA overnight and then for 3 days in 30% sucrose (sucrose in 1X phosphate buffered saline), and then at −80°C.
1.2 Drugs and vehicles
The following drugs were obtained from Sigma (Sigma-Aldrich, MO, USA): BW723C86 (5-HT2b receptor agonist), 2,5-dimethoxy-4-iodoamphetamine (DOI, a 5-HT2a agonist), SB206553 (5-HT2b antagonist), ketanserin (5-HT2a antagonist) and the NADPH oxidase inhibitors apocynin and DPI. All drugs were initially dissolved in dimethylsulphoxide (DMSO) on the day of the experiment and then diluted in artificial cerebral spinal fluid (aCSF) at DMSO:aCSF ratios determined by the solubility of each compound. However, DMSO was never used in excess of 20% aCSF, which was made fresh on the day of each experiment, although sometimes the remaining aCSF was stored refrigerated and used the following day. Prior to use, the aCSF was bubbled with a gas mixture consisting of 95% O2 and 5% CO2. Apocynin, DPI, ketanserin and DOI were mixed fresh each day, whereas aliquots of the other compounds remained viable for up to a week if stored frozen in DMSO, after which the drugs were discarded. In these situations, the mixture was thawed prior to each experiment and diluted by the appropriate amount of aCSF.
1.3 Protocol
Following stabilization of nerve signals, a baseline blood sample was taken, followed by 3 consecutive intrathecal injections (6µl each injection at 5 minute intervals) of agonists selective for the 5-HT2a (DOI) or 2b (BW723C86) receptor. Dose response curves were determined for each agonist to determine the effective dose. For DOI: 1µM (N=4), 10 µM (N=5), 100µM (N=6) and 1mM (N=5); and BW723C86: 10µM (N=5), 100µM (N=4), 1mM (N=6) and 10mM (N=4). In a previous study, micro-injections of the 5-HT2a (α-methyl-5-HT) or 2b (BW723C86) receptor agonists into the XII motor nucleus of neonatal rat brainstem slices enhanced tonic activity of the XII nerve (Gunther et al. 2006). In the same study, an injection of BW723C86 directly into the pre-Bötzinger complex also increased respiratory burst frequency suggesting 5-HT2 receptors can modulate the excitability of different brainstem respiratory regions. In our studies, assessment of XII nerve activity, therefore, served as an important internal control to determine if intrathecal injections near the phrenic motor nucleus resulted in unintended drug distribution to brainstem regions, as outlined previously (Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009). Thus, for either agonist, since we did not observe any changes in XII nerve activity (see Fig. 2) or changes in respiratory frequency (data not shown) even at doses that elicited phrenic motor facilitation, we could reasonably conclude that each agonist exerted localized effects to the phrenic motor region, rather than via unintended effects on brainstem respiratory regions.
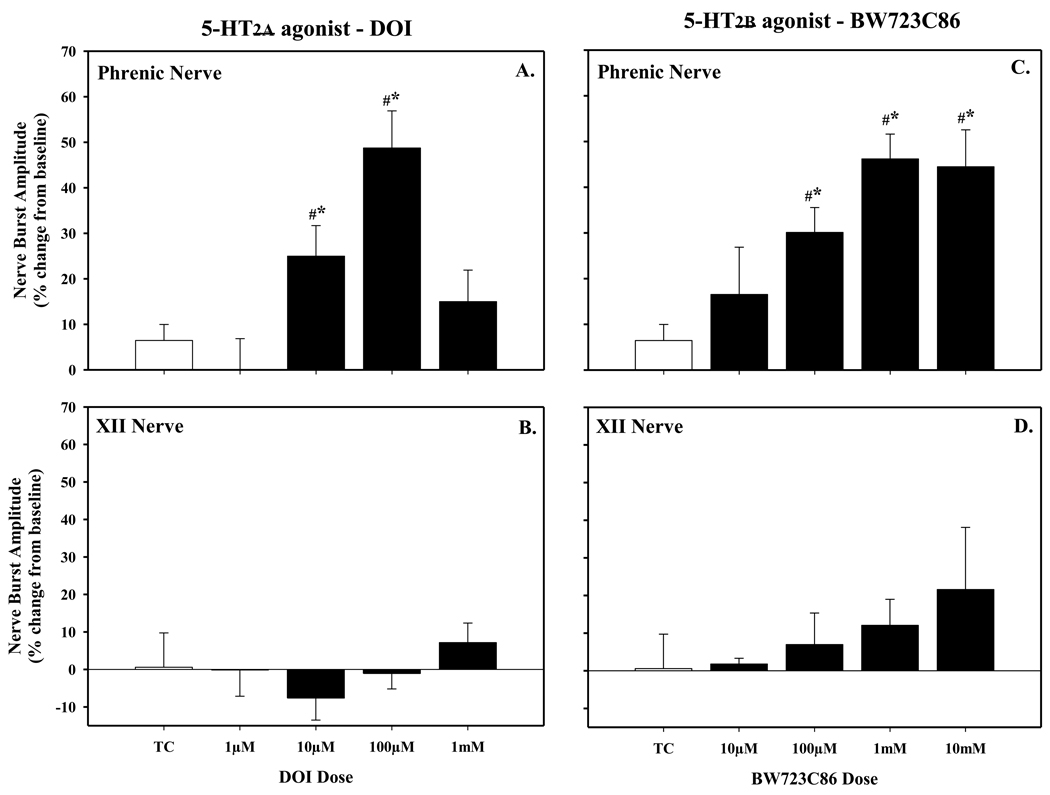
Figure 2.
Dose-response curve of phrenic motor facilitation (pMF) evaluated at 90 minutes following episodic (3×6µl at 5 minute intervals) intrathecal injections of the 5-HT2a agonist DOI (A) or 5-HT2b agonist (C) Neither episodic DOI or BW723C86 had any effect on XII nerve activity (B and C, respectively). DOI-induced pMF was maximal at 100µM dose, and subsequently chosen for additional experiments; at doses higher than 100µM, however, pMF was reduced, revealing a bell-shaped dose-response curve. Episodic BW723C86 also elicited dose-dependent pMF and was maximal at 1mM, whereas a higher dose had no further effect on the magnitude of pMF. The dose of 1mM of BW723C86 was also chosen for additional experiments. Values are means ± 1 SEM and expressed as % change from baseline amplitude (i.e. 0%). *significant difference from time control (TC, open bar) treated rats (P<0.05); #significant difference from baseline (P<0.05). Note, the TC rats (N=5) received episodic intrathecal injections of vehicle (i.e. DMSO:aCSF).
Additional groups of rats were pre-treated with relatively selective antagonists for each of the respective receptor subtypes. BW723C86-treated rats were pre-treated (20 minutes prior) with an injection of the 5-HT2b-selective antagonist, SB206553 (300µM, N=5) via a second intrathecal catheter. DOI-treated rats were pre-treated (20 minutes prior) with the 5-HT2a-selective antagonist ketanserin (500µM, N=3) instead. The same doses of each antagonist were also cross-checked against the opposing agonist (i.e. BW723C86 versus ketanserin (N=5) and DOI versus SB206553 (N=3)) to increase confidence in the relative selectively of the agonists. Appropriate time controls (TC’s) were performed where rats received only episodic vehicle (3×6µl, N=5) or bolus injection of the antagonists (SB206553, N=2; ketanserin, N=2). Additional groups of rats were used to test if the effective dose for each agonist required NADPH oxidase activity. BW723C86 (1mM) injected rats were pre-treated with a single bolus injection of apocynin (600µM, 15µl, N = 5) or diphenylenodium (DPI; 1mM, 15µl, N = 5) via a second intrathecal catheter ~20 minutes prior to episodic injection of the agonist. These experiments were also performed for DOI (100µM) injected rats (Apocynin, N = 5; DPI, N = 5). DPI and apocynin are functionally distinct inhibitors of NADPH (Hancock and Jones, 1987; O’Donnell et al. 1993) and these same doses were previously shown to inhibit AIH-induced pLTF (MacFarlane et al. 2008) and episodic 5-HT-induced pMF (MacFarlane and Mitchell, 2009). Time control (TC) experiments demonstrated that neither apocynin nor DPI affect phrenic nerve activity have been presented elsewhere (MacFarlane and Mitchell, 2009).
1.4 Immunohistochemistry
Immunohistochemistry was performed on four rats injected with a retrograde tracer, Cholera toxin B fragment (CtB, List Biologicals, Campbell CA, USA) to identify phrenic motor neurons in the cervical spinal cord. Each rat received an intrapleural injection of CtB for retrograde labeling of spinal phrenic motor neurons using a technique similar to that described previously (Mantilla et al. 2009). In brief, each rat was anaesthetized with isoflurane, and placed in the supine position on a surgical table while breathing into a face mask that supplied isoflurane (1–1.5%) on a background of O2 (100% O2). The rib cage was palpated in order to identify the fifth intercostal space at the anterior axillary line and a sterilized 27G needle attached to a 50µl Hamilton syringe was used to inject 25µl of a 0.2% CtB (dissolved in sterile injectable saline) on the left and another 25µl on the right side of the chest into the pleural space. The needle was modified so that it could be inserted no further than 6mm below the surface of the skin. After the injections, the animals were monitored closely for signs of respiratory distress associated with pneumothorax, isoflurane was discontinued and the rats were allowed to recover and monitored for a further 30 minutes. All rats recovered well and showed no sign of respiratory distress.
At 3 days post-injection, rats were again anesthetized with isoflurane, euthanized (0.3ml Beuthanasia-D, i.c., Schering-Plough, NJ. USA) and transcardially perfused with heparinized saline (4 IU/ml) followed by 4% PFA solution (freshly made in 0.1M phosphate buffer, pH adjusted to 7.4). 3 days post-CtB injections is sufficient time to allow for adequate retrograde tracing of the fragment from the site of injection (pleural space/diaphragm) to the spinal cord. The cervical spinal cord (~C3-C5) was removed and placed in 4% PFA overnight at 4°C, then transferred to 20% sucrose (PBS buffered) until it sank (typically overnight). Serial coronal sections (40µm thick) were prepared using a freezing microtome (Leica SM2000R) and stored in a cryoprotectant solution (30% ethylene glycol, 30% glycerol in PBS 0.1M). For double labeling with CtB and 5-HT2a receptor, free floating sections were washed with PBS 0.1M, incubated in a blocking solution (5% normal donkey serum, 0.1% triton in PBS for 30 min) and incubated overnight at 4°C with goat anti CtB antibody (1:10000, Calbiochem) and rabbit anti-5-HT2a receptor (1:200, courtesy of Dr. M. Brownfield). After several washes, the sections were incubated with a secondary antibody (Alexa 594 anti goat 1:1000 for CtB; Alexa 488 anti-rabbit for 5-HT2a 1:200; Molecular probes, Eugene, OR, USA) at room temperature for 2hr 30min. The sections were washed several times in PBS and mounted on slides (superfrost) using an antifade solution (Prolong Gold antifade reagent, Invitrogen, OR, USA).
For double labeling of CtB with 5-HT2b, two different 5-HT2b antibodies from two different species were used: mouse anti-5-HT2b (1:200, BD Pharmigen, lot 00173, San Jose, CA, USA) or rabbit anti-5-HT2b (1:200, ABCAM, AB32994). All tissues were processed as described previously, and incubated in a secondary antibody (1:200, Alexa 488 anti rabbit, Alexa 488 anti mouse, Molecular probes, Eugene, OR, USA).
Slides were examined with a confocal microscope (Eclipse TE 2000-U, Nikon, Japan) with EZ-C1 software (Nikon, Japan). Z-stacks were taken (30µm thick, 2µm steps) with 20× magnification objective and images were rendered with the max intensity finished using the 3D project from the EZ-C1 software.
1.5 Statistical analysis
Peak integrated nerve amplitude and frequency (bursts per minute) of phrenic and XII nerve activity were averaged in 1min bins at each recorded data point (baseline, 30, 60 and 90 minutes post-episodic injections of 5-HT agonists). Values were expressed as a % change from baseline. For changes in phrenic and XII nerve activity, within rat comparisons were made with baseline; whereas comparisons were also made between TC treated rats at all time points. Statistical comparisons were made for time and drug treatment using two-way, repeated measures ANOVA. The Bonferroni post hoc test was used to identify statistically significant individual comparisons. Differences were considered significant at p < 0.05. All values are expressed as mean ± 1 SEM.
2.0 Results
2.1 5-HT2a and 5-HT2b agonists induce pMF
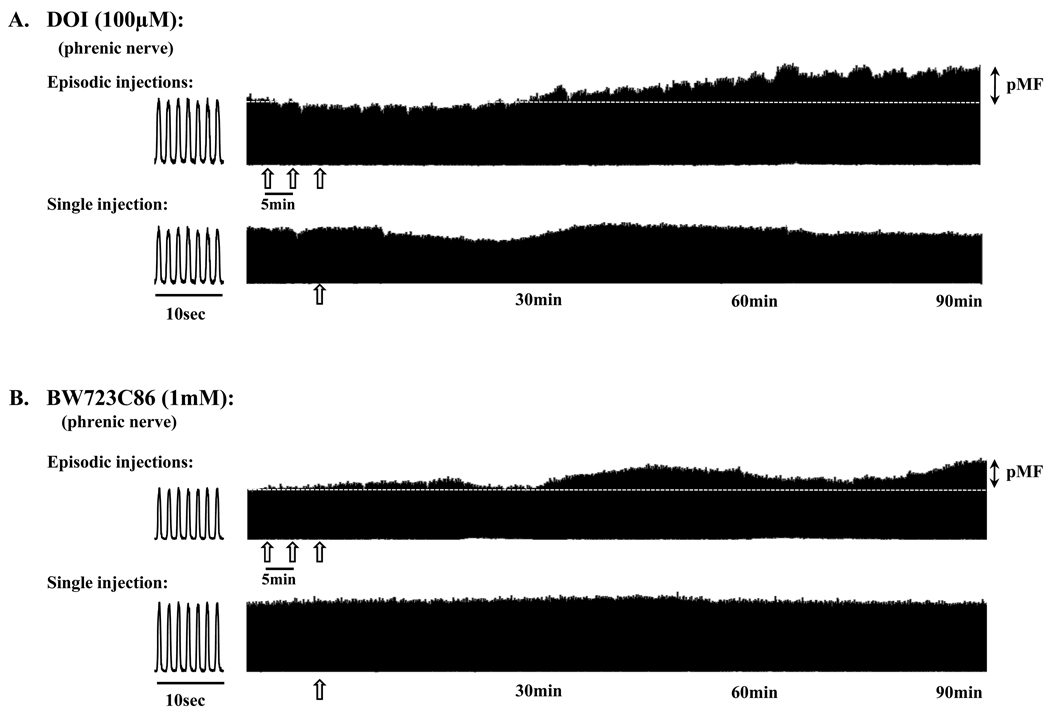
Episodic intrathecal injections (3×6µl) of the 5-HT2a agonist, DOI caused a progressive increase in phrenic (Fig. 1A) nerve burst amplitude lasting at least 90 minutes post-injection (i.e. phrenic motor facilitation, pMF). The magnitude of DOI-induced pMF was dose-dependent (Fig. 2A), increasing with each dose up to 100µM (48.7 ± 8.2% above baseline). A single injection of DOI failed to elicit pMF and there were also no obvious short-term neuromodulatory effects of DOI on phrenic nerve activity (Fig. 1A). pMF was no longer present above 100µM (1mM, 15.0 ± 6.9%), revealing a bell-shaped dose-response curve (Fig. 2A) similar to serotonin (MacFarlane and Mitchell, 2009). DOI had no effect on XII nerve activity at any dose studied (Fig. 2B) suggesting that the drugs had not distributed to the brainstem and increased descending respiratory drive (ie. XII acts as an internal control for drug distribution).
Figure 1.
Representative phrenic neurograms from four rats that received episodic (top traces) or a single (bottom traces) intrathecal injection of agonists selective for (A) the 5-HT2a receptor (DOI, 100µM) or (B) the 5-HT2b receptor (BW723C86, 1mM). Note the increase in phrenic nerve burst amplitude (i.e. phrenic motor facilitation, pMF) up to 90 minutes following the last injection for each agonist, whereas a single injection had no effect. Open arrows indicate an injection of the agonist. In experiments that addressed the effects of episodic intrathecal injections, each injection was administered at 5 minute intervals (6µl/injection). Dashed line extrapolates baseline (i.e. pre-injection) nerve burst amplitude. 10 second bins illustrating several breaths for each rat are provided at the beginning of the traces.
Similar results were observed following episodic intrathecal injections (3×6µl) of the 5-HT2b agonist, BW723C86, which also caused a progressive increase in phrenic (Fig. 1B) nerve burst amplitude lasting at least 90 minutes post-injection (i.e. phrenic motor facilitation, pMF). A single injection of BW723C86 did not elicit pMF and there were no short-term neuromodulatory effects of BW723C86 injections on spontaneous phrenic nerve activity at the doses studied (Fig. 1B). The magnitude of pMF was also dose-dependent and highest at 1mM (46.2 ± 5.4%, above baseline, Fig. 2C). A higher dose (10mM) had no further effect on pMF magnitude (44.5 ± 8.1%). The magnitude of BW723C86-induced pMF was similar in magnitude to DOI-induced pMF (c.f. BW723C86, 1mM, 46.2 ± 5.4%), albeit at an order of magnitude higher dose (1mM vs 100µM). Interestingly, in contrast to DOI, there was no bell-shaped dose-response curve with BW723C86. At all doses tested, XII nerve activity was unaffected (Fig. 2D).
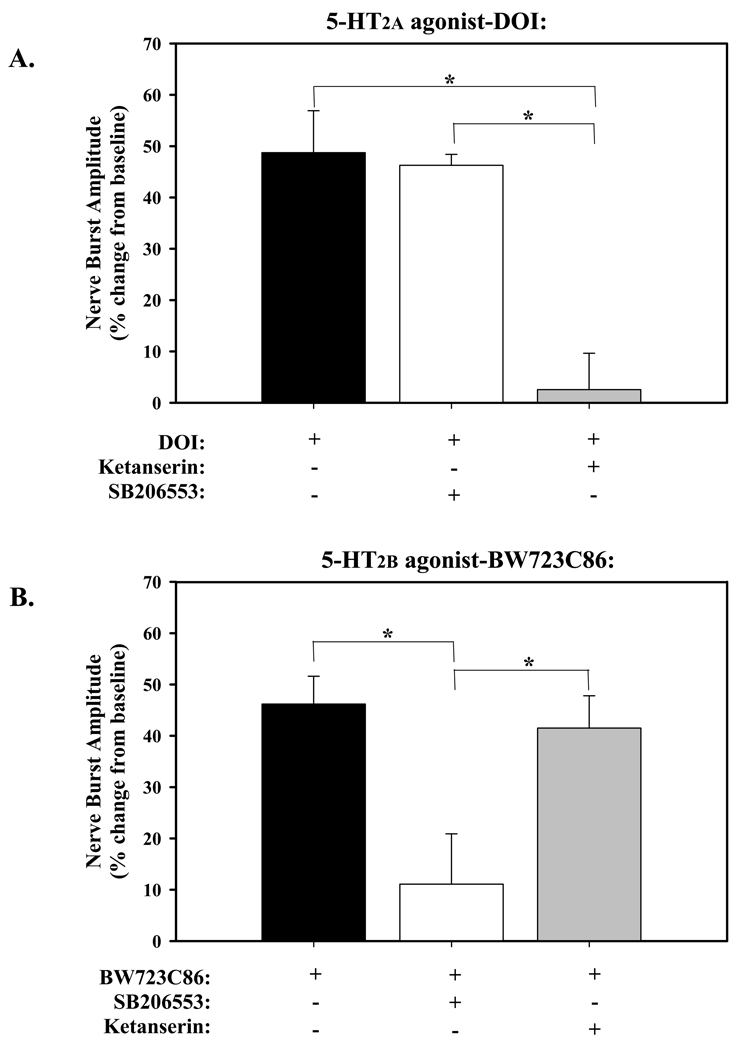
2.2 Selective antagonists block pMF
pMF elicited by the 5-HT2a agonist, DOI, was blocked by a 500µM intrathecal injection of the 5-HT2a selective antagonist, ketanserin (2.6 ± 7.1%; Fig. 3A). However, the same dose of ketanserin did not affect the magnitude of BW723C86-induced pMF (41.5 ± 6.3%; Fig. 3B). pMF elicited by the 5-HT2b agonist, BW723C86, was blocked by pre-treatment with a 300µM intrathecal injection of the 5-HT2b selective antagonist, SB206553 (11.1 ± 9.8%; Fig. 3B). In contrast, SB206553 (at the same dose) had no effect on DOI (5-HT2A)-induced pMF (46.2 ± 2.1%; Fig. 3A).
Figure 3.
Effects of pre-treatment with 5-HT2 receptor antagonists on pMF. A) 5-HT2a agonist-DOI: pre-treatment with ketanserin (5-HT2a antagonist, 500µM) blocked DOI-induced pMF, whereas pre-treatment with the SB206553 (5-HT2b antagonist, 300µM) had no effect. B) 5-HT2b agonist-BW723C86: pre-treatment with the dose of ketanserin that successfully blocked DOI-induced pMF had no effect on BW723C86-induced pMF, whereas pre-treatment it was blocked by SB206553. Values are means ± 1SEM and expressed as a % change from baseline (evaluated at 90minutes post-injection of agonists). *significant difference between treatment groups as indicated (P<0.05). Each antagonist was 12µl in volume and injected 20 minutes prior to episodic injections of the agonist.
These results confirm that DOI and BW723C86 were selective with respect to their intended target receptors, and that 5-HT2a and 5-HT2b receptors independently elicit pMF.
2.3 5-HT2a and 5-HT2b receptor immunoreactivity in the phrenic motor nucleus
Retrogradely labeled phrenic motor neurons (Cholera toxin-B immuno-positive) were identified in clusters in the ventral-lateral gray matter in coronal sections of ~C4 in all four rats receiving intrapleural injections. A representative section from one rat indentifying CtB-positive phrenic motor neurons co-labeled with the 5-TH2b receptor is provided in Figure 4A. 5-HT2b receptor labeling was observed in the neuropil and at the membrane surface of CtB-positive phrenic motor neurons (Fig. 4D) as well as intensely stained in their nuclei (Fig. 4D). Similar staining is seen in different (inter-) neurons of the same slices. Even though we used two different antibodies for the 5-HT2b receptor, the pattern of staining was the same with both of them (data not shown). The 5-HT2a receptor labeling was also observed in phrenic motor neurons although it is difficult to definitively determine if they are also located on the membrane (Fig. 4E). 5-HT2a receptor labeling was also diffusely in the neuropil and in unlabeled neurons near neighboring the phrenic motor neurons. Thus, identified phrenic motor neurons express both 5-HT2a and 5-HT2b receptors, consistent with the observation that both 5-HT2a and 5-HT2b agonists are both capable of eliciting pMF, although the role of these receptors in cell types other than phrenic motor neurons cannot be ruled out using our approach.
Figure 4.
Double immunofluorescent staining of 5-HT2a and 2b receptors (green) with the retrograde tracer (red) Cholera toxin B fragment (CtB) in phrenic motor neurons in the cervical spinal cord (~C4 region). (A) 4× magnification of the C4 spinal cord area containing CtB-labeled phrenic motor neurons (red) located bilaterally in the ventral horn and labeled with the 5-HT2b receptor (green); scale bar: 1mm. (B) Higher magnification (20×) of the clustered phrenic motor neurons within the dashed square region outlined in (A), and a 20× magnification (C) of phrenic motor neurons labeled with 5-HT2a receptors in a different rat. In (B), note the CtB positive phrenic motor neurons (red) are co-labeled with intense nuclear staining (arrows) as well as in the surrounding neuropil for the 5-HT2b receptor (green); scale bar: 100µm. Under 100× magnification (D), the 5-HT2b receptor also appears to be in the membrane (middle and right panels) of phrenic motor neurons (arrow heads). Note, 100× magnification (E) of CtB positive phrenic motoneurons (red) co-labeled with the 5-HT2a receptor (green), which is located in the cytoplasm (arrow heads) of phrenic motor neurons and the surrounding neuropil; scale bar: 10µm.
2.4 NADPH oxidase activity is necessary only for 5-HT2B-induced pMF
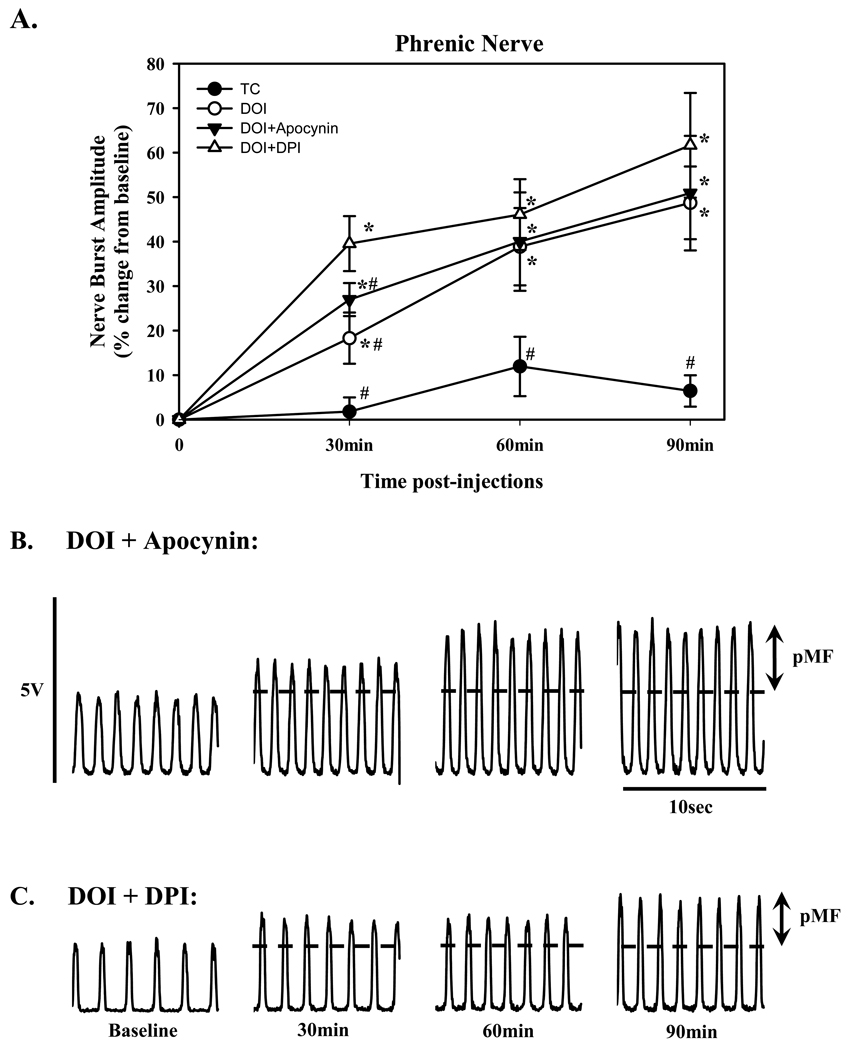
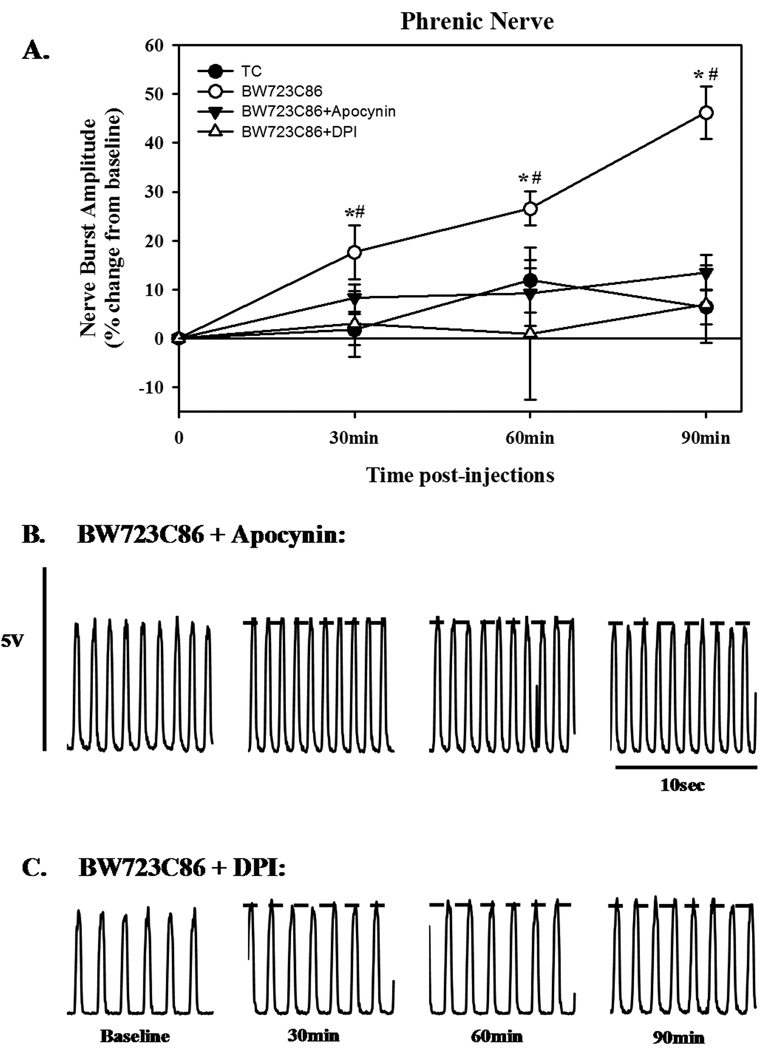
Optimal doses of BW723C86 (1mM) and DOI (100µM) were chosen for experiments testing whether NADPH oxidase activity is necessary for 5-HT receptor induced pMF. The effects of intrathecal injections of apocynin and DPI were similar to one another, but had differential effects on BW723C86- versus DOI-induced pMF. Neither apocynin (50.9 ± 12.9%) nor DPI (61.7 ± 11.8%) had any significant effect on DOI-induced pMF (Fig. 5). In contrast, apocynin (600µM) or DPI (1mM) significantly attenuated (13.5 ± 3.6% and 7.7 ± 11.2 %, respectively) BW723C86-induced pMF (Fig. 6).
Figure 5.
Phrenic motor facilitation (pMF) following episodic (3×6µl at 5 minute intervals) intrathecal injections of the 5-HT2a agonist, DOI (100µM) and the effects of pre-treatment with intrathecal injections of the NADPH oxidase inhibitors, apocynin and DPI. A) pMF evaluated at various time-points (as indicated) following episodic DOI (open circle). Note, that pre-treatment with either apocynin (solid triangle, 600µM) or DPI (open triangle, 1mM) 20 minutes prior to DOI had no effect on the agonist-induced pMF. TC treated group is also included (Closed circle). Values are means ± 1 SEM and expressed as % change from baseline amplitude (i.e. 0%). *significant difference from baseline value (P<0.05); #significant difference from DOI-treated rats. Below are representative neurograms (10 sec bins at baseline, 30, 60, and 90 minutes), illustrating the effects of pre-treatment with apocynin B) and DPI C) on DOI-induced pMF.
Figure 6.
Phrenic motor facilitation (pMF) following episodic (3×6µl at 5 minute intervals) intrathecal injections of the 5-HT2b agonist, BW723C86 (1mM) and the effects of pre-treatment with intrathecal injections of the NADPH oxidase inhibitors, apocynin and DPI. A) pMF evaluated at various time-points (as indicated) following episodic BW723C86 (open circle). Note, that pre-treatment with either apocynin (solid triangle, 600µM) or DPI (open triangle, 1mM) 20 minutes prior to BW723C86 inhibited the agonist-induced pMF. TC treated group is also included (Closed circle). Values are means ± 1 SEM and expressed as % change from baseline amplitude (i.e. 0%). *significant difference from baseline value (P<0.05); #significant difference from time control-treated rats. Below are representative neurograms (10 sec bins at baseline, 30, 60, and 90 minutes), illustrating the effects of pre-treatment with apocynin B) and DPI C) on pMF.
3.0 Discussion
Our results demonstrate that: 1) episodic spinal 5-HT2a or 2b receptor activation (without hypoxia/re-oxygenation) are both sufficient to elicit phrenic motor facilitation (pMF) in vivo; 2) both receptors are expressed in identified phrenic motoneurons; 3) the magnitude of agonist-induced pMF is dose-dependent, although only DOI-induced pMF revealed a bell-shaped curve; and 4) 5-HT2b, but not 5-HT2a receptor induced pMF requires spinal NADPH oxidase activity. These data provide important insight concerning the varied Gq-coupled receptor mechanisms whereby serotonin elicits spinal respiratory plasticity.
3.1 Episodic 5-HT2a and 2b-induced pMF
Episodic intrathecal injections of 5-HT2a or 2b agonists (DOI and BW723C86, respectively) elicit pMF lasting at least 90 minutes post-injection, an effect similar in magnitude and time course to AIH-induced phrenic long-term facilitation (pLTF) and 5-HT induced pMF (MacFarlane and Mitchell, 2009). There was no effect on XII nerve activity (i.e. no facilitation) with either agonist at any dose tested, suggesting that effective doses did not reach the brainstem through unintended drug distribution. Single injections of either agonist had no effect on phrenic nerve discharge, suggesting episodic 5-HT2a and 5-HT2b receptor activation is necessary to induce pMF. In contrast to a previous study, a single intravenous injection of the 5-HT2a agonist DOI was sufficient to restore activity in the phrenic nerve ipsilateral to C2 spinal “hemisection” for up to 2 hrs after the injection (Zhou et al., 2001). The sustained effects of a single injection of DOI in a rodent model of a spinal injury are difficult to compare with our model, but could be related to the drastic effects that spinal injury has on the spinal cord. Collectively, our data support previous findings that episodic spinal 5-HT injections elicit pattern-sensitive pMF (MacFarlane et al. 2009). We speculate that the same mechanisms underlie AIH-induced phrenic LTF, although this hypothesis remains to be tested (see below).
Both 5-HT2a and 2b receptor activation elicited dose-dependent pMF. However, the shape of those dose response curves were quite different since only the 5-HT2a receptor agonist revealed a bell-shaped curve whereas 5-HT2b receptor did not. Although we cannot be sure that higher doses of the 5-HT2b agonist would not reveal a bell-shaped curve, the 5-HT2a effect is reminiscent of 5-HT induced pMF shown previously (MacFarlane and Mitchell, 2009). In that study, we demonstrated that the declining phase (i.e. at higher doses) of the pMF dose response curve was determined by the activation of opposing 5-HT receptors. For example, 5-HT2 receptor activation initiates cellular processes that promote pMF, whereas other receptors, specifically the 5-HT7 subtype, inhibit pMF to an extent that depends on the availability of extracellular 5-HT (MacFarlane and Mitchell, 2009). Similarly, at the highest dose of DOI studied, this agonist may lose selectivity and activate other 5-HT receptors that inhibit 5-HT2a induced pMF in a way shown previously with progressively higher doses of 5-HT (MacFarlane et al. 2009).
The differential dose-dependent effects of DOI and BW723C86 further support the idea that these agonists elicit pMF via different receptor sub-types. This relative selectivity was confirmed by the ability of selective antagonists (ketanserin vs SB206553) to block the appropriate form of pMF, but not pMF elicited by the other receptor agonist (see Fig. 3). Specifically, the 5-HT2a selective antagonist ketanserin completely blocked DOI-induced pMF, but had no effect on BW723C86-induced pMF. The converse was true for the 5-HT2b selective antagonist, SB206553, which had no effect DOI-induced pMF (Fig. 3). Collectively, there is strong supportive data to suggest that the supposedly selective 5-HT2 receptor agonists had their intended effects without any cross-over to the other receptor subtype. Since selective spinal activation of each receptor subtype elicits pMF, we wondered if they do so via the same cellular mechanism since both are Gq-protein coupled metabotropic receptors (Hannon and Hoyer, 2008). We tested this idea by exploring their requirement for spinal NADPH oxidase activity.
3.2 Only 5-HT2B-induced pMF requires spinal NADPH oxidase activity
Since BW723C86-induced pMF (1mM) was attenuated by intrathecal DPI or apocynin, and 5-HT2a induced pMF was not, there is clear evidence that these receptor subtypes differ in the details of the mechanisms giving rise to pMF. The requirement of 5-HT2b induced pMF for spinal NADPH oxidase activity and ROS formation is consistent with AIH-induced pLTF, which also requires spinal 5-HT receptor activation (Fuller et al. 2001; Baker-Herman and Mitchell, 2002) and ROS formation (MacFarlane et al. 2008) via NADPH oxidase activity (MacFarlane et al. 2009). Since key subunits of the NADPH oxidase complex are localized in putative phrenic motor neurons (MacFarlane et al. 2009), we propose that pMF following episodic 5-HT2b receptor activation reflects the same underlying mechanism as AIH-induced pLTF (MacFarlane et al. 2009). However, the requirement of AIH-induced pLTF for selective 5-HT2a vs 2b receptor activation has not been thoroughly explored.
NADPH oxidase activity is important for multiple forms of neuroplasticity, including hippocampal LTP (Kishida et al. 2006; see Kishida and Klann, 2007) and sensory facilitation of the carotid body (Peng et al. 2006; 2009). In the latter case, sensory facilitation of the carotid body following CIH was mimicked by episodic application of 5-HT and was blocked by the 5-HT2a receptor antagonist, ketanserin (Peng et al. 2006). This finding is in contrast to the current study in which only the BW723C86-induced pMF required NADPH oxidase activity, whereas DOI-induced pMF did not. Mechanistic distinctions giving rise to differential NADPH oxidase dependence of the two forms of plasticity is unknown. 5-HT2b receptors have been shown to increase NADPH oxidase activity. For example, angiontensin II stimulated a 5-HT2b-dependent increase in NADPH oxidase activity in cardiac fibroblast cells and antagonists to the 5-HT2b receptor prevented an angiontensin II-induced increase in ROS formation and ventricular hypertrophy in vivo (Monassier et al. 2008). 5-HT2b receptor activation also increased NADPH oxidase-derived ROS formation in 1C11 cell lines (Schneider et al. 2006). The differential effects of apocynin/DPI on receptor-induced pMF confirm that the respective agonists (DOI and BW723C86) elicit pMF via different receptors (and different downstream mechanisms). Although NADPH oxidase activity is a common theme to AIH-induced pLTF and CIH-induced carotid body sensory LTF, receptor antagonist studies do not allow us to determine if perhaps both receptor subtypes must be activated to achieve the full response. If both receptors must be activated, then the response could be blocked with antagonists targeting either receptor subtype alone, or by inhibitors of mechanism initiated by a single receptor subtype, for example, inhibition of NADPH oxidase. Thus, there are currently no studies available to test the hypothesis that both 5-HT2a and 2b receptors must be activated to induce either AIH-induced pLTF or sensory LTF after CIH pre-treatment. The results revealing that successful blockade of these forms of plasticity with ketanserin alone (Fuller et al. 2001; Peng et al. 2006), therefore, does not exclude the possibility that multiple serotonin receptors do not play a role.
3.3 Conclusions
We provide evidence that episodic activation of either spinal 5-HT2a or 2b receptors is sufficient to elicit phrenic motor facilitation, but that they do so via mechanisms that differ in their requirement for NADPH oxidase activity. Our data are consistent with reports concerning the role of spinal serotonin receptors (Fuller et al. 2001; Baker-Herman and Mitchell, 2002) and NADPH oxidase (MacFarlane et al. 2009) in AIH-induced pLTF, suggesting that both forms of plasticity share a common mechanism (MacFarlane and Mitchell, 2009). Several lines of evidence indicate that agonists used successfully targeted the intended receptor including: 1) different shapes of the dose/response curves, 2) the ability of selective antagonists to block the relevant agonist-induced pMF, but not pMF induced by the other receptor agonist; and 3) differential sensitivity to NADPH oxidase inhibitors.
We provide evidence of 5-HT2a receptors within and around positively identified phrenic motor neurons, which is consistent with previous reports (Basura et al., 2001). On the other hand, although very little is known about 5-HT2b receptor distribution anywhere in the CNS, we provide the first evidence of its existence located in phrenic motor neurons and, specifically within the membrane. Although the presence of 5-HT2b (and 5-HT2a) receptors on phrenic motor neurons supports their involvement in spinal mechanisms of respiratory plasticity (e.g. following AIH), their localisation in the surrounding neuropil and in neighbouring (inter-) neurons means we are unable to conclusive exclude the involvement of neighbouring cells involved in the expression of pMF. Further work is necessary to determine differences in the cellular pathways that underlie DOI- and BW723C86-induced pMF. Collectively our data provide further insight concerning mechanisms giving rise to spinal mechanisms of respiratory plasticity.
4.0 Acknowledgements
This work was funded by a grant from the NIH (NIH HL-80209). We thank Brad Hodgeman for technical assistance. Special appreciation is extended to Safraaz Mahammed for his custom-designed computer program used for data analysis. Peter MacFarlane was a recipient of a Parker B Francis Fellowship from the Family Francis Foundation. Stéphane Vinit is supported by a Craig H. Neilsen Foundation Fellowship.
Abbreviations
- AIH
acute intermittent hypoxia
- 5-HT
serotonin
- ROS
reactive oxygen species
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- pMF
phrenic motor facilitation
- pLTF
phrenic long-term facilitation
- C4
cervical spinal section 4
- MAP
mean arterial blood pressure
- PO2
partial pressure of Oxygen
- PCO2
partial pressure of Carbon Dioxide
- XII
hypoglossal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol (Lond) 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol. 2001;129:25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Baker-Herman T, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22(14):6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7(1):48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10(5):373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169(2):255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4(7):895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Dinger B, Hea L, Chen J, Liua X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol & Neurobiol. 2007;157:45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001;21:1532–1537. doi: 10.1523/JNEUROSCI.21-05-01532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 1991;567:11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fabian RH, DeWitt DS, Kent TA. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood Flow Metab. 1995;15(2):242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Perez-Polo RJ, Kent TA. Extracellular superoxide concentration increases following cerebral hypoxia but does not affect cerebral blood flow. Int J Devl Neurosci. 2004;22:225–230. doi: 10.1016/j.ijdevneu.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic nerve output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Selected contribution: phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, special learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Günther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2b receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66(9):949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Br Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Henderson LM, Chappell JB. NADPH oxidase of neutrophils. Biochim Biophys Acta. 1996;1273:87–107. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. TRK receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huber KM, Sawtell NB, Bear MF. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology. 1998;37(4–5):571–579. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. The J Biol Chem. 2007;28(48):34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxyamidotryptamine. Am J Physiol. 1999;277:R658–R666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog. Neuropsychopharmacol Biol Psychiatry. 1999;23(3):359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Klann E, Roberson ED, Knapp LT, Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273(8):4516–4522. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002;22(3):674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science. 2002;295(5560):1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol & Neurobiol. 2008;164(1–2):64–71. doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neurosci. 2008;152(1):189–197. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol & Neurobiol. 2008;164(1–2):263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol. 2009;587(9):1931–1942. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587(Pt 22):5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labelling of phrenic motor neurons by intrapleural injections. J Neurosci Methods. 2009;182(2):244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka N, Nakajima Y, Hayakawa Y, Ohgame S, Hamano S, Nagaishi M, Yamamoto T. Transplacental effects of allopurinol on suppression of oxygen free radical production in chronically instrumented fetal lamb brains during intermittent umbilical cord occlusion. J Matern Fetal Neonatal Med. 2005;18(1):1–7. doi: 10.1080/14767050500127716. [DOI] [PubMed] [Google Scholar]
- Millhorn DA, Eldridge FE, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42:171–198. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90(6):2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94(1):358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Monassier L, Laplante MA, Jaffré F, Bousquet P, Maroteaux L, de Champlain J. Serotonin 5-HT(2B) receptor blockade prevents reactive oxygen species-induced cardiac hypertrophy in mice. Hypertens. 2008;52(2):301–307. doi: 10.1161/HYPERTENSIONAHA.107.105551. [DOI] [PubMed] [Google Scholar]
- Pellmar TC. Peroxide alters neuronal excitability in the CA1 region of guinea pig hippocampus in vitro. Neurosci. 1987;51:1960–1963. doi: 10.1016/0306-4522(87)90068-6. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body of intermittent hypoxia: implications for recurrent apneas. PNAS. 2003;100(17):10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol. 2001;281(3):L524–L528. doi: 10.1152/ajplung.2001.281.3.L524. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem. 2004;385:217–221. doi: 10.1515/BC.2004.015. [DOI] [PubMed] [Google Scholar]
- Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCε signaling axis in pulmonary artery smooth muscle cells. Free Rad Biol Med. 2008;45(9):1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Ourv TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ward N, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci. 2005;23(6):1665–1670. doi: 10.1111/j.1460-9568.2006.04682.x. [DOI] [PubMed] [Google Scholar]
- Wilkerson JER, Baker-Herman TL, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation following sustained hypoxia. FASEB J. 2006 doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126(2):313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557(3):773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001a;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol (Lond) 2001b;531:509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91(6):2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]